Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The nervous system actively participates in, regulates, and integrates all the other body systems. The nervous system is broadly divided into:
The central nervous system (CNS) (see Ch. 3 ).
Composed of brain and spinal cord.
The peripheral nervous system (PNS) (see Ch. 4 ).
Composed of the autonomic nervous system (ANS) and somatic nervous system.
This chapter focuses on the structure and cellular physiology of the functional unit of the nervous system, neurons (nerve cells).
The neuron is the basic cellular unit of the nervous system. The human brain contains approximately 10 11 neurons, with each making roughly 1000 synapses with other neurons. Thus there are about 10 14 synaptic connections in the human brain, forming a “neural network” allowing the highly interconnected nerve cells to communicate with one another.
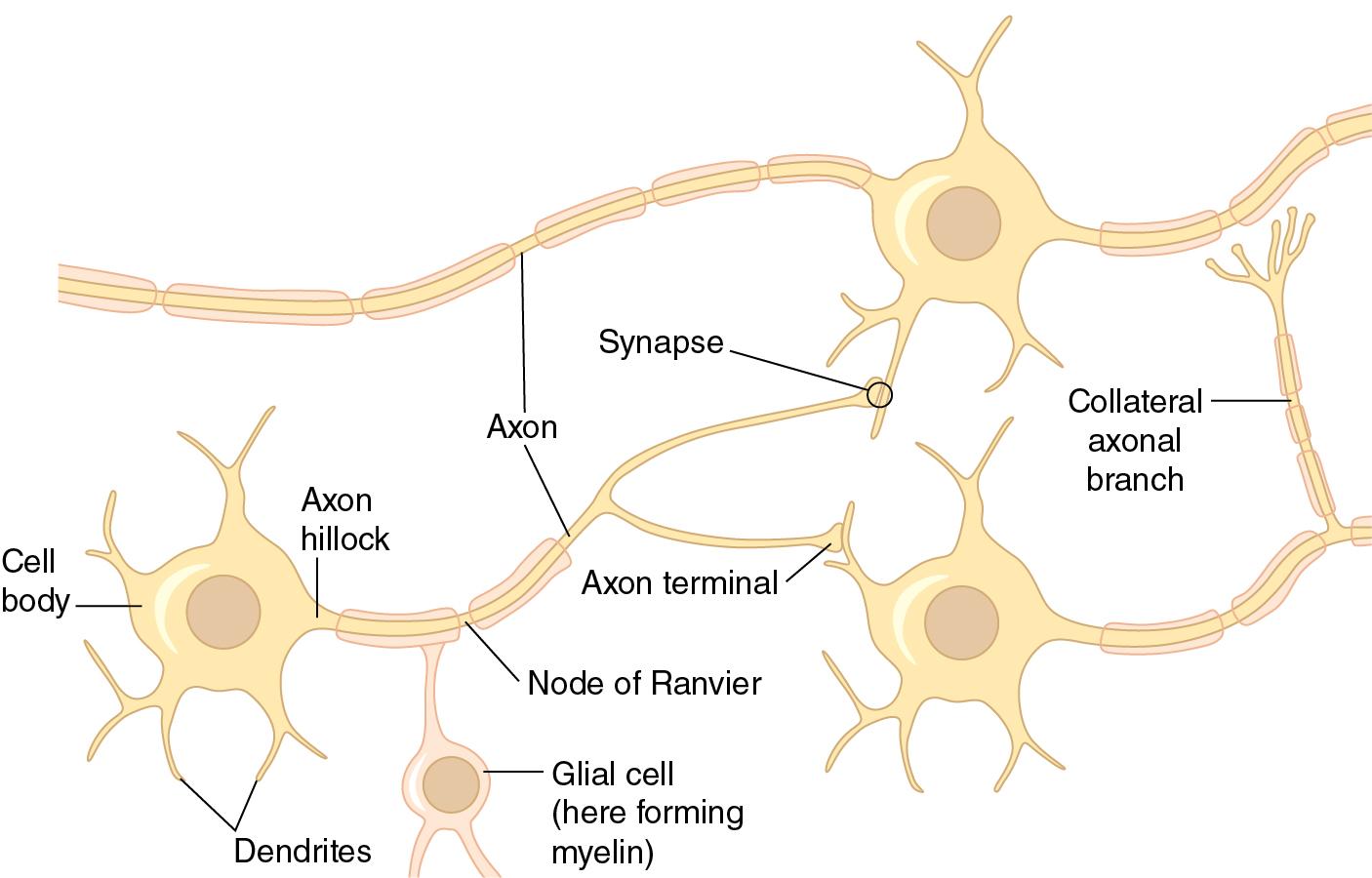
Although neurons share many characteristics similar to other cells, they also possess specialized structures that enable information to be processed in an organized manner. Although the precise structure of neurons is dependent on their function, all share several basic features ( Fig. 2.1 and Table 2.1 ):
Dendrites: Short, highly branched cytoplasmic extensions.
