Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter offers a review of the anatomy and physiology of normal hematopoiesis that is intended to provide a basis for understanding the marrow failure syndromes described at length in the following chapter. In this chapter we briefly discuss the phylogeny of hematopoiesis and describe marrow anatomy and the egress of recognizable hematopoietic cells from the marrow into the peripheral blood. A more detailed analysis then follows of the cellular bases of erythrocyte, granulocyte-macrophage, and megakaryocyte development, including discussions of the pluripotent stem cell and its niche, the more committed but still undifferentiated progenitor cells, and the differentiated precursors of the mature formed elements of the blood. Much of this chapter is devoted to the interactions of growth factors and the cells that produce them in the upregulation of hematopoiesis. The mechanisms of downregulation of hematopoiesis by cell interactions and cytokines are touched upon here, but they are less well understood despite the fact that they are likely to influence the pathophysiology of aplastic anemia and other marrow failure syndromes.
That “blood is life” was appreciated by Empedocles in the fifth century BC. The theory that the vasculature contains blood, phlegm, black bile, and yellow bile, all revealed when freshly let blood is permitted to separate, is attributed to Polibus, the son-in-law of Hippocrates.
Servetus recognized the systemic and lesser circulations in the sixteenth century. He was burned at the stake, in part because he did not accept the dogma that blood must pass through the intraventricular cardiac septum. (Grant disapproval and, more recently, approval without funding have been substituted for immolation. The effects are not entirely dissimilar.)
In view of the present growth of knowledge of hematology, it is remarkable to realize that the concept of the circulation of the blood was finally established by Harvey in 1628, not even 400 years ago. This began the clinical application of blood transfusion, of which Pepys wrote “it gave rise to many pretty wishes as of the blood of a Quaker to be let into an Archbishop and such like.”
In the mid–seventeenth century, Swammerdam observed red blood corpuscles in the microscope and Malpighi discovered the capillary circulation in the lung and in the omentum. But it was not until the nineteenth century that the source of blood cell production began to be successfully explored. Houston suggested that red cells were derived from leukocytes in the lymphoid system. Zimmerman believed that erythrocytes were derived from platelets, an opinion shared by Hayem. Addison, perhaps not surprisingly, attributed red cell production to the adrenals, and Reikert finally suggested that red cells might be produced in the embryonic liver. In fact, it was not until 1868 that Neumann demonstrated that red cells arise from precursors in the marrow. The modern understanding of the physiology of hematopoiesis then began.
* For an entertaining review from which this precis was in part drawn, see Robb-Smith AHT: The growth of knowledge of functions of the blood, in MacFarlane RG, Robb-Smith AHT, editors: Functions of the blood , Oxford, 1961, Blackwell Scientific Publications. For more details, see also Blood pure and simple by the late Maxwell M. Wintrobe, New York, 1980, McGraw-Hill.
Much can be learned about the physiology of hematopoiesis from study of the evolution of oxygen transport, a subject reviewed by Lehman and Huntsman.
One of the major advantages of mammalian life over that of invertebrates is the capacity to package large amounts of hemoglobin within cells. This permits the delivery of oxygen to tissues without the enormous increase in oncotic pressure that would be induced by a similar concentration of high–molecular-weight hemoglobin free in the plasma. The renewal rate of red cells is a function of metabolic rate or basal heat production. This is illustrated dramatically in studies of the animal kingdom, ranging from the turtle to the pygmy shrew, and by comparisons of red cell renewal in marmots during periods at ambient and cold temperatures, in rats, and in frogs.
The production of blood cells in bone marrow is a late development in phylogeny. Red cells are found in the coelomic cavity of the worm, and are produced in the kidneys of the goldfish. The influence of oxygen demand on the production of red cells is illustrated by the effects of hyperoxia on bled rats and the behavior of the European eel, one of the few vertebrate forms that ordinarily lacks erythrocytes in its juvenile state. When the adult eel struggles against the current up the rivers of Europe, nucleated cells containing hemoglobin appear in its plasma. This influence of oxygen demand on respiratory pigment production is also illustrated in organisms that do not produce red cells, such as Daphnia , the English water flea, a creature that produces high–molecular-weight hemoglobin in its ovaries when it is exposed to low oxygen tension in stagnant ponds. The discovery of transcription factors that function as oxygen sensors provides a potential molecular explanation for these regulatory mechanisms.
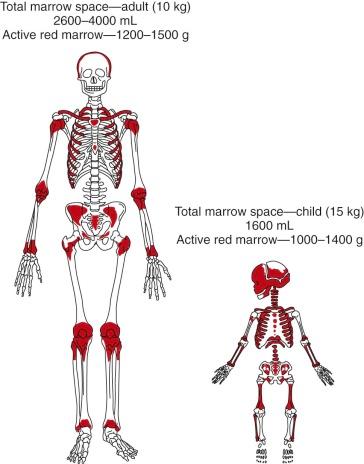
The active red marrow space of a child is much greater than that of an adult, presumably because the high requirements for red cell production during neonatal life demand the resources of the entire production potential of the marrow. During postnatal life the demands for red cell production ebb, and much of the marrow space is slowly and progressively filled with fat ( Fig. 1-1 ). In certain disease states that are usually associated with anemia, such as myeloid metaplasia, hematopoiesis may return to its former sites in the liver, spleen, and lymph nodes and may also be found in the adrenals, cartilage, adipose tissue, thoracic paravertebral gutters, and even in the kidneys.
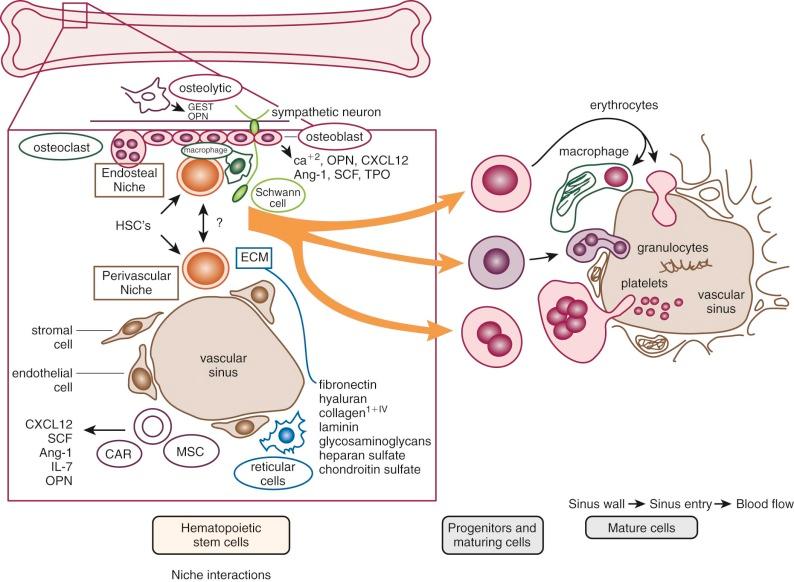
The microenvironment of the marrow cavity is a vast network of vascular channels or sinusoids in which float fronds of hematopoietic cells. This complex area of cell biology and anatomy has been the subject of several reviews. The vascular and hematopoietic compartments are joined by reticular fibroblastoid cells that form the adventitial surfaces of the vascular sinuses and extend cytoplasmic processes to create a lattice on which blood cells are found. The lattice itself is illustrated by reticulin stains of marrow sections ( Fig. 1-2 ). The conformation of the meshwork of reticulin and the location of hematopoietic cells in the network of vascular sinuses are best illustrated by scanning electron microscopy ( Fig. 1-3 ). The fibroblastoid network has two major functions: (1) it provides an adhesive framework onto which the developing cells are bound, and (2) it participates in the production by these cells of essential hematopoietic colony-stimulating factors, to be discussed below. Cell-cell adhesion may be mediated by binding of the hematopoietic very late antigen 4 (VLA-4) integrin to stromal fibronectin or vascular cell adhesion molecule 1 (VCAM-1). In addition, cytokine receptors such as KIT can bind to the membrane-bound form of stem cell factor (SCF), and the extracellular matrix proteins secreted by stromal cells may actually provide a binding site for some growth factors or for hematopoietic cells. In addition, chemokines, a family of small molecules, play major roles in stromal function. Specifically, stromal-derived factor 1 (SDF-1) is a potent attractant for hematopoietic cells (both mature leukocytes and progenitor cells that express its CXC chemokine receptor 4 [CXCR4]). Gene disruption studies in mice show that both ligand and receptor are lethal in the embryonic stage, with defects in B lymphopoiesis and myelopoiesis. These relate to a critical role for CXCR4 in bone marrow engraftment in immunodeficient nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice ; SDF-1 has been shown to activate the integrins VLA-4, VLA-5, and lymphocyte function–associated antigen 1 (LFA-1) on CD34+ cells. Last, SDF-1 plays a role in the egress of progenitor cells from bone marrow to blood during stem cell mobilization. Indeed, pharmacologic inhibition of the SDF-1 receptor CXCR4 with the drug plerixafor is employed clinically to enhance mobilization.
