Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Anemia is defined as a hemoglobin (Hb) value less than the lower limit of normal not explained by the state of hydration. This definition has physiologic validity, in that it is the amount of Hb per unit volume of blood that determines the oxygen-carrying capacity of blood. The normal Hb level for the adult female is 14.0 ± 2.0 g/dL. Defining anemia as a Hb level of less than 12 g/dL, the incidence of anemia was 6.9% in US nonpregnant women younger than 50 years of age. The incidence of anemia (Hb level <12 g/dL) was significantly higher in Black women than in White women, with an incidence of 24% versus 3%, respectively. This higher prevalence in US Black women persists in data from 2003–12, where Black reproductive-age women had a four- to seven-fold higher incidence of anemia compared to White women. In another study, 32% of women presenting at less than 7 weeks’ gestation had an Hb value lower than 12 g/dL, suggesting that the prevalence of anemia is high. In pregnancy, because of the relatively greater increase in plasma volume compared with red cell mass (i.e., hemodilution of pregnancy), anemia is defined by the Centers for Disease Control and Prevention (CDC) as an Hb concentration lower than 11 g/dL in the first and third trimesters or lower than 10.5 g/dL in the second trimester. Of note, definitions of anemia in pregnancy vary across publications. Between 1995 and 2011, the global incidence of anemia (Hb level of less than 11 g/dL throughout pregnancy) fell from 43% to 38%, and in one-half of cases anemia was caused by iron deficiency. In this same study, the incidence of anemia in pregnant women from high-income regions was 22% and approximately 17% in the United States.
Symptoms caused by anemia result from tissue hypoxia, the cardiovascular system’s attempts to compensate, or an underlying disease. Tissue hypoxia produces fatigue, lightheadedness, weakness, and exertional dyspnea. Cardiovascular compensation leads to a hyperdynamic circulation, with attendant symptoms of palpitations and tachycardia. Clinical conditions commonly associated with anemia in pregnancy include malnutrition, multiple pregnancy, trophoblastic disease, chronic renal disease, chronic liver disease, and chronic infection. In obstetric patients, however, anemia is most commonly discovered not because of symptoms but because a complete blood count (CBC) is obtained as part of a routine laboratory evaluation.
Anemia is not a diagnosis; rather, like fever or edema, it is a sign. The key issue in the evaluation of anemia is to discover the underlying mechanism or pathologic process. Although a mild anemia caused by iron deficiency during pregnancy is of little consequence to either the mother or the fetus, a similarly mild anemia caused by carcinoma of the colon has grave implications. Furthermore, many anemias, such as the hemoglobinopathies and thalassemias, have genetic implications for the fetus.
Box 55.1 presents a classification of anemia based on the pathophysiologic mechanism involved. Although a mechanistic classification of anemia provides an exhaustive catalog of diagnoses, it does not lend itself to a systematic investigation of an individual patient. Rather, when the patient is anemic, one wants to know (1) the morphology of the anemia, and (2) the reticulocyte count. Determining the answers to these questions allows one to make a first approximation of a specific diagnosis and to answer the following questions:
What is the mechanism of the anemia?
Is there an underlying disease?
What treatment is appropriate?
Dilutional (expansion of the plasma volume)
Pregnancy
Hyperglobulinemia
Massive splenomegaly
Decreased RBC production
Bone marrow failure
Decreased building blocks or stimulation
Iron or protein deficiency
Chronic infection, chronic renal disease
Decreased erythron
Hypoplasia (hereditary, drugs, radiation, toxins)
Marrow replacement (tumor, fibrosis, infection)
Ineffective production
Megaloblastic (vitamin B 12 and folate deficiency, myelodysplasia, erythroleukemia)
Normoblastic (refractory anemia, thalassemia)
Increased RBC loss
Acute hemorrhage
Hemolysis
Intrinsic RBC disorders
Hereditary
Hemoglobinopathies
RBC enzyme deficiency
Membrane defects
Porphyrias
Acquired
Paroxysmal nocturnal hemoglobinuria
Lead poisoning
Extrinsic RBC disorders
Immune
Mechanical
Infection
Chemical agents
Burns
Hypersplenism
Liver disease
The CBC and the reticulocyte count provide answers to the first two questions. These data allow a morphologic classification of the anemia and indicate whether the marrow is hyperproliferative or hypoproliferative. The patient’s Hb value is determined by converting the hemoglobin to cyanmethemoglobin and quantitating the amount spectrophotometrically. The rest of the CBC values are either obtained by direct measurement via electronic resistance detection and flow cytometry, or are calculated from directly measured parameters.