Function: Receive afferent signals from the environment.
Located near the cell body (soma).
Cell body (soma).
Function: Contains nucleus and molecular machinery necessary for gene expression, protein production, and cell metabolism.
Axon: Long, cytoplasmic extension.
Function: Transmits signals from soma to end organs or other neurons.
Proteins and other substances are ferried along microtubule “highways” in the axon by specific transport proteins.
Kinesins (anterograde transport).
Dyneins (retrograde transport) (see Clinical Correlation Box 2.1 and ( Fast Fact Box 2.1 ).
Myelin: Phospholipid-rich sheath surrounding axons.
Function: Insulates neurons and facilitates nerve transmission over long distances.
Composed of the plasma membrane of specialized glial support cells ( Fig. 2.1 and Table 2.2 ).
In the CNS, support cells are called oligodendrocytes and support many neurons.
In the PNS, support cells are called Schwann cells and support one neuron.
| Neuroglia | Description | Function |
|---|---|---|
| Astrocytes | Stain positive for glial fibrillary acidic protein (GFAP) | Repair neurons, provide nutritional support, maintain the blood-brain barrier, regulate CSF composition |
| Ependymal cells | Single cell layer, lines the ventricles | Produce CSF in the choroid plexus, circulate CSF |
| Microglia | Irregular nuclei, little cytoplasm | Become phagocytic in response to tissue damage |
| Oligodendroglia (oligodendrocytes) | Found in the CNS | Myelinate up to 30 neurons each |
| Schwann cells | Found in the PNS. Gaps between cells are called nodes of Ranvier | Myelinate only one axon each. Secrete growth factors and create a pathway for axonal regeneration |
Phospholipid fat in the myelin sheath gives axons a white appearance.
White matter = myelinated axons.
Grey matter = unmyelinated cell bodies.
Terminal boutons: Specialized endings of axon.
Function: Allows communication to target tissues or other neurons via synapse.
Synaptic cleft.
Function: Maintains association of presynaptic and postsynaptic elements.
Extensive cytoskeletal elements allow maintenance of structure.
Site of enzymatic degradation of excess neurotransmitter.
Certain pathogens exploit retrograde transport to invade the nervous system. Upon exposure to distal axon terminals, they are able to “hitch-hike” back to the cell soma. Known examples include tetanus toxin, herpes simplex virus, rabies virus, and poliovirus. Thus onset of symptoms will be delayed according to the length of time required for retrograde transport.
Anterograde: Substances move away from the soma.
