Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The immune system protects the organism from infectious microbes and avoids responses that produce damage to host tissues.
The immune system is divided into the innate and adaptive systems. The adaptive immune system recognizes different specific antigens, adapts to changing environments, and provides immunologic memory.
The innate immune system includes all aspects of the host defense mechanisms, such as barrier mechanisms (epithelium, mucous layer, mucociliary transport) and soluble bioactive molecules (complement proteins, defensins, cytokines, mediators, and enzymes).
Cell-mediated immune responses are orchestrated by T cells and their cytokines and mount appropriate reactions to offending antigens.
Allergic rhinitis is an example of an IgE-dependent, mast cell–mediated immune reaction, whereby the release of mast cell or basophil mediators is followed by a complex inflammatory process that determines the response to allergens to which the individual is sensitized.
Allergic rhinitis is a common disease that leads to significant impairment of quality of life and a large healthcare expenditure.
The pathophysiology of allergic rhinitis revolves around mast cell release of inflammatory mediators followed by a chronic inflammatory response, in which eosinophils and lymphocytes play a predominant role and which leads to hyperresponsiveness of the nasal mucosa to subsequent stimulation by antigens or irritants.
H 1 antihistamines are effective agents for the treatment of allergic rhinitis but do not completely control the bothersome symptom of nasal congestion.
Leukotriene receptor antagonists are effective in controlling the symptoms of allergic rhinitis, and their efficacy parallels that of antihistamines. They are not recommended as monotherapy for allergic rhinitis but might be useful for patients with allergic rhinitis and asthma.
Intranasal steroids are potent anti-inflammatory agents that control almost all aspects of allergic nasal inflammation and are superior in efficacy to antihistamines and leukotriene receptor antagonists in controlling symptoms of the disease and improving quality of life.
Intranasal steroids should be considered first-line therapy for allergic rhinitis, except in the mildest cases of the disease.
The most effective combination for the treatment of allergic rhinitis is an intranasal steroid with an intranasal antihistamine.
Immunotherapy is the most effective treatment of allergic rhinitis and can be administered subcutaneously or by the sublingual route.
Immunotherapy is the only allergic rhinitis therapy that has been shown to alter the natural course of the disease.
The importance of the immune system in health and disease has long been recognized. Not only does the immune system protect the organism from infectious microbes with its diverse collection of pathogenic mechanisms, it also avoids responses that produce damage to host tissues. This basic property of the immune system relies on detecting structural features of the pathogen that are distinct from those of host cells. When this process fails, immunopathology and disease results. In this chapter, we first provide an overview of the human immune system and its components. We then discuss immunopathology, with a focus on allergic rhinitis (AR), which is the most common manifestation of immunopathology in the upper aerodigestive tract. Workup and management of AR will be discussed in detail.
Broadly defined, the innate immune system consists of all aspects of the host defense mechanisms encoded in the germline genes of the host. These include barrier mechanisms such as epithelial cell layers that express tight cell-cell contact, the secreted mucous layer that overlays the epithelium, and the epithelial cilia that sweep away this mucous layer. The innate response also includes soluble proteins and small bioactive molecules that are either constitutively present in biologic fluids, such as the complement proteins and defensins, or are released from activated cells and include cytokines, chemokines, lipid mediators of inflammation, and bioactive amines and enzymes. Activated phagocytes—including neutrophils, monocytes, and macrophages—are also part of the innate immune system. Because the recognition molecules used by the innate system are expressed broadly on a large number of cells, this system is poised to act rapidly after an invading pathogen is encountered.
The second set of responses constitutes the adaptive immune response. The adaptive system is composed of small numbers of cells with specificity for any individual pathogen, so the responding cells must proliferate after encountering the pathogen to attain sufficient numbers to mount an effective response against the microbe; thus the adaptive response generally expresses itself temporally after the innate response in host defense. A key feature of the adaptive system is that it produces long-lived cells that persist in an apparently dormant state but that can reexpress effector functions rapidly after repeated encounters with an antigen. This feature provides the adaptive response with immune memory that permits it to contribute to a more effective host response against specific pathogens when they are encountered a second time.