Based on the size of the red blood cells (RBCs) or the mean corpuscular volume (MCV), anemia can be classified as microcytic, normocytic, or macrocytic. The appearance of the RBCs may also provide a clue to the mechanism of the anemia. For example, hypochromic microcytic cells associated with a low reticulocyte count suggest iron deficiency, thalassemia trait, sideroblastic anemia, or lead poisoning. Oval macrocytes combined with a low reticulocyte count and hypersegmented neutrophils suggest megaloblastic anemia (vitamin B 12 or folate deficiency). Hyperchromic spherocytes and an elevated reticulocyte count are characteristic of hereditary spherocytosis. Various poikilocytes, such as sickle cells, blister cells, target cells, and schistocytes, suggest sickle cell disease, oxidant stress–related acute hemolysis such as in glucose-6-phosphate dehydrogenase deficiency, hemoglobinopathies including thalassemias, and mechanical RBC destruction, respectively. Although the CBC is an excellent first step in the approximate diagnosis of anemia, additional studies are usually necessary to confirm the diagnosis. Table 55.1 lists laboratory studies frequently used in the evaluation of an anemic patient.
| Laboratory Study | Reference Range (by Trimester When Available) |
|---|---|
| Red blood cell (RBC) count | 3.42–4.55 × 10 12 /L (first) 2.81–4.49 × 10 12 /L (second) 2.72–4.43 × 10 12 /L (third) |
| Mean corpuscular volume (MCV) | 81–96 μm 3 (first) 82–97 μm 3 (second) 81–99 μm 3 (third) |
| Mean corpuscular hemoglobin concentration (MCHC) | 32–35 g/dL |
| Reticulocyte count | 48–152 × 10 9 /L (0.5%–1.5%) |
| Lactate dehydrogenase (LDH) | 78–433 U/L (first) 80–447 U/L (second) 82–524 U/L (third) |
| Serum haptoglobin | 30–200 mg/dL |
| Total bilirubin | 0.1–0.4 mg/dL (first) 0.1–0.8 mg/dL (second) 0.1–1.1 mg/dL (third) |
| Direct Coombs test | Negative |
| Hb electrophoresis | >98% A |
| <3.5% A 2 | |
| <2% F | |
| Serum ferritin | >20 μg/L |
| Serum iron | 72–143 μg/dL (first) 44–178 μg/dL (second) 30–193 μg/dL (third) |
| Total iron-binding capacity | 235–408 μg/dL (first) 302–519 μg/dL (second) 580–597 μg/dL (third) |
| Transferrin saturation | 16%–60% |
| Folate level: | |
| Serum | 26–150 μg/L (first) 8–240 μg/L (second) 14–207 μg/L (third) |
| Red blood cells | 137–589 ng/mL (first) 94–828 ng/mL (second) 109–663 ng/mL (third) |
| Serum vitamin B 12 | 118–438 pg/mL (first) 130–656 pg/mL (second) 99–526 pg/mL (third) |
| Anti–intrinsic factor (AIF) antibody | Negative |
Lactate dehydrogenase (LDH) and haptoglobin levels are useful in defining intravascular hemolysis. If haptoglobin is absent or low in conjunction with an elevated LDH level, the presence of intravascular hemolysis is supported. Undetectable haptoglobin values are virtually pathognomonic of hemolysis, except in the rare individuals with congenital haptoglobin deficiency. Further studies are necessary to rule in or rule out specific causes of intravascular hemolysis, such as severe autoimmune hemolytic anemia (direct Coombs test), paroxysmal nocturnal hemoglobinuria (PNH; flow cytometry), hemoglobinopathies including sickle cell disease and thalassemia (high-performance liquid chromatography [HPLC]), and other microangiopathic hemolytic anemias.