Retrograde: Substances return to the soma.
| Classification | Description |
|---|---|
| Unipolar | One dendrite or one axon (i.e., sensory neurons) |
| Pseudo-unipolar | One process that branches into a dendrite and an axon |
| Bipolar | One dendrite and one axon |
| Multipolar | Multiple dendrites and axons |
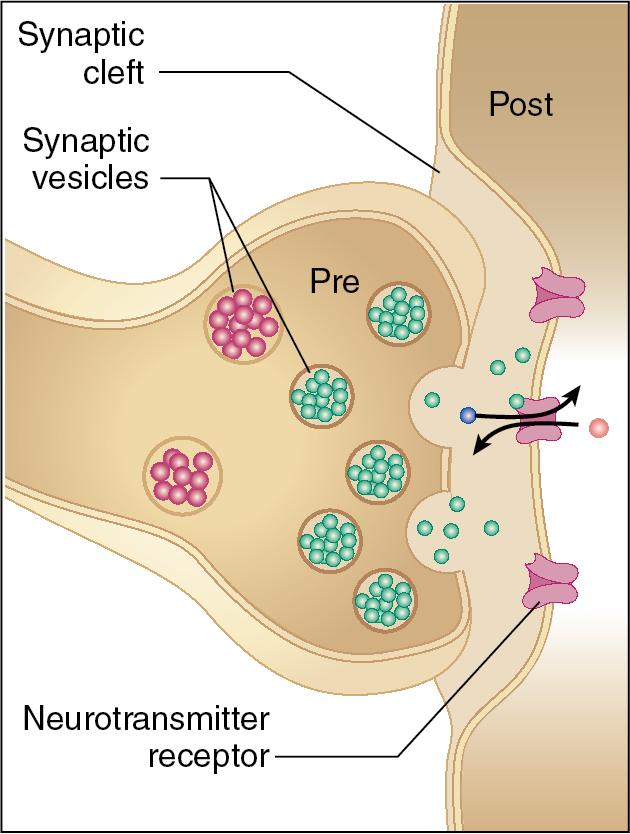
The presynaptic axon terminal, synaptic cleft, and postsynaptic dendrite comprise the synapse ( Fig. 2.2 ). Fundamentally, there are two different types of synapses:
Chemical: Convert an electrical signal into a chemical signal, which is then received by chemical receptor. More highly regulated.
Electrical: Allow ions to flow directly between neurons from the presynaptic to postsynaptic membranes without loss of signal strength.
As in other cells, the cell membrane is a lipid bilayer with embedded proteins. In neurons, the plasma membrane serves three crucial roles:
Maintains the integrity of the intracellular environment.
Allows reception of signals.
Regulates changes in electrochemical state of the nerve cell.
Two classes of membrane proteins essential to nerve cell function are ion channels and neurotransmitter receptors:
Ion channels are found in specialized regions of the axon cell membrane and regulate the passage of ions, such as Na + , K + , Cl − , and Ca 2+ into or out of the cell.
Ungated channels are constituitively open to their specific ion.
Gated channels
Exist in three possible states ( Fig. 2.3 ):
Closed = specific ion cannot cross the cell membrane.
Open = specific ion is permitted to cross the cell membrane.
Locked = specific ion cannot cross the cell membrane and the receptor cannot open.