Mammalian toll-like receptor (TLR) family members are transmembrane proteins that contain repeated leucine-rich motifs in their extracellular portions. Mammalian TLR proteins contain a cytoplasmic portion that is homologous to the interleukin (IL)-1 receptor and can therefore trigger intracellular signaling pathways. TLRs are pattern-recognition receptors that recognize pathogen-associated molecular patterns present on a variety of bacteria, viruses, and fungi. The activation of TLRs induces expression of costimulatory molecules and the release of cytokines that instruct the adaptive immune response. TLRs directly activate host defense mechanisms that combat the foreign invader or contribute to tissue injury.
TLRs were initially found to be expressed in all lymphoid tissue but are most highly expressed in peripheral blood leukocytes. In the airway, TLRs are expressed in a wide variety of cells including epithelial cells, macrophages, mast cells, eosinophils, and dendritic cells. TLRs consist of a large family with at least 11 members. Different TLRs are recognized/activated by different pathogens. Examples include TLR2 and gram-positive cell wall components, TLR4 and lipopolysaccharides (LPSs) of gram-negative bacteria and pneumolysin, a major virulence factor of Streptococcus pneumoniae . Mammalian TLR3 mediates the response to double-stranded ribonucleic acid (RNA), a molecular pattern expressed by many viruses during infection. Activation of TLR3 induces interferons (IFN)-α and IFN-β, cytokines important for antiviral responses. In addition to their important role in innate immunity, activation of TLRs as part of the innate response can influence and modulate the adaptive T-cell response and modify the shaping of the TH1/TH2 balance. As well as their protective effects, TLRs expressed on dendritic cells, epithelial cells, eosinophils, and mast cells contribute to airway inflammation, including asthma.
The function of antimicrobial peptides (AMPs) is essential to the mammalian immune response. They participate primarily in the innate immune system and are used as a first-line immune defense by many organisms, including plants, bacteria, insects, and vertebrates. AMPs directly kill a broad spectrum of microbes, including gram-positive and gram-negative bacteria, fungi, and certain viruses. In addition, these peptides interact with the host to trigger events that complement their role as antibiotics. AMPs have also been shown to have chemotactic properties and cytotoxicity against a wide range of normal and malignant targets, and in vitro studies have demonstrated their ability to enhance cellular proliferation and cytokine responses of CD4 + T cells and to modulate expression of costimulatory molecules. Most of these peptides achieve their effects by direct killing of pathogens by virtue of their antimicrobial activity but also stimulate the cellular immune response to generate tissue inflammation, which contributes to the defense. Two major families of AMPs have been characterized in mammals—defensins and cathelicidins.
Natural killer (NK) cells are present in the peripheral circulation and in the spleen, lungs, and liver but they are not found in lymph nodes and do not recirculate through the thoracic duct lymph. These cells are usually larger than typical lymphocytes and display less nuclear material and more cytoplasm. The role of NK T cells is the killing of viral-infected and malignant cells in the host. NK cells have no antigen-specific receptors; their cytotoxic activity is inhibited by encountering self major histocompatibility complex (MHC) molecules through inhibitory receptors on the surface of NK cells that recognize class I; thus they kill self cells that have downregulated class I molecule expression. This factor is important in host defense because several viruses have developed mechanisms to downregulate class I expression in infected cells as a strategy to avoid CD8 + cell killing; thus NK cells are active in killing those virally infected cells.