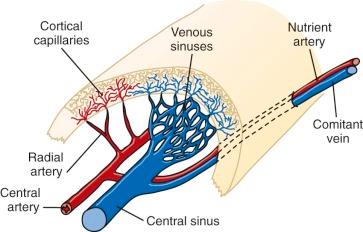
A schema of the marrow circulation is shown in Figure 1-4 . The central and radial arteries ramify in the cortical capillaries, which in turn join the marrow sinusoids and drain into the central sinus. Cells that egress from the marrow sinusoids then join the venous circulation through comitant veins. The inner, or luminal, surface of the vascular sinusoids is lined with endothelial cells, the cytoplasmic extensions of which overlap, or interdigitate, with one another. The escape of developing hematopoietic cells into the sinus for transport to the general circulation occurs through gaps that develop in this endothelial lining and even through endothelial cell cytoplasmic pores.
The location of the different hematopoietic cells is not random. Clumps of megakaryocytes are found adjacent to marrow sinuses. They shed platelets, the fragments of their cytoplasm, directly into the lumen. This reduces the requirement for movement of bulky mature megakaryocytes, a mobility characteristic of the granuloid- and erythroid-differentiated precursors as they approach the point at which they egress from the marrow. A schema that illustrates the transfer of hematopoietic cells into the sinus is shown in Figure 1-5 . Disruption of the function of microenvironmental cells inhibits long-term murine marrow cultures. Such disruptions may be responsible for certain cases of aplastic anemia. In recent years significant attention has been paid to the cellular components of the hematopoietic stem cell (HSC) niche, with several reports of the role of osteoblasts as supportive microenvironmental cells that liberate critical cytokines such as SDF-1. However, alternative views argue for a major role of vascular endothelium of the marrow sinusoids in sustaining HSCs (see Fig. 1-5 ). See the review by Krause and Scadden for further details.
The concept that sustained hematopoiesis derives from pluripotent stem cells was first suggested in 1949 by Jacobson, who showed that mice can be protected from the lethal affects of whole-body irradiation by exteriorization and shielding of the spleen. This protective effect was shown to be cell mediated by the observation that the injection of spleen cells could initiate recovery and reestablish hematopoiesis in irradiated animals. The clonal nature of hematopoiesis and the concept that a single pluripotent stem cell exhibits the capacity to repopulate the entire hematopoietic system was first demonstrated experimentally by Till and McCulloch, who also used the mouse as an experimental system. They found colonies of hematopoietic cells containing erythrocytes, granulocytes, macrophages, and megakaryocytes in the spleens of irradiated recipients within 10 days after transplant. Subsequent experiments using karyotypically marked donor cells confirmed the clonal origin of the differentiated cells in the colony, proving that a single multipotent stem cell had given rise to these differentiated cells. It was also shown that each colony contained a number of stem cells that could again form a colony of differentiated progeny in a second irradiated recipient, demonstrating their self-renewal capacity. This is true only of spleen colonies that are present on days 12 to 14 (colony-forming unit–spleen,[CFU-S 12 ]). Colonies observed on days 7 to 8 after marrow infusion are transient, disappear by day 12, and are neither multipotential nor self-maintaining. Under steady-state conditions no more than 10% of the CFU-S become committed to differentiation during any given 3-hour period. The demonstration of a stem cell that can differentiate to form progenitor cells for erythropoiesis, granulopoiesis, and megakaryopoiesis is consistent with subsequent observations in disease states such as chronic myelogenous leukemia and polycythemia vera, in which a clonal origin of abnormal erythroid, granulocytic, and megakaryocytic precursor cells and lymphocytes can be demonstrated.
The demonstration of a multipotent stem cell in adult bone marrow led to a systematic search for the ontogenic origins of HSCs. Experiments performed by Moore and Metcalf demonstrated the presence of cells capable of repopulating the adult marrow in the yolk sac and the murine fetal liver. Subsequent work has corroborated these observations. It is widely accepted that the first blood cells that circulate in the mammalian embryo arise from the yolk sac. This so-called “primitive” blood lineage consists primarily of nucleated erythrocytes that express embryonic forms of hemoglobin. The classic view of the developmental origins of HSCs holds that the first progenitors of the yolk sac later populate the fetal liver, where they mature to become the “definitive” HSCs of the adult bone marrow, whose erythroid progeny express mature adult forms of beta hemoglobin. However, painstaking dissections of midgestation mouse embryos prior to the onset of blood circulation suggest that the HSCs that can engraft the bone marrow of an irradiated adult mouse first arise within the embryonic trunk. Refinements of the anatomic location of these nascent definitive HSCs show their emergence from a specialized hemogenic endothelium along the base of the developing aorta, where the first pulsations of the heartbeat serve as a trigger to activate an endothelial-to-hematopoietic transition. Debate persists, and some argue that HSCs may arise simultaneously in the yolk sac and intraembryonically from the hemogenic endothelium of the aorta. Complicating the true origins of the first definitive HSCs further, the placenta also appears to be a source of HSCs during development, although it remains possible that the stem cells arrive through the circulation from intraembryonic sources such as the hemogenic endothelium of the aorta. Alternatively, given its rich network of arteries, the placenta may indeed be a de novo producer of blood stem cells. The placenta is quantitatively a large source of HSCs during development.