Total bilirubin is elevated modestly in hemolytic anemia (rarely more than 4 mg/dL). The increase results predominantly from an increase in the indirect fraction. However, significant hemolysis can occur without an elevation in the bilirubin. Therefore, the bilirubin level is helpful only when it is elevated.
The direct Coombs test uses anti–human immunoglobulin to detect immunoglobulins attached to the surface of RBCs. A positive test indicates an immune cause for a hemolytic anemia. In such cases, it is important to search for underlying causes of autoimmunity, such as connective tissue disease, lymphoma, and carcinoma.
Iron studies including serum iron, total iron-binding capacity (TIBC), percentage saturation, and ferritin are the most routinely utilized tests in establishing a diagnosis of iron deficiency, with other biomarkers such as soluble transferrin receptor (sTfR) and hepcidin showing promise as indicators of iron status. In the iron-deficient state, serum iron decreases, TIBC increases, and percentage saturation decreases. In contrast, in anemia of chronic disease, both serum iron and the TIBC are decreased but the percentage saturation usually remains normal. Ferritin correlates closely with body iron stores, and many investigators support the use of serum ferritin as the best single test to make a diagnosis of iron deficiency even prior to the development of anemia. , A ferritin level of 12 μg/L or lower is consistent with iron deficiency anemia, and some authors use values of less than 15 to 20 μg/L. , While ferritin is currently the preferred test to evaluate stored iron, it is also an acute phase reactant, and therefore it may not be a good measure of stored iron in patients with chronic inflammatory disorders. The sTfR assay may be considered if a routine iron panel and ferritin results do not provide conclusive information regarding iron stores. Since sTfR is not affected by chronic disease, an increased value supports iron deficiency anemia (IDA). However, there are limitations to its interpretation due to lack of assay standardization and common reference ranges, including in pregnant women. An elevated sTfR is not as specific for iron deficiency as a very low ferritin level. Blacks as well as those living at higher elevations may have moderately increased sTfR levels. Additionally, it is elevated in alpha thalassemia trait. , Serum iron and ferritin levels are both increased after ingestion of iron. , Therefore iron therapy must be discontinued for 24 to 48 hours before these studies are carried out.
Iron deficiency and its effects at the cellular level occur prior to the development of anemia. Therefore there is increasing interest in identifying biomarkers of iron status, and those currently being studied include the ratio of sTfR to serum ferritin, otherwise known as the total-body iron stores (TBI) model reported as part of the National Health and Nutrition Examination Survey (NHANES), hepcidin, and percentage total iron-binding capacity saturation (%TSAT). ,
Serum folate, RBC folate, and serum vitamin B 12 levels are useful in defining the cause of macrocytic anemia. Either serum folate or RBC folate may be measured; however, serum folate quickly returns to normal levels with dietary supplementation and may be high in patients with vitamin B 12 deficiency. RBC folate more accurately reflects the body’s folate stores over time. However, it can be falsely decreased in the presence of vitamin B 12 deficiency. In early or mild vitamin B 12 deficiency, vitamin B 12 levels may be in the low-normal range, and an elevated methylmalonic acid (MMA) test may be used to help diagnose vitamin B 12 deficiency in such cases. Identifying the cause of vitamin B 12 deficiency is the next step in evaluation. The presence of anti–intrinsic factor antibodies is specific for pernicious anemia. However, the sensitivity is low, and they may be undetectable in approximately 40% of cases, so the absence of these antibodies does not rule out a diagnosis of pernicious anemia. Although the classic two-step Schilling test has historical importance in understanding the pathophysiology of pernicious anemia, its utility is limited because of the need to use a radiolabeled reagent.
Although a bone marrow aspiration and biopsy can add useful information, it is rarely done today in pregnant anemic women. In addition to providing a ratio of myeloid to erythroid production (normal, approximately 3:1), it provides a measure of iron stores, allows a differential count of myeloid and erythroid precursors, and provides evidence of involvement by a primary or secondary neoplasm or other infiltrative process, such as infection.