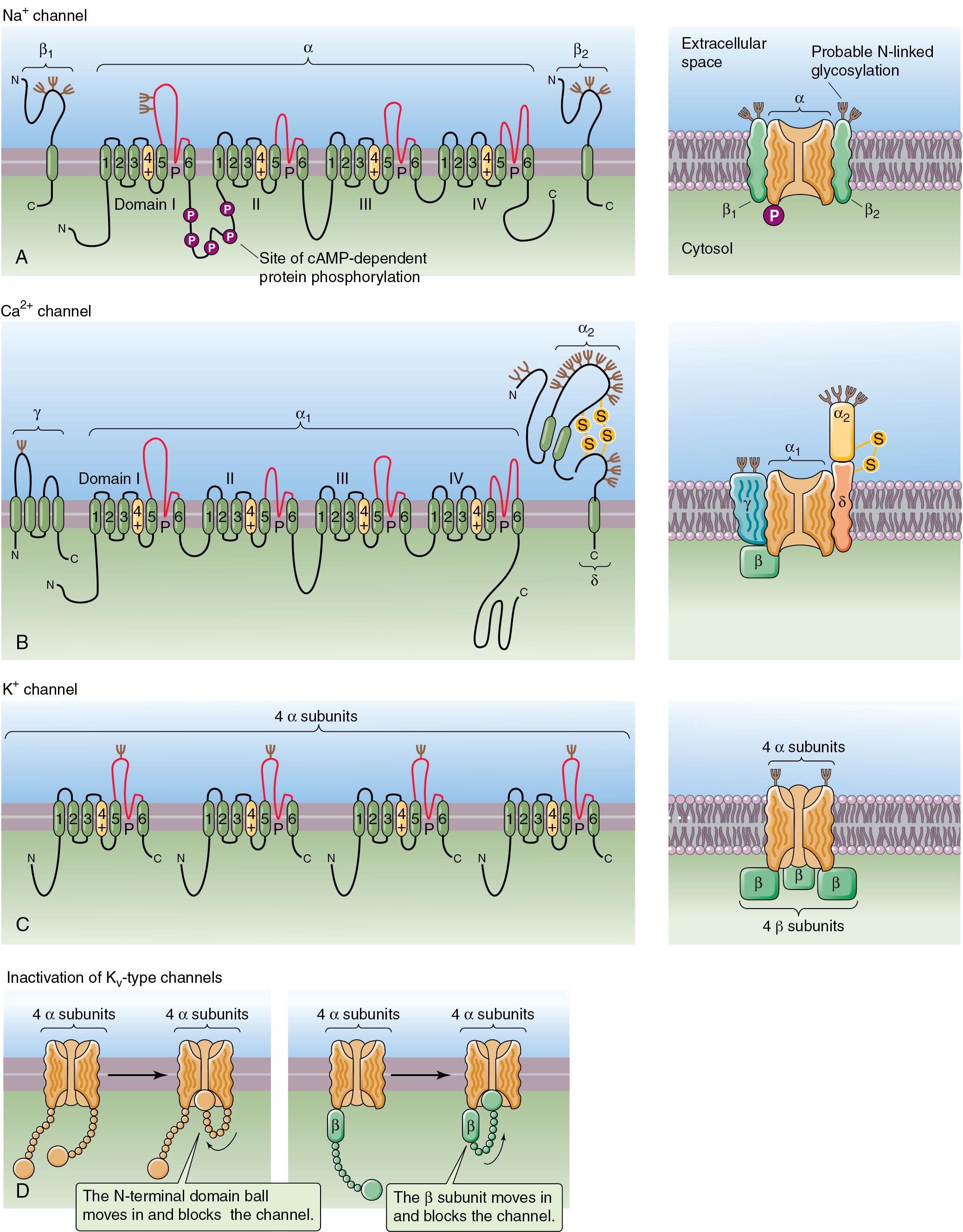
Two subclasses are essential to neuronal function:
Voltage-gated ion channel is more likely to be open when there is a change in the electrical potential of the nerve cell membrane.
Ligand-gated ion channel is more likely to be open when a specific neurotransmitter binds to it.
Neurotransmitter receptors are present on the dendrites and on the plasma membrane of nonneuronal target cells. These receptors bind their respective neurotransmitter ligands that have been released into the synaptic cleft (see Fig. 2.2 ).
The plasma membrane is semipermeable, allowing some substances to pass between the intracellular and the extracellular spaces while restricting the movement of others.
Two types of gradients exist in driving the movement of substances across the plasma membrane:
Chemical gradients occur when the concentration of a solute differs on one side of the membrane from the other.
Solute tends to move from the area of higher concentration to that of lower concentration until equilibrium is established.
Electrical gradients occur when the solute possesses an electrical charge.
Na + , K + , Ca 2+ , Cl − , and HCO 3 − are electrically charged solutes whose intracellular and extracellular concentrations are tightly controlled.