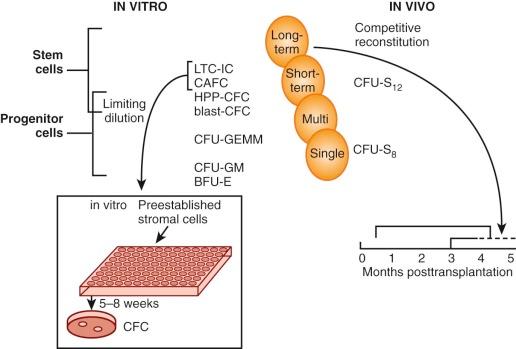
The most primitive cells with the greatest self-renewal capacity reconstitute long-term hematopoiesis after transfer into irradiated recipient mice. These cells, termed long-term reconstituting hematopoietic stem cells (LTR-HSCs), were shown to be separable from CFU-S 12 in a limiting-dilution assay designed to detect and enumerate “cobblestone areas.” This assay is derived from the original “Dexter” technique for long-term culture of murine marrow in which CFU-S, colony-forming unit–granulocyte-macrophage (CFU-GM), and burst-forming unit–erythroid (BFU-E) flourish for many months on and within an adherent stromal monolayer. The areas of active hematopoiesis have a “cobblestone” appearance. In the limiting-dilution assay, different concentrations of bone marrow cells are plated onto a series of microwells that contain a preestablished stromal monolayer, and at 5 weeks the cobblestone areas that comprise proliferating blast cells within the stromal cell layer are counted. In cell separation experiments, their numbers correlate with a cell fraction that is characterized by low mitochondrial mass per cell (minimal retention of the supravital fluorochrome rhodamine-123); this Rho− cell fraction is enriched for marrow repopulating cells but depleted of CFU-S 12 . Intermediate and rapidly sedimenting cells contained more than 99% CFU-S 12 as well as the cells responsible for short-term reconstitution. In contrast, long-term reconstituting cells (>60 days) came from a slowly sedimenting fraction that contained only 0.25% CFU-S 12 .
The stem cell model of hemopoiesis has parallels in other organ systems. That rapidly self-renewing epithelial tissues such as skin and intestine have stem cells that continually replenish the cells lost by differentiation is well described. However, the demonstration of the existence of neural stem cells has raised the possibility that many organ systems might retain a population of self-renewing stem cells. Muscle satellite cells also appear to fulfill this role. These organ- or tissue-specific stem cells arise during early fetal development from embryonic stem cells of the inner cell mass of the blastocyst that are pluripotent and give rise to all cell types in the body.
Many assays have been proposed as “surrogate” stem cell assays, but until homogeneous populations can be evaluated in both in vitro and in vivo assays it will be impossible to determine the precise cell type measured by these methods. Fauser and Messner and others demonstrated colonies in semisolid media that contain granulocytes, erythrocytes, monocytes, and megakaryocytes (CFU-GEMM) in methylcellulose cultures of human bone marrow. A unique type of in vitro blast cell colony that comprises small numbers of blast cells with higher self-renewal capacity (secondary colonies on replating) than CFU-GEMM has been described. Evidence for the presence of pluripotent HSCs is also derived from the human “Dexter” technique for liquid culture of marrow, in which myeloid progenitors (mostly CFUs-GM) are sustained for about 2 months on and within an adherent stromal monolayer. The progenitors can be detected by replating into methylcellulose with several growth factors at 5 to 8 weeks, thereby demonstrating that unipotent and multipotent cells are generated in this culture system. Eaves and colleagues have adapted this long-term culture technique to a limiting=dilution assay in which long-term culture-initiating cells (LTC-ICs) can be quantitated after culture at different concentrations on a stromal layer for 5 weeks, followed by replating in methylcellulose to score for the number of wells that do not contain colonies. The analogous cobblestone area–forming cell (CAFC) assay has also been adapted to human cells. The high proliferative potential colony-forming cell (HPP-CFC) assay that gives rise to macroscopically visible (>5 mm) in vitro colonies is another method that measures the enormous proliferative capacity of primitive progenitor cells. In an effort to establish a more direct measure of the ability of human stem cells to reconstitute hematopoiesis, Dick and colleagues developed an in vivo assay. In this method human cells are injected into immunodeficient nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice, and 5 to 8 weeks later the animals are sacrificed and tested for the presence of human cells in blood and progenitors in bone marrow. By limiting-dilution assay these SCID reconstituting cells (SRCs) can be quantitated. Because NOD/SCID mice develop thymic lymphomas that prevent long-term studies and have natural killer (NK) cell activity that confers graft resistance, improvements in the model have been achieved by using mice with additional truncated (NOG) or deleted (NSG) interleukin 2 receptor (IL-2R) common gamma chains ; these animals support a -fivefold higher level of CD34+ engraftment. Cell fractionation and gene marking studies provide some evidence that SRCs are more primitive than LTC-ICs. Although SRCs may still be a heterogeneous population of cells that includes long-term repopulating HSCs, this assay provides a better measure of stem cell properties than the LTC-IC assay, especially with regard to the multipotent (i.e., myeloid and lymphoid) and self-renewal properties of HSCs. A tentative relationship of the cells measured in these different assays to the stem cell is shown in Figure 1-6 .
Application of these assays and analysis of HSCs, in general, has been hindered by the low frequency of the cell in the hematopoietic population and the lack of reagents to identify stem cells. However, it is now possible to purify murine stem cells by several methods. Murine HSCs can be highly purified by density gradient centrifugation combined with labeling with antibodies, lectins, or intracellular dyes (alone or in combination) followed by separation using fluorescence-activated cell-sorting (FACS), immunopanning, or immunomagnetic beads. Immunologic reagents that define murine stem cell populations include antibodies to Thy-1 (CD90), Kit, and stem cell antigen (Sca-1). Weissman and coworkers used the FACS in combination with negative expression of T-cell, B-cell, granulocyte, and monocyte lineage-specific markers; low expression of Thy-1; and expression of Sca-1 to isolate HSCs. Transplantation of single Kit + , Thy-1 lo , Lin−, Sca-1 + (KTLS) cells can ensure long-term survival of lethally irradiated syngeneic recipients, and approximately 20% of such cells are LTR-HSCs. Murine HSCs can be even further enriched using cell surface receptors of the signaling lymphocyte activation molecule (SLAM) family. HSCs are CD150+, CD244−, and CD48−, whereas multipotent progenitors lose CD150 expression and begin to express the other family members CD244 and CD48. Purification of CD150+, CD48−, and CD41− cells (CD41 is also expressed on megakaryocyte lineage cells) provides a population of cells 45% of which are LTR-HSCs.