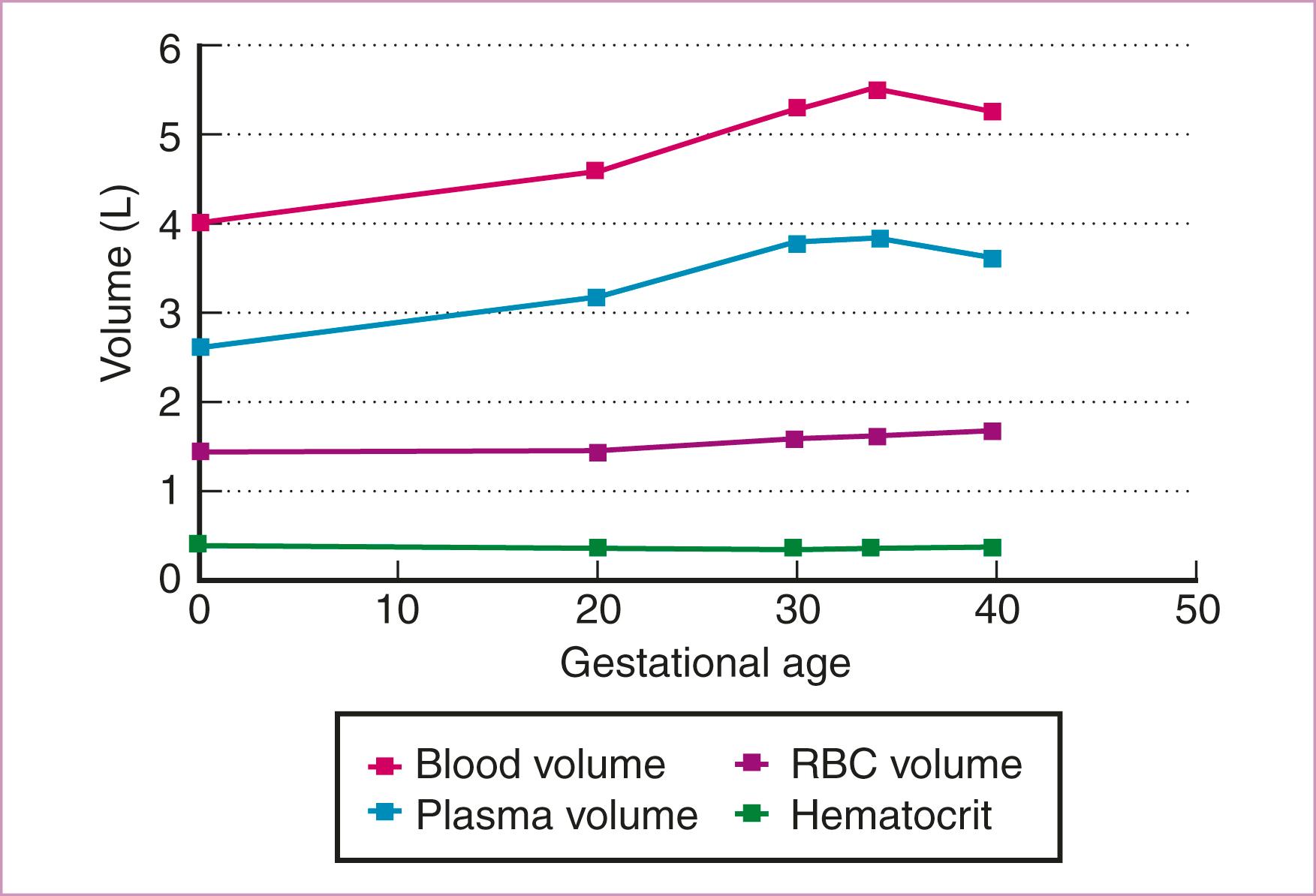
During pregnancy, there is normally a 40%–45% increase in blood volume, the maximum being reached at 34 weeks’ gestation. , The plasma volume increases by 47%, but the RBC mass increases only by 17%; the latter reaches its maximum at term. As shown in Fig. 55.1 , this disparity produces a relative hemodilution throughout pregnancy, which reaches its maximum between 28 and 34 weeks. Although this dilutional effect lowers the Hb, hematocrit (Hct), and RBC count, it causes no change in the MCV or in the mean corpuscular Hb concentration (MCHC). Therefore serial evaluation of these two indices is useful in differentiating dilutional anemia from progressive IDA during pregnancy. In the former the indices do not change, and in the latter they decrease progressively.