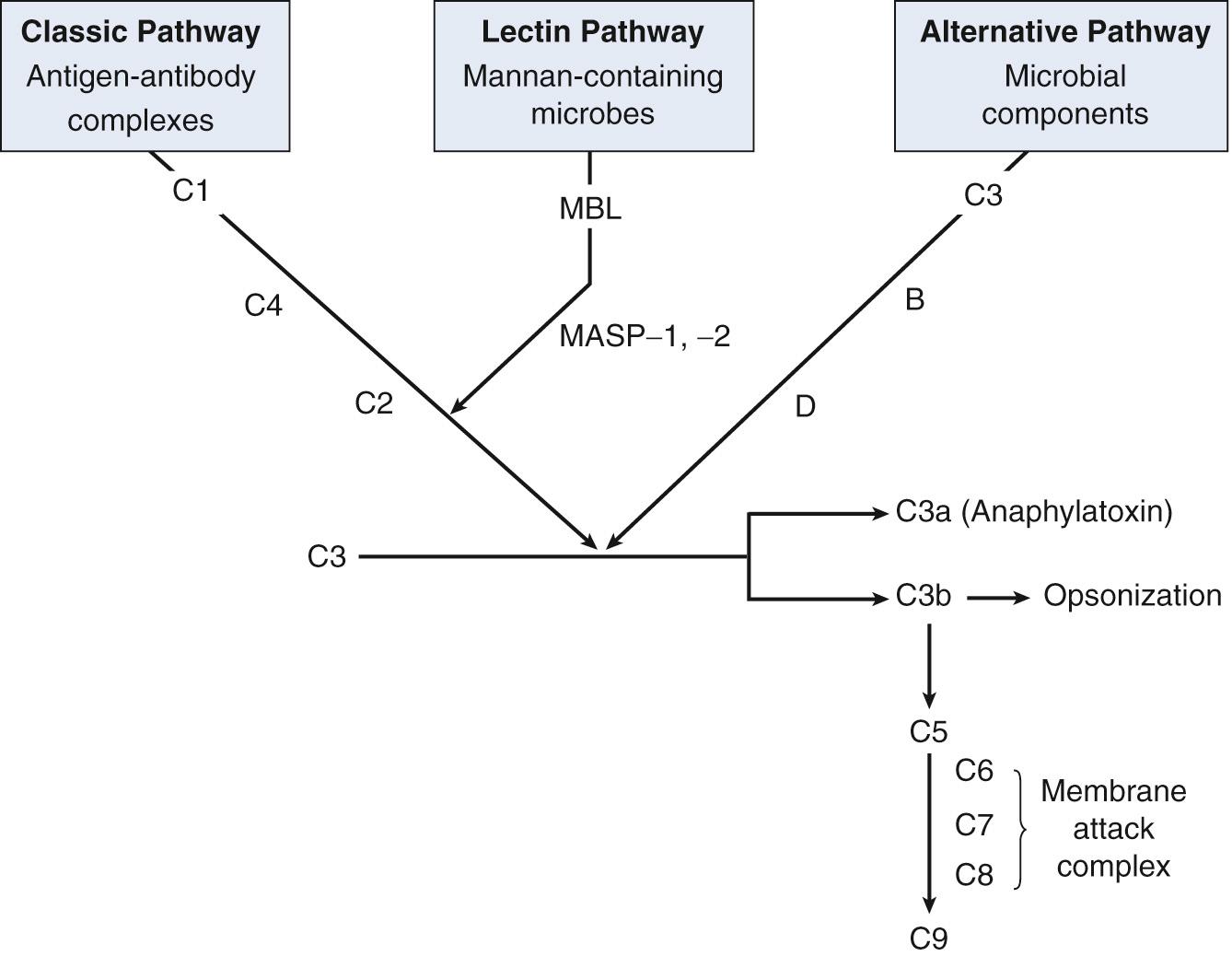
The complement system is an important effector of both the innate and adaptive immune responses. It consists of more than 25 plasma and cell-surface proteins that are sequentially activated and that can interact with one another, with antibodies, and with cell membranes. These interactions mediate functions such as immune adherence, phagocytosis, chemotaxis, and cytolysis. Complement system proteins constitute approximately 15% of the globulin fraction of plasma and circulate as inactive molecules. Complement activation centers on cleavage of C3, which can be achieved by classic, alternative, or lectin pathways ( Fig. 35.1 ). The classic pathway is activated by antigen-antibody complexes, the alternative pathway is activated by microbial structures, and the lectin pathway is triggered by microbial cell wall components containing mannans. All three pathways lead to cleavage of C3, which results in the release of the small C3a fragment—a potent anaphylatoxin that induces mast cell degranulation, creates edema, and recruits phagocytic cells—and the larger C3b fragment, which covalently attaches to the activating antigen, marking it for destruction. C3b serves both as a site for attack of the complement membrane attack complex (MAC), a self-assembling, pore-forming complex of the plasma proteins C5, C6, C7, C8, and C9 that kills targets by osmotic lysis, and as an opsonin, enhancing phagocytosis by its binding to complement receptors on the surfaces of neutrophils and macrophages. The importance of the complement system is underscored by the phenotypes of inherited deficiencies of individual components. For example, deficiencies of components of the MAC lead to increased susceptibility to Neisseria infections, deficiency of C3 results in life-threatening susceptibility to pyogenic infections that are often fatal during childhood, deficiency of C4 or C2 causes a lupus-like immune complex disease, and deficiency of the serum inhibitor of C1 leads to episodic mast cell–independent episodes of angioedema.