Transmembrane movements are the basis of electrochemical signaling in neurons and muscle cells.
At baseline, the intracellular space is electrically negative compared with the extracellular space. Three factors play into this.
The interior of a cell contains negatively charged solutes, such as proteins and nucleic acid macromolecules such as DNA and RNA that cannot traverse the cell membrane.
The Na + ,K + -ATPase enzyme in the plasma membrane transports three Na + out of the cell and two K + into the cell, meaning a net loss of one positive charge from the cytoplasm.
Leakage of K + out of the cell down its chemical gradient through membrane channels removes more positive charges from the cell.
The baseline electrical polarization of the cell membrane is called the resting membrane potential (Em). Mathematically, Em is conventionally defined as the difference in electrical potential between the inside and the outside of the cell: E m = E in – E out . A typical human neuron has an E m of – 60 to – 70 mV.
A combined electrochemical gradient acting on each of the major ions thus regulates its movement ( Fig. 2.4 ). The sum of the chemical and electrical potential of ion X is called the electrochemical potential (μ) and is defined by the equation:

μ 0 is the reference state electrochemical potential in mV; R = 8.3145 J/(°K)(mol) is the universal gas constant; T is the absolute temperature in degrees Kelvin, approximately 310° K at body temperature; ln[X] is the natural logarithm of the concentration of the ion X; z is the electrical charge on ion X (+1 for K + and Na + , +2 for Ca 2+ and Mg 2+ , and –1 for Cl − and HCO 3 − ); F = 9.6485 × 10 4 Coulombs/mol is the Faraday constant; and E is the electrical potential in mV.
The difference in electrochemical potential across a cell membrane is defined as Δμ =μ in – μ out . Because μ 0 is constant, substituting Equation 2.1 and combining like terms yields:
Δμ is called the electrochemical potential difference. It quantifies the electrochemical gradient, the combined chemical and electrical forces acting on an ion X. As Equation 2.2 depicts, the electrochemical gradient acting on a particular ion with respect to a particular cell depends on:
The ratio of the intracellular and extracellular concentrations of the ion.
The ion’s charge.
The E m of the particular cell (see Fig. 2.4 ).
When the ion is in chemical and electrical equilibrium (i.e., no net flow in either direction), there is no net charge generated and thus Δμ must equal 0. Given this, we can rearrange Equation 2.2 to yield the following equation:
Converting from natural logarithm to log10 and substituting in the known values of R, T, and F results in the Nernst equation.
This is the Nernst potential, or equilibrium potential that results from an ion being in equilibrium at the measured intracellular and extracellular concentrations. The Nernst potential depends on the particular ion’s charge and its distribution across the cell membrane ( Table 2.3 ). The Nernst equilibrium potential is a simplification of a more complex equation describing the steady state potentials for every ion gradient in the cellular and extracelular space. This equation, known as the Goldman equation, is as follows: Em = (−61.54 mV)log[(P K* [K + ] in /P K* [K + ] out ) + (P Na+* [Na + ] in /P Na+* [Na + ] out ) +(P Cl−* [Cl − ] out /P Cl−* [Cl − ] in ) + ...)]. The contribution of each ion is determined by the magnitude of the electrochemical gradient and the permeability or conductance of the membrane to that ion. In the resting potential state, where the membrane permeability to K + is much greater (P K+ >>> P for other ions) than that of other ions because the K + channels are open while those for other ions are closed, the resting membrane potential is very near the Nernst potential for K + , as the Goldman equation simplifies to something close to the K + Nernst equation. The Goldman equation describes a steady state situation, while the Nernst equation is a simplified, special case of that state that describes an equilibrium situation.
| X | z | [X] in | [X] out | E X |
|---|---|---|---|---|
| K + | +1 | 120 mM | 4 mM | −91 mV |
| Na + | +1 | 10 mM | 140 mM | +70 mV |
| Cl − | −1 | 9 mM | 105 mM | −65.6 mV |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here