The classic study by Scott and Pritchard showed that iron stores in healthy women are marginal at best. These authors evaluated iron stores in the bone marrow of healthy, White college students who had never been pregnant and had never donated blood. Approximately two-thirds had minimal iron stores. In another study, almost 50% of healthy primigravidas had minimal iron stores in the marrow during the first trimester. Other reports confirm continued high rates of iron deficiency. , In a prospective report of more than 400 women in early pregnancy, one-third had a ferritin level of less than 20 μg/L, and 15% of them had severe iron depletion with ferritin value less than 12 μg/L. In addition, despite the high rate of iron depletion, only 6% of the women with a ferritin level of less than 20 μg/L also had anemia with a Hb level of less than 11 mg/L, suggesting that measuring only Hb is an inadequate screen for iron deficiency. The incidence of iron deficiency as measured by total-body iron, which considers body weight, was 18% in the first trimester and rose to 29% by the third trimester in US women in NHANES for 1999 to 2006.
The major reason for poor iron stores in women is likely menstrual loss. Data from Hallberg and associates indicated that typical menstrual blood loss is 28 to 36 mL per cycle. This is equivalent to 14 to 18 mg of elemental iron, because each milliliter of blood contains 0.5 mg of iron. To meet the iron loss accruing with menses alone, a woman must absorb 1.5 to 2.0 mg of elemental iron from her diet each day. Because only 10% of dietary iron is usually absorbed and the average diet contains only 6 mg/1000 kcal, a woman’s iron balance is precarious at best.
Pregnancy presents substantial demands on iron balance, exceeding that saved by 9 months of amenorrhea. Table 55.2 lists the iron requirements for pregnancy. If available iron stores are insufficient to meet the demands of pregnancy, iron-deficient erythropoiesis results. In a prospective study of 35 nonanemic women, ferritin levels were measured before and during pregnancy to determine the relationship of iron stores to developing anemia. Approximately 60% of the women with a ferritin concentration of less than 20 μg/L before pregnancy were anemic by 20 weeks’ gestation, compared with 25% of women with normal prepregnancy ferritin levels. Fenton and colleagues used serum ferritin levels to evaluate iron stores in pregnant women and found significantly higher ferritin levels in women who were receiving iron supplementation than in those who were not. The usual sequence of iron deficiency occurs in three stages. In the first stage, or prelatent iron deficiency, there is a reduction in marrow storage iron as detected by a decreased serum ferritin. During the second stage, or latent iron deficiency, abnormalities in iron studies are detected (decreased iron, increased TIBC, decreased percentage saturation), but the Hb level remains normal. In the final, third stage, anemia becomes apparent with a decreased Hb and, finally, decreased MCV.
| Required for | Average (mg) | Range (mg) |
|---|---|---|
| External iron loss | 170 | 150–200 |
| Expansion of red blood cell mass | 450 | 200–600 |
| Fetal iron | 270 | 200–370 |
| Iron in placenta and cord | 90 | 30–170 |
| Blood loss at delivery | 150 | 90–310 |
| Total requirement | 1130 | 580–1340 |
As noted, most women enter pregnancy with marginal iron stores. Further, although the estimated average requirement of daily iron intake in pregnant women between 14 and 18 years of age is 23 mg/d of iron and for pregnant women between 19 and 50 years it is 22 mg, the National Academy of Medicine reported the median intake in pregnancy was only 15 mg. Thus pregnancy places a large demand on iron balance that cannot be met with the usual diet. In the absence of supplementation, iron deficiency will develop, and in many women anemia will also develop. Although supplementation with 60 mg of elemental iron per day during the second and third trimesters meets the daily requirement, a prospective study reported that despite a mean supplementation of 49 mg/d of iron, iron deficiency increased from 8% in the first trimester to 62% by the third trimester. However, iron supplementation reduces the incidence of anemia by as much as 73%. , The National Academy of Medicine recommends that supplementation be offered only to women whose serum ferritin level is less than 20 μg/L. Although this is a valid recommendation scientifically, the high cost of the screening limits its applicability.