Pluripotent stem cells are derived from the yolk sac and ultimately reside in the bone marrow; these are the progenitor cells from which all cells of the immune system are derived ( Fig. 35.2 ). These pluripotent stem cells give rise to lymphoid and myeloid stem cells. Lymphoid stem cells differentiate further into the three major populations: T cells, B cells, and NK cells. T cells are defined by their cell-surface expression of the T cell receptor (TCR), a transmembrane heterodimeric protein that binds processed antigen displayed by antigen-presenting cells (APCs). B cells are phenotypically defined by their expression of the B cell receptor for antigen, membrane-bound immunoglobulin. NK cells are defined morphologically as large granular lymphocytes. They are distinguished by their lack of either TCR or surface immunoglobulin. They recognize their virus-infected or tumor-cell targets through the use of a complex collection of activating and inhibitory cell-surface receptors. Lymphocytes represent approximately 25% of leukocytes in the peripheral blood. The relative contribution of each subtype to this percentage is as follows: T lymphocytes, 80%; B lymphocytes, 10%; NK large granular lymphocytes, 10%.
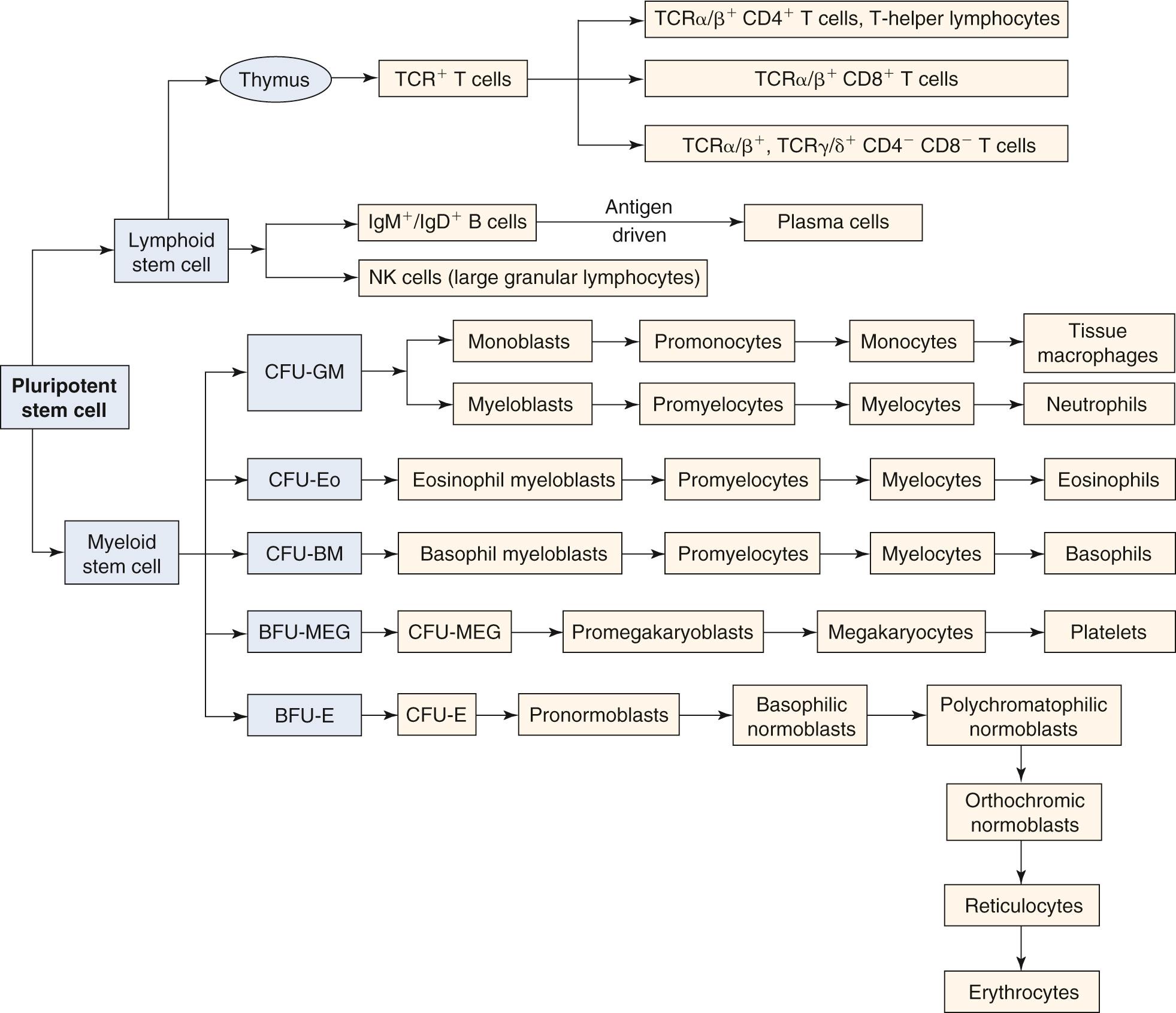
Myeloid stem cells give rise to different forms of granulocytes, megakaryocytes, and erythrocytes. Cells of the granulocyte lineage that play prominent immune roles include neutrophils, monocytes, eosinophils, basophils, and mast cells. Differentiation of the myeloid stem cell occurs in the bone marrow, as does the development of B lymphocytes and NK lymphocytes. In contrast, T-cell progenitors leave the bone marrow and migrate to the thymus, where they differentiate into mature T lymphocytes.