Expression of the CD34 antigen has been used to enrich for human stem cells. Although most colony-forming cells (CFCs) express both the CD34 and CD33 antigens, cells that give rise to CFC in long-term bone marrow cultures (i.e., pre-CFCs) can be separated by their expression of CD34, lack of expression of CD33, and intermediate forward light-scattering properties. A G 0 CD34+ cell population has been isolated by exploiting the resistance of these cells to 5-fluorouracil (5-FU) in the presence of stem cell factor (SCF) and interleukin-3 (IL-3). The G 0 cells are also positive for KIT, interleukin-6 receptor (IL-6R), and IL-1R, and they do not form progenitor-derived colonies upon direct culture in methylcellulose; after 5 weeks in culture on stromal cells, however, they do form primary colonies in methylcellulose 40% of which are replatable. The importance of CD34-positive marrow cells is emphasized by in vivo simian studies. Similar to human bone marrow, the CD34 antigen is expressed by a minority of baboon cells, and infusion of these cells isolated by immunoabsorption chromatography and FACS can reconstitute lymphohematopoiesis in lethally irradiated baboons. The cloning of the murine CD34 complementary DNA (cDNA) has cast some doubt on expression of CD34 by LTR-HSCs, at least in the mouse. A monoclonal antibody raised to murine CD34–glutathione-S-transferase (GST) fusion protein was used to separate purified Sca-1+, Kit+, lin− bone marrow cells into CD34 lo /−, CD34 lo , and CD34+ fractions. Interestingly, long-term multilineage reconstitution was observed after transplantation of the CD34 lo /− cells, whereas the CD34+ fraction gave early but unsustained multilineage reconstitution. These data are supported by experiments demonstrating that a tiny subset of murine bone marrow cells that exclude the Hoechst 33342 dye (called the side population ) contains all the LTR-HSC activity but is CD34−. It is possible that murine and primate LTR-HSCs differ in their expression of CD34; however, the human and primate transplants have not used very highly purified cells, and so it is also possible that CD34 lo /− cells could account for the long-term engraftment. Primate and human studies have also raised the possibility that HSCs do not express CD34. When primitive human lin− cells are separated into CD34+ and CD34− fractions, the capacity to reconstitute hemopoiesis in immunodeficient mice (SRC) is found in both cell fractions. A resolution to this controversy may come from the demonstration that resting murine HSCs are CD34−, whereas HSCs activated with 5-FU or cytokines such as G-CSF express the CD34 antigen. Most interestingly, transplantation of activated CD34+ HSCs showed that these HSCs can lose CD34 expression after return of the recipients to the resting steady state and still retain the capacity to reconstitute secondary recipients, demonstrating that CD34 expression is reversible. The current state of the art for HSC purification in the mouse includes the following marker profile: CD34−, CD150+, CD48−, CD41−, flt3−, and CD49b lo ; whereas in the human system, the current purification would exploit a different marker profile: CD34+, CD38−, CD45RA−, CD90+, and CD49f+, with further enrichment (capacity for single-cell engraftment) in a Rho − fraction of these cells.
The long-term repopulating HSCs of the marrow (LTR-HSCs) slowly self-replicate while occasionally (and stochastically) differentiating into cells that are multipotent but have reduced self-renewal capacity (called myeloid lymphoid progenitors in humans) and then into either common lymphoid progenitors or common myeloid progenitors. These cells can be prospectively isolated according to their expression of unique combinations of cell surface markers ( Fig. 1-7 ). Lymphoid differentiation will not be further considered here. The common myeloid progenitor differentiates into all of the progenitors of the blood cells other than lymphoid cells. These include more committed progenitors of the granulocyte, monocyte (GMPs), and eosinophil lineages or progenitors of the megakaryocyte, erythroid, and basophil lineages (MEPs). These cells can be isolated prospectively, and transplantation into lethally irradiated mice can confer transient but not long-term hematopoiesis.
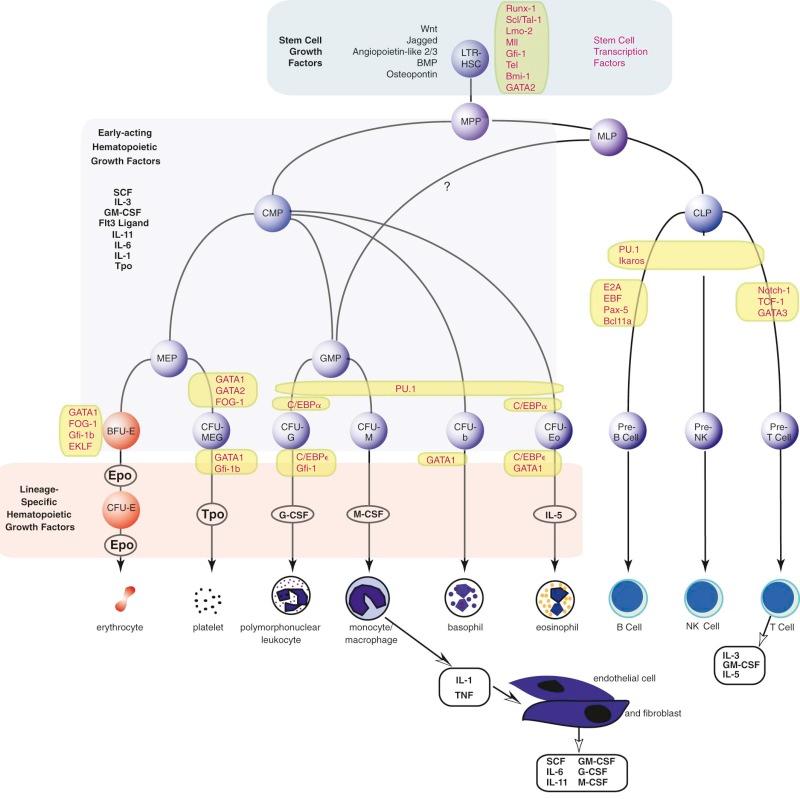
The current view of the mouse hierarchy derived from transplant studies has defined a linear track from LTR-HSCs to short-term reconstituting hematopoietic stem cells (STR-HSCs) (the latter most likely equivalent to the human myeloid lymphoid progenitor), and finally a multipotential progenitor, and then several branch points. There is a lymphocyte-myeloid multipotent progenitor (LMPP) that gives rise to common lymphoid progenitors and GMPs, whereas GMPs can also arise from common myeloid progenitors. There is certainly heterogeneity in these populations, and future fate mapping and sorting techniques may further define the populations.
The erythroid progenitor compartment is invisible under the light microscope. Erythroid progenitors are committed progenitors of a single lineage derived from the stochastic differentiation of bipotential or multipotential progenitors that are, in turn, derived from a tiny population of multipotential stem cells (see Fig. 1-7 ). In humans, the most primitive single-lineage committed erythroid progenitor is the erythroid burst-forming unit (BFU-E). In response to the combination of erythropoietin and either SCF, IL-3, or GM-CSF in vitro in semisolid (methylcellulose) cultures, BFU-E divide several times while still motile, thereby forming subpopulations of erythroid colony-forming units (CFU-E). Then, each of the latter forms a large colony of proerythroblasts that go on to form more mature erythroblasts and even reticulocytes. The burstlike morphology of the colony is responsible for the name of the progenitor. The entire process requires about 2 weeks in vitro. Bone marrow also contains the more mature CFU-E that, under the influence of erythropoietin, form small colonies of erythroblasts in 7 days.
The murine erythroid progenitor phenotype (pre–CFU-E) can be defined as Sca-1−, Kit+, CD41−, CD150+, and endoglin+. The human BFU-E counterpart is CD34+, CD38+, IL-3Rα−, and CD45RA−.
The first colony assays relevant to the study of the production of granulocytes and monocytes in the mouse were described in 1965 by Pluznik and Sachs and in 1966 by Bradley and Metcalf. Analogous assays have been developed in the human system. These groups demonstrated that individual cells derived from mouse spleen or bone marrow could give rise to colonies of up to several thousand differentiated granulocytes and/or macrophages in a soft agar medium. A period of 7 to 8 days was required for full maturation of these colonies (12 to 14 days is required in humans). Appropriate studies were performed to demonstrate the single-cell origin of the colonies. These studies also demonstrated that a single progenitor cell, which was termed the colony-forming unit in culture, or CFU-C, was capable of differentiation into both granulocytes and macrophages, giving rise to the designation CFU-GM. Unit gravity sedimentation and other separation methods have been used to demonstrate that CFU-GM represent a cell population distinguishable from the pluripotent stem cell. Long-term liquid bone marrow cultures have been particularly helpful in defining humoral and cell-cell interactions that induce myeloid differentiation. CFU-GM give rise to the more mature granulocyte and macrophage colony-forming units, CFU-G and CFU-M, respectively. In addition, CFU-GM can be distinguished from the eosinophil progenitor, CFU-Eo, each arising independently from the common myeloid progenitor (CMP). Human CFU-GM are CD34+, CD38+, IL-3Rα lo , and CD45RA−.
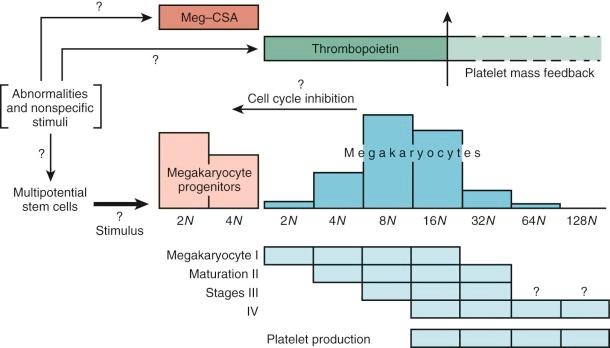
Figure 1-7 provides an accepted if idealized schema of lineage-restricted megakaryocyte progenitor development. Evidence strongly suggests that the initial phase of differentiation into erythrocyte and megakaryocyte commitment involves a single progenitor capable of giving rise to colonies of differentiated cells, all of which express a nuclear transcription factor known as GATA1. Of great interest is the fact that another transcription factor, nuclear factor, erythroid 2 (NF-E2), has been shown to regulate platelet production. Mice rendered NF-E2 deficient have increased numbers of immature megakaryocytes in the marrow but die from bleeding resulting from absence of platelets. The thrombocytopenia is due to a block late in megakaryocyte maturation. Interestingly, these animals do not show an increase in thrombopoietin levels, suggesting that the megakaryocyte mass rather than the platelet count regulates thrombopoietin production.
The erythroid precursor or erythroblast pool represents about one third of the marrow cell population in the normal child above the age of 3 years or in the adult. Proerythroblasts are the earliest recognizable forms. These divide and mature through various stages that involve nuclear condensation and extrusion and hemoglobin accumulation. On average, each erythroblast can form about eight reticulocytes. Measurement of the total marrow proerythroblast content and daily reticulocyte production shows that under normal conditions, replicating proerythroblasts largely maintain the reticulocyte pool being renewed from the progenitor compartment at a rate of about 10% per day.
Up to this point we have discussed the nondescript progenitors of erythropoiesis without reference to their physical appearance or to the appearance of their differentiated daughter cells. The best evidence to date suggests that hematopoietic progenitors or stem cells look like lymphoblasts, and studies of peripheral blood have shown that BFU-E reside in the nonadherent “null” lymphocyte population.
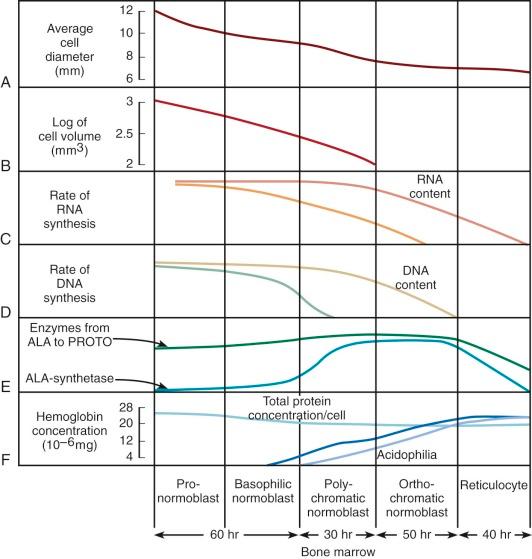
The pathway of erythroid precursor differentiation between the development of proerythroblasts and the mature red cell is known as the erythron and includes the functioning differentiated precursor cells observed in bone marrow aspirates and biopsies. The morphology of erythroid precursor maturation is well described in several texts and will not be repeated here. The salient features of the morphologic changes during cell development are related to biochemical and kinetic alterations that were reviewed by Granick and Levere and are shown in Figure 1-8 . The residence times spent in each morphologic compartment are shown at the bottom of the figure, but the average transit time from proerythroblast to emergence of the reticulocyte into the circulation is approximately 5 days. In acute anemia, the transit time may decrease to as little as 1 or 2 days by means of skipped divisions. The red cells that emerge are macrocytic and may bear surface i antigen and other fetal characteristics because the abbreviated time in the marrow compartment does not permit complete conversion of i antigen to I antigen or acquisition of certain other adult characteristics. The cells also contain excessive burdens of the rubbish that normally accumulates during cell assembly, because less time is available for the cleansing action of cell proteases and nucleases. Thus stress erythropoiesis is associated with circulating Pappenheimer bodies (iron granules), basophilic stippling (ribosomes), Heinz bodies (hemoglobin inclusions), and Howell-Jolly bodies (nuclear remnants).
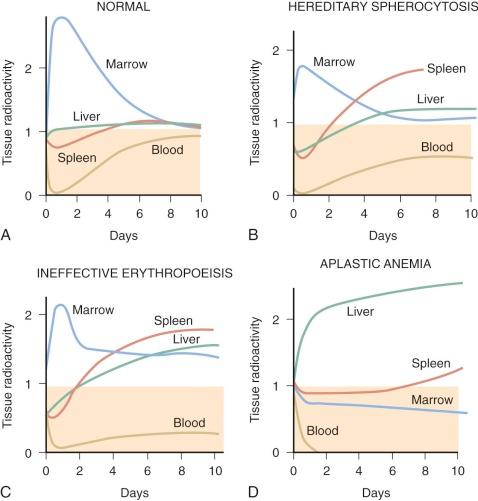
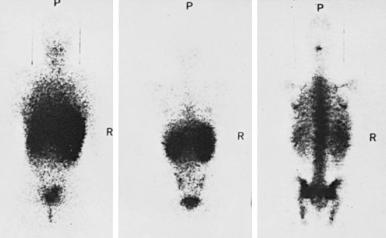
The kinetics of erythropoiesis can be monitored by the use of radioactive iron and surface scanning. The various ferrokinetic patterns in human diseases are shown in Figure 1-9 . The total distribution of erythroid marrow can be determined by scintigraphy using 111 InCl bound to transferrin, as shown in Figure 1-10 . Both 59 Fe kinetics and 111 InCl scintigraphy can be useful in the diagnosis of marrow failure, but these techniques are rarely necessary. The initial uptake of 59 Fe in marrow is found primarily in proerythroblasts and early basophilic erythroblasts.
Mouse proerythroblasts; basophilic, polychromatic, and orthochromatic normoblasts; reticulocytes; and mature erythrocytes can be separated on the basis of increasing expression of glycophorin A (Ter119) and loss of expression of CD71. Highly purified populations can be obtained by substituting CD44 for CD71, since there is a thirtyfold loss of expression of CD44 during erythroid maturation compared with a fourfold decrease in CD71 expression.
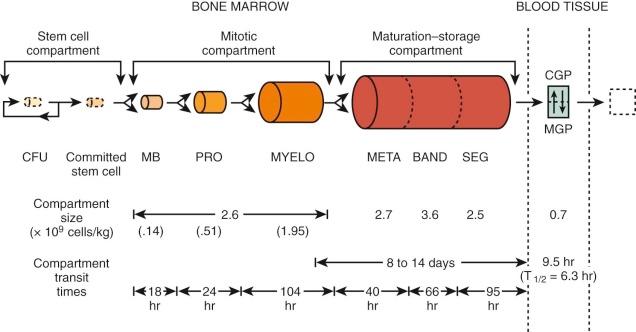
A model that describes the production and kinetics of neutrophils in humans is shown in Figure 1-11 . It is highly compartmentalized. The relatively tiny peripheral blood pool is divided into two components in equilibrium; the circulatory pool (CGP) and the marginating pool (MGP). These pools provide entrance into the tissues. The level of circulating cells is buffered by an immense marrow reserve of identifiable precursors, some of which are in the mitotic compartment and others in a maturing storage compartment. The transit times within each compartment are relatively long, so that a huge reserve remains available. The responses of these compartments to various diseases are detailed in Chapter 22 . The kinetics of proliferation of recognizable cell precursors have been studied using labeled precursors of DNA. The so-called labeling indices from which measurements of cell cycle times can be made have served as important approaches to the study of the pharmacology and toxicity of chemotherapy (see Chapter 45 ).
The final stages of granulocyte production and their release from the marrow are also multifaceted. At least four factors may influence granulocyte egress: the organization and localization of the cells in relation to vascular channels; the development of nuclear and cytoplasmic changes that increase cell deformability; factors that cause cell release; and, finally, the regulation of blood flow through vascular channels in the marrow.
Despite the fact that the morphology of megakaryocyte development is fairly well established ( Table 1-1 ), bone marrow examinations can be of limited value in the various platelet disorders. Bone marrow smears and biopsies provide insufficient data about thrombopoiesis because the final stage of platelet production, extrusion of cytoplasm into the sinusoid and shedding of platelets (to be described later), is not appreciated by these techniques; only the relative numbers of megakaryocytes and their size and ploidy can be appreciated by routine morphologic methods. It is not surprising that such information is only loosely correlated with platelet production ( Fig. 1-12 ). Furthermore, sampling errors can be responsible for serious misinterpretations. This is a particular hazard in aspirates of neonatal marrow, in which megakaryocytes may be hard to detect whether platelet production is normal or not. Furthermore, megakaryocytes are not evenly distributed in marrow smears. They are more readily found around the edges of the particles, and they may be mistaken for broken cells by the untrained observer. Megakaryocyte nuclei are often found lying free in marrow smears, where they may be erroneously scored as tumor cells. Biopsy sections provide more accurate assessments of megakaryocyte number and distribution than smears, though the latter are usually sufficient (except in neonates) if examined carefully. Biopsies should not be attempted in neonates merely to define megakaryocytes; clinical judgment is a safer tool in these patients.
| Stage | Nuclear Morphology | Cytoplasmic Staining (Wright-Giemsa) | Approximate Size Range | Demarcation Membranes | Granules | Suggested Name |
|---|---|---|---|---|---|---|
| I | Compact (lobed) | Basophilic | 6-24 µm | Present by electron microscopy | Few present by electron microscopy | Megakaryoblast |
| II | Horseshoe | Pink center | 14-30 µm | Proliferating to center of cell | Starting to increase | Promegakaryocyte |
| III | Multilobed | Increasingly more pink than blue | 15-56 µm | Extensive but asymmetric | Great numbers | Granular megakaryocyte |
| IV | Compact but highly lobulated | Wholly eosinophilic | 20-50 µm | Evenly distributed | Organized into “platelet field” | Mature megakaryocyte |
Examinations of routine marrow smears and biopsies, though instructive, are limited by their two-dimensional views and the thickness of the sections. Megakaryocytes have a peculiar predilection to lie next to the endothelial cell lining of the fronds of developing marrow cells, perhaps because thrombopoietin is produced by these cells. In general, megakaryocytes are too large to squeeze through the sinusoidal meshwork, so they merely push their cytoplasms through the fenestrations. These cytoplasmic structures, called proplatelets, serve as microtubule conduits for platelet packaging and release at sinusoids. The protruding cytoplasms form demarcation lines and then shatter into platelets, which are swept into the blood, regulated in part by fibrinogen interacting with platelet integrin αIIbβ3. The megakaryocyte nuclei rarely make the transfenestration journey into the sinusoids and thence the blood. If they do, they may be mistakenly interpreted by the unwary microscopist as tumor cells in the blood. Intact or partial megakaryocytes are regularly observed in the blood of patients with marrow-invasive diseases such as certain leukemias, metastatic cancers, granulomatous disorders, and fibrosis.
Since the pioneering work in the early 1960s by Metcalf and Sachs and their coworkers, it has been recognized that normal and leukemic blood progenitor cells can be propagated in culture in the presence of soluble protein growth factors. These factors were originally termed colony-stimulating factors, or CSFs, based on their ability to support the formation of colonies of blood cells by bone marrow cells plated in semisolid medium. During the 1970s and 1980s, it was recognized that there exist multiple types of CSFs based on the different types of blood cells found in the colonies that grew in the presence of the different factors, leading to the hypothesis that the growth and differentiation of different lineages of blood cells are controlled, at least in part, by exposure of progenitor cells to CSFs having different lineage specificities. With the molecular cloning of the genes for many of these factors and their receptors during the 1980s and 1990s it became possible to study in detail the structure, function, and biology of the recombinant CSFs as well as the molecular biology of their respective genes. This analysis, along with similar work on the regulation of cells in the immune system, led to the realization that there exist a large number of interacting regulatory molecules now generally known as cytokines or lymphohematopoietic cytokines that together serve to control the hematopoietic and immune systems and to integrate the responses of these systems with those of many other physiologic systems. These control molecules serve to regulate the growth, development, differentiation, activation, and trafficking of cells within the hematopoietic and immune systems. With the elucidation of the sequence of most of the human genome in the final years of the 1990s, even more cytokine and growth factor genes have been discovered, providing new insight into how all these systems have evolved with common themes but also providing further challenges to cell biologists in their attempts to understand the functions and interactions of all of the different molecules.
The cytokine gene families that contain at least one member that functions within the hematopoietic or immune system include the lymphohematopoietic cytokines (including many interleukins and CSFs ); the receptor tyrosine kinase ligand CSF-1, the stem cell factor (SCF) (also known as steel factor ), and the ligand for the Flk2/Flt3 receptor (FLT-3) ; the IL-1 gene family ; the chemokines ; the tumor necrosis factors ; the interferons ; the IL-10 gene family ; the transforming growth factor β (TGF-β) ; and the IL-17 gene family. We will briefly describe these families and the molecules among the family members selected as hematopoietic growth factors (HGFs) for further discussion.
For our purposes, the lymphohematopoietic cytokines are defined as the protein ligands for the members of the hematopoietin receptor gene family, also known as the cytokine receptor class I gene family . This gene family is characterized by an extracellular domain with pairs of conserved cysteine residues and the distinctive amino acid sequence element Trp-Ser-Xaa-Trp-Ser (WSXWS). These receptors all signal at least partly through the JAK-STAT pathway. This rather large family of hematopoietins now includes many interleukins (IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-11, IL-12, IL-13, IL-15, IL-21, and IL-23), granulocye-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), erythropoietin (EPO), thrombopoietin (Tpo), thymic stromal thymopoietin (TSLP), leukemia inhibitory factor (LIF), oncostatin M (OSM), cardiotropin 1 (CT-1), and ciliary neurotrophic factor (CNTF). The cytokine receptors on the cell surface signal as multimers, either homomeric or heteromeric, and the family can be further divided into subfamilies based on the molecular composition of their receptor complexes. We will focus on cytokines that have major actions on hematopoietic cells. Those with homodimeric receptors include EPO, Tpo, and G-CSF. A second subgroup comprising GM-CSF, IL-3, and IL-5 in the human system have receptor complexes that share a common subunit known as the beta common chain that forms heterodimers with unique cytokine alpha subunits to give selectivity for the respective cytokines. A third group includes IL-6 and IL-11 (as well as LIF, OSM, CNTF, and CT-1, not considered further); all share a common receptor-signaling chain known as gp130, with unique chains that provide ligand specificity.
Three HGFs, macrophage CSF (M-CSF, also known as CSF-1 ), stem cell factor (SCF), and the ligand for FLT3 (FLT3L) have been identified that signal through related receptors, which themselves have tyrosine kinase activity. The receptors for these HGFs are all evolutionarily related, as are the ligands themselves, and each plays an important role in controlling blood cell production.
The IL-1 gene family has grown substantially and now includes at least many IL-1s (IL-1α through IL-1ξ), the IL-1 receptor antagonist (IL-1RA), and IL-18. IL-1α, IL-1β, and IL-18 all have potent ability to induce cytokine production (IL-18 particularly interferon gamma (IFN-γ) in combination with IL-12 ), but the two IL-1s have also been shown to synergize with other HGFs in support of early blood cell proliferation and, therefore, will be considered further here. The functions of the other novel members of the IL-1 gene family are still unknown.
The chemokines represent a large family of generally small proteins (usually 8 to 12 kDa) readily identifiable by sequence homology, including twin characteristic cysteine residues near the amino termini that separate the family into CC chemokines if the cysteines are adjacent or CX chemokines if they are separated by one residue. Currently, there are more than 50 members of the gene family that have demonstrated important roles in cell trafficking in many systems. The receptor gene family for this cytokine family comprises about 20 members of the G-protein–coupled receptor (GPCR) family. However, their biologic function is not limited to chemotaxis, because several have been shown to effect gene transcription, apoptosis, and cell proliferation. IL-8 is a prominent CX chemokine that regulates neutrophil trafficking and therefore plays a role in inflammatory responses. However, two chemokines, SDF-1 and macrophage inflammatory protein 1α (MIP-1α) have been reported to inhibit the cycling of very early stem cells and therefore may be important molecules in halting cycling stem cells and returning them to their normal, quiescent state. SDF-1 is clearly very interesting and important in the hematopoietic system as a chemotactic factor for very primitive stem cells, providing possible mechanisms for the homing of cells both as hematopoiesis moves from the fetal liver to the bone marrow and during transplantation of cells in adults.
The TNF gene family now includes TNFα, lymphotoxin α and β, CD27Ligand, CD30Ligand, CD40Ligand, FasLigand, TRAIL, and Trance. Many of these molecules are involved in regulating activation and death of various cells, including T lymphocytes and erythroid progenitors. TNFα itself has many biologic activities, particularly in inflammation. However, it is mentioned here because of its potent and important role in promotion of dendritic cell differentiation and activation and its capacity to depress hematopoiesis.
The type I interferons (13 species of IFN-α, IFN-β, and IFN-ω) are closely linked on human chromosome 9. The type II interferon gamma (IFN-γ) demonstrates little if any sequence homology with the type I interferons, but the receptors for all of these cytokines are members of cytokine receptor class 2, which is related to but distinct from cytokine receptor class I (HGF receptors) because class 2 receptors lack the distinctive WSXWS sequence. In addition to their antiviral activities, both type I and type II interferons have multiple other regulatory activities with many cell types, including T cells. In the human system, IFN-γ plays a prominent role in polarizing T helper cells toward the type 1 (Th1) phenotype. However, none of these molecules appear to have prominent roles in directly controlling blood cell production and will not be considered further here.
The large TGF-β gene family includes TGF-β1, TGF-β2, and TGF-β3; the bone morphogenetic proteins (BMPs) 2 through 16; the growth and differentiation factor (GDF) genes 1 to 10; activin; and inhibin. Like those for many of the other cytokines, the receptors for the TGF-β family members are multimers comprised of two different members of the serine/threonine kinase receptor gene family. Receptors of different ligand specificities are generated by forming these gene products in different combinations. TGF-β1 itself is a very important regulator of cell cycling in many cell systems, including the hematopoietic system, and will be considered here further as a negative regulator of hematopoiesis. Mutations in its receptor cause Loeys-Dietz syndrome. The BMPs and GDFs have been implicated in many developmental systems. Interestingly, BMP-4 has been shown to be essential in Xenopus species for the embryonic development of HSCs, and it seems at least possible that this molecule might also play a role in mammals in blood cell development during embryogenesis if not in adult hematopoiesis, an area worth following. However, for our purposes and the sake of simplicity, we will only include TGF-β1 as an HGF.
Early on, it was recognized that different colony-stimulating factors could selectively support the growth of specific types of hematopoietic colonies. Thus when human bone marrow cells are cultured in semisolid medium in the presence of G-CSF, colonies emerge 7 to 8 days later that consist largely of mature neutrophilic granulocytes and their precursors. This finding led to the model that G-CSF, to a large degree, interacts with relatively late hematopoietic progenitors that have already committed to the neutrophil lineage (CFU-G) and thus G-CSF supports their growth and final maturation into functional neutrophils. Similar analysis has revealed that the other major hematopoietic cell lineages have analogous, lineage-specific, late-acting factors and that these molecules frequently serve as important if not primary regulators of the respective pathways. Thus M-CSF supports monocyte/macrophage colony growth and is important in supporting the growth and maturation of monocyte progenitors (CFUs-M) ; IL-5 supports eosinophilic granulocyte colony formation and therefore supports the growth and maturation of eosinophil progenitors (CFUs-Eo) as well as activating eosinophils ; EPO is necessary for the growth and maturation of both earlier (BFU-E) and later (CFU-E) progenitors of the erythroid lineage ; and TPO directly supports the growth and maturation of megakaryocyte progenitors (CFUs-meg) and the subsequent production of functional platelets.
Although the regulation of the respective blood cell pathways by the lineage-specific HGFs is likely to be their major function, in no case is this lineage specificity absolutely maintained. G-CSF has been found to influence the migration and proliferation of endothelial cells, cells that express high-affinity receptors for this cytokine. IL-5 serves as a growth factor for activated B cells, particularly in the mouse, and affects the type of immunoglobulin secreted by mature B cells. Epo and TPO have been noted to interact with megakaryocyte and erythroid progenitors, respectively. M-CSF appears to be important in trophoblast development. Finally, populations of early HSCs have been found to express receptors for many cytokines; typically, these cells do not respond to single factors but require combinations of factors to trigger them into cycle. “Lineage-specific” factors that have been reported to act in various combinations to trigger cycling of early “stem” cells include G-CSF, M-CSF, and TPO, demonstrating that the molecules are not strictly “lineage specific” even within the hematopoietic system. Mice deficient in either the Tpo receptor ( Mpl ) or G-CSF are deficient in levels of all progenitor cells, consistent with the idea that these factors indeed are involved in expansion of early lineage cells. However, when administered in vivo each of these molecules largely influences the growth and development of the expected lineage, so that the designation of lineage specificity seems warranted.
Initial analysis of human bone marrow cell cultures grown in the presence of GM-CSF revealed that a variety of different colony types develop over a period of 10 to 14 days. Mature blood cells that could be readily identified included neutrophils, monocytes/macrophages, and eosinophils; this finding led to the designation of the molecule as a “granulocyte-macrophage” colony-stimulating factor, or GM-CSF. In comparison with G-CSF, it was found that it took longer to produce colonies with identifiable neutrophils but the ultimate variety of cell types was greater. This led to a model in which GM-CSF acts on progenitors committed to produce either neutrophils or monocytes (CFU-GM), a precursor to the G-CSF–responsive CFU-G and the M-CSF–responsive CFU-M. These later progenitors apparently retain responsiveness to GM-CSF as well because mature monocytes and neutrophils can be observed in cultures supported by GM-CSF alone. That this model is not strictly correct was shown when recombinant GM-CSF was introduced into human bone marrow cultures in the presence of EPO and it was found that this combination of factors was very effective in supporting the development of erythroid colonies (murine GM-CSF is somewhat less effective in this regard). Thus despite its name, GM-CSF generally interacts with intermediate multilineage progenitors that yield neutrophils, eosinophils, monocytes, erythroid cells, and megakaryocytes (CFU-GEMM). At the time, these activities were similar to those ascribed to IL-3 in the murine system. When human IL-3 was identified, it proved to have abilities to support multilineage colony formation similar to those of human GM-CSF, indicating that it interacts with slightly different but strongly overlapping subsets of progenitors. In comparison with GM-CSF, IL-3 is somewhat more effective in supporting multilineage, erythroid, and megakaryocyte colony formation and GM-CSF is slightly more effective with granulocyte and monocyte/macrophage colony formation. In serum-free conditions, the ability of IL-3 to support final neutrophil and monocyte maturation is significantly depressed, indicating that the later-acting factors, G-CSF or GM-CSF in the case of neutrophils or M-CSF or GM-CSF in the case of monocytes, are necessary for final end cell production.
In addition to acting slightly earlier than GM-CSF, IL-3 is clearly distinguished in its activity by its ability to support the growth and maturation of mast cells and basophils. In the mouse, this was one of the first recognized activities of IL-3, and when IL-3 was first administered to primates, basophilia was one of the most prominent findings. Thus IL-3 appears to be capable of supporting the growth and development of basophil and mast cell progenitors. In the human system, both IL-3 and GM-CSF can support the development and differentiation of dendritic cells of either myeloid or lymphoid origin, especially in combination with SCF, FLT3L, and TNF.
IL-4 in mice and humans has been reported to support multilineage colony formation, including colonies that contain cells from the erythroid, megakaryocytic, neutrophilic, and monocytic lineages. IL-4 in the mouse supports mast cell growth and therefore shares many activities with IL-3. However, IL-4 plays very important roles in the development and maturation of T cells and B cells, particularly in the polarization of cytokine production by helper T cells toward the type 2 (Th2) response. Therefore, on balance IL-4 is likely to be more important in controlling immune cell development and function and will not be discussed further here. IL-9 in both the murine and human systems has been shown to enhance erythroid colony formation in the presence of Epo and appears to play a role in T-cell and B-cell development as well. More recently, IL-9 has been shown to have some effects on the growth and activation of mast cells and may play some role in the pathology of asthma.
Stem cell factor (SCF) and FLT3 ligand (FLT3L), both receptor tyrosine kinase ligands, interact with a variety of hematopoietic progenitor cells, perhaps most importantly with very early stem cell populations. SCF also plays an important role in melanocyte growth and development, which is reflected in the coat color effects of mutations in SCF or its receptor, KIT. Genetic analysis of mice clearly showed that mice defective in either SCF (Sl mice ) or in Kit (W mice ) have serious hematopoietic (and many other) defects, including macrocytic anemia, mast cell deficiencies, and deficiencies in the stem cell compartment (reviewed in reference 152). These early studies had already indicated the critical importance of SCF in the survival and development of stem cells. Mutations in the human KIT gene lead to a similar phenotype in melanocyte development in humans known as the piebald mutation; however, these individuals do not have any hematologic problems, probably because severe mutations in this locus are likely to be lethal. In vitro, the activities of SCF are generally most evident when combined with other HGFs; the proliferative activity of SCF with hematopoietic cells in culture as a single factor is minimal. In fact, culture of murine bone marrow cells in SCF alone ultimately yields largely mast cells. However, SCF acts synergistically to enhance the activities of most of the other HGFs in culture and is particularly effective when combined with HGFs such as IL-3, IL-1, or IL-11 at promoting the expansion of “blastlike cells” that retain considerable potential for yielding multilineage colonies in secondary culture. These colonies, when replated under conditions that support B-lymphocyte development or when transplanted into animals also yield B and T lymphocytes, indicating that SCF-responsive cells include primitive stem cells with both lymphoid and myeloid potential. SCF has also been implicated in combination with IL-2 or IL-7 in early stages of T-cell development in the thymus, with IL-7 in pre–B-cell growth, and with IL-7 in enhancing NK cell responsiveness to IL-2. However, the fact that none of these lineages are dramatically affected in W or Sl mice indicates that SCF-independent mechanisms can compensate in these systems.
SCF, M-CSF, and FLT3LG are all expressed as both membrane-bound and soluble forms. In the case of SCF, expression of membrane-bound forms of the molecule in the marrow microenvironment provides a nice model for how this growth factor might act locally. Indeed, cell lines that express membrane-associated SCF exclusively are much more effective in supporting long-term hematopoiesis in vitro than cell lines that produce soluble forms of the molecule exclusively. This interaction of membrane-associated SCF with KIT provides one mechanism for the adherence of hematopoietic cells to stroma; binding of human megakaryocytes to fibroblasts can be blocked by antibodies to KIT. Finally, if membrane-associated forms of SCF are present in which the cytoplasmic domain is essentially missing, the result is male but not female sterility, suggesting that the cytoplasmic domain may have an as yet undetermined important biologic function.
As shown by the early genetic studies and confirmed through analysis of the recombinant protein, SCF is not specific for the hematopoietic system. It is an important growth factor for melanocytes and primordial germ cells, and it appears to play a role in development of the nervous system, perhaps as a neuronal guidance factor, although it has been difficult to demonstrate neurologic defects in W or Sl mice other than the fact that the mice are severely constipated due to SCF dependence of intestinal Cajal cells.
The FLT3 receptor tyrosine kinase was originally identified as a novel receptor present in HSCs; with human marrow, the expression is largely limited to the CD34+ cell population. FLT3L alone yields low numbers of CFU-GM colonies from human bone marrow but acts synergistically with other cytokines, including IL-3, GM-CSF, EPO, and SCF, to yield enhanced colony formation, both in terms of size and numbers of colonies. The synergy observed between FLT3L and the other HGFs is comparable to that observed with SCF in similar systems, with the exception that FLT3L has little effect on BFU-E. Multifactor combinations with SCF have been used for expansion of colony-forming cells in long-term cultures; FLT3L has effects comparable to those of SCF when combined with IL-1, IL-3, IL-6, and EPO in 4-week cultures. Like SCF, FLT3L in combination with other cytokines such as GM-CSF supports dendritic cell development from CD34+ bone marrow cells. In contrast to SCF, FLT3L does not support the growth and development of mast cells. Despite this overlap in bioactivities, mice in which the Flt3 receptor tyrosine kinase has been disrupted appear to have normal hematopoiesis, with the only detectable defects observed within the B-lymphocyte lineage. However, mice in which both the Kit and Flt3 receptor tyrosine kinase genes have been disrupted display more severe hematologic complications than mice with mutation of only one of these genes, suggesting that the two pathways can to some degree compensate for one another. The importance of FLT3L in B-cell growth has also been shown with cultures of primitive B-cell progenitors (CD43+, B220 low ) in combination with either IL-7 or SCF. Altogether, these findings argue for an important role for FLT3L in hematopoiesis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here