The fetal compartment preferentially obtains iron, folate, and vitamin B 12 from—and at the expense of—the mother. Maternal iron is transferred to the fetus via serum transferrin. Transferrin binds to receptors on the apical surface of placental syncytiotrophoblast, where the iron is released and subsequently binds to ferritin in placenta cells. It is then transferred to apotransferrin, which enters into the fetal circulation as holotransferrin. If maternal iron status is low, expression of placental transferrin receptors increase to facilitate enhanced uptake.
Folic acid, a water-soluble vitamin, is widely available in the diet. Dietary folates are a family of compounds that appear as polyglutamates. In humans, the only source of folate is the diet, and absorption occurs primarily in the proximal jejunum, facilitated by pancreatic conjugases in the intestine. The activity of conjugase is decreased by use of anticonvulsants, oral contraceptives, alcohol, or sulfa drugs. Therefore, in addition to an absolute diminution in dietary intake, the combination of increased need (e.g., multiple pregnancy, hemolytic anemia) and decreased absorption can lead to folate deficiency. , , Folate deficiency is defined as a serum level of less than 2.5 to 3 ng/mL.
The folate requirement of 400 μg/d for a nonpregnant woman increases to 600 μg/d during pregnancy. , Because adequate folate intake before and during the first weeks of pregnancy reduces the occurrence of neural tube defects, all women considering pregnancy should consume 600 μg of folate per day, which can be achieved by the combination of a nutritious diet plus a supplement containing 400 μg/d. One study estimated that a folate supplementation of 400 μg/d would reduce the risk for neural tube defects by 36% and that 5 mg/d would reduce the risk by 85%. If a previous pregnancy was complicated by a neural tube defect, the mother’s intake of folate should be 4 mg/d in the next pregnancy, starting at least 4 weeks before conception and continuing through the first 3 months of pregnancy. , When folate depletion occurs, blood abnormalities such as decreased serum folate, individual macroovalocytes then increased MCV, hypersegmentation of neutrophil nuclei, and decreased RBC folate may be detected before the development of anemia.
Vitamin B 12 , also abundantly available in the diet, is bound to animal protein. Absorption requires hydrochloric acid and pepsin to free the cobalamin molecule from protein. Intrinsic factor is also essential for absorption. After absorption, transport occurs via binding to transcobalamin II, and most of the vitamin B 12 is stored in the liver. The recommended daily intake of vitamin B 12 is 2.4 μg. Vitamin B 12 deficiency is defined by a serum level of less than 160 to 200 pg/mL.
The microcytic anemias are characterized by abnormal Hb synthesis with normal RBC production. A logical progression of diagnostic steps requires, first, that IDA be ruled out (see Iron Deficiency Anemia, later). If iron deficiency anemia is diagnosed rather than ruled out, it is important to consider gastrointestinal bleeding as the cause, although it is rare in pregnant women. This can be accomplished by performing a fecal occult blood test. If a microcytic anemia is not the result of iron deficiency, another cause should be sought, such as hemoglobinopathy including thalassemia, chronic infection, or inflammatory disorder, or one of the sideroblastic anemias. For this purpose, the following tests should be considered:
Peripheral blood smear examination
Serum iron studies (iron, TIBC, percentage saturation) and ferritin
HPLC
Whole blood lead level
Molecular testing for α- and β-thalassemias
Bone marrow examination
As noted, iron deficiency anemia is associated with decreased serum iron, increased TIBC (>400 μg/dL), decreased percentage saturation, and decreased ferritin concentration (<20 μg/L). Because iron deficiency is the most common anemia in pregnancy, it is reasonable and cost-effective to screen all women with microcytic anemia with ferritin initially. A ferritin level of less than 20 μg/L is generally diagnostic of IDA. However, IDA may still be present when the serum ferritin level is greater than 20 μg/L, particularly in the setting of other conditions including chronic disease. Additional tests to confirm iron deficiency may be warranted in such settings. Anemia of chronic disorders is associated with decreased serum iron level but paradoxically normal or increased ferritin and decreased TIBC. If iron studies and ferritin are normal, the patient should be evaluated for thalassemia or a sideroblastic anemia. Hb evaluation by high-performance liquid chromatography (HPLC) can help diagnose thalassemias and hemoglobinopathies; however, concurrent iron deficiency may mask HPLC abnormalities seen in thalassemia, and therefore iron deficiency should be evaluated first and treated before performing HPLC. Molecular testing for thalassemia confirmation may also be considered. Ring sideroblasts are present in the bone marrow of individuals with hereditary or acquired sideroblastic anemia (e.g., lead poisoning, alcohol abuse, arsenic, excess dietary zinc).