Differentiation of lymphoid and myeloid stem cells depends on their interaction through their surface receptors with soluble ligands (cytokines) or surface ligands (cell interaction molecules). Therefore, proliferation and differentiation along one of the myeloid or lymphoid lineages are controlled (1) through the spatially and temporally regulated exposure of these stem cells to different ligands or factors and (2) through the differential expression of receptors on the stem cells. Cytokines have pleiotropic effects on the development of lymphoid and myeloid cells, which affects both growth and maintenance of pluripotent stem cells and their development and differentiation to specific lineages. Stromal cells within the bone marrow and thymus also regulate cell growth and differentiation by releasing cytokines, such as IL-4, IL-6, IL-7, and IL-11 and granulocyte-macrophage colony-stimulating factor (GM-CSF). They also participate in cell-cell interactions with progenitors through engagement of cell-surface molecules that provide additional regulatory stimuli and participate in the development of the intercellular matrix (e.g., collagen, fibronectin).
T cells in the blood and lymphoid organs can be divided into two major categories: CD8 + cells (cytotoxic T lymphocytes) and CD4 + cells (helper T cells). These two categories of cells have distinct and important functions within the adaptive immune system.
CD8 + cells are cytotoxic against cells infected with intracellular microbes and against tumor cells; CD8 + cells are the pillars of the cell-mediated immune response. CD8 + effector T-cell function is distinguished by antigen-specific cytotoxicity restricted by MHC class I molecules. On priming, CD8 + T cells produce cytotoxic proteins, including perforin and granzymes, and secrete them at the point of contact with the target cell, which results in specific killing without bystander cell damage. The perforin-dependent pathway of cytotoxicity is largely responsible for cell-mediated clearance of infectious viruses and certain intracellular bacterial infections and for the rejection of allogeneic tissue grafts and tumors.
CD4 + cells work to activate both humoral immune responses (B-cell help) and cellular responses (delayed-type hypersensitivity responses). After recognizing antigens presented by MHC class II molecules, CD4 + cells become activated to secrete IL-2, partly in response to monocyte-derived IL-1 and partly in response to autocrine stimulation by IL-2 as part of a positive feedback loop. Activated CD4 cells interact with other CD4 + or CD8 + cells by secreting IL-2 and with B cells by secreting B-cell growth and differentiation factors (IL-2, IL-4, and IL-6); thus CD4 + cells augment immune responses by stimulating B cells sensitized by antigen and by stimulating CD8 + cells sensitized by binding of antigen in the context of MHC class I molecules. Classically, CD4 + effectors were viewed in the context of the TH1/TH2 paradigm but other subsets have emerged and include IL-17-producing T cells (TH17), IL-22-secreting T cells (TH22), IL-9-secreting T cells (TH9), and T cells with regulatory functions (regulatory T cells [Tregs]).
TH2 cells produce cytokines such as IL-4, IL-5, and IL-13, and they specialize in facilitating B cell antibody responses. They drive B cell proliferation by IL-4 and contact-dependent CD40:CD40 ligand binding, which increases humoral defenses against extracellular pathogens. Moreover, IL-4