Because of the diverse nature of normocytic anemia, it is the most difficult type to evaluate. The reticulocyte count varies according to whether RBC production is increased, normal, or decreased. If erythropoiesis is increased, one must differentiate between hemorrhage and an increased rate of destruction. The blood smear may reveal a type of RBC shape that can be virtually diagnostic. Schistocytes are seen in microangiopathic hemolysis—as in the HELLP syndrome ( h emolysis, e levated l iver enzymes, l ow p latelets) and thrombotic thrombocytopenic purpura—and in association with prosthetic heart valves. Other types of poikilocytes that may be encountered on peripheral blood smear examination and that may suggest an etiology include sickle cells, target cells, stomatocytes, ovalocytes, spherocytes, elliptocytes, and acanthocytes and should warrant further evaluation by a hematologist.
The Coombs test differentiates immune from nonimmune causes of hemolysis. Immune hemolysis is related to alloantibodies, drug-induced antibodies, and autoantibodies. Nonimmune causes of hemolysis include various hereditary disorders such as hemoglobinopathies, disorders of the RBC membrane (hereditary spherocytosis and hereditary elliptocytosis), or deficiency of an RBC enzyme, and acquired nonimmune hemolytic anemias may be caused by PNH or lead poisoning.
Bone marrow examination can be helpful for evaluation of patients who have hypoproliferative anemias with normal iron studies and folate and vitamin B 12 levels. If increased ring sideroblasts are identified, both acquired and hereditary forms of sideroblastic anemia must be considered. If erythropoiesis is normoblastic, etiologic mechanisms fall into two major categories. The first category has myeloid-to-erythroid production ratios greater than 4:1 and includes red cell aplasia, primary marrow-based disorder (e.g., chronic myeloid leukemia), effects of chronic diseases, infection (e.g., parvovirus), and endocrine disorders such as hypothyroidism and hypopituitarism. In contrast, the myeloid-to-erythroid ratio is decreased (e.g., 2:1 or less) when erythroid hyperplasia is present, as with relatively acute hemolysis or myelodysplastic syndrome (MDS) if in conjunction with significant dysplasia. If there is megaloblastic erythropoiesis and erythroid hyperplasia, considerations include nutritional deficiencies such as folate and vitamin B 12 deficiencies, MDS, drugs, particularly those that interfere with nucleotide synthesis, and toxins (benzene, arsenic).
Macrocytic anemia is associated with either (1) an increased rate of RBC production and release of less than fully mature RBCs or (2) disorders of impaired DNA synthesis. Abnormal serum vitamin B 12 or serum and RBC folate levels allow a diagnosis of vitamin B 12 or folate deficiency. If a diagnosis of folate deficiency is confirmed, the various causes of decreased deconjugation of the polyglutamate and malabsorption must be considered. Folic acid, the polyglutamate present in food, must be deconjugated by intestinal enzymes such as dihydrofolate reductase for absorption. Causes of decreased deconjugation and hence poor absorption of folate include alcoholism and folate antagonists (methotrexate, pyrimethamine, trimethoprim), which inhibit dihydrofolate reductase. Additional causes of malabsorption causing folate deficiency are celiac sprue, inflammatory bowel disease, and gastric bypass surgery. If vitamin B 12 deficiency is diagnosed, causes of malabsorption such as pernicious anemia or small bowel malabsorption should be considered. Pernicious anemia, the most common cause of B 12 deficiency, is diagnosed when anti–intrinsic factor antibodies are present. These antibodies bind intrinsic factor produced in the stomach and thus inhibit B 12 absorption. If anti–intrinsic factor antibodies are negative, referral to gastroenterology for appropriate evaluation for small bowel malabsorption is appropriate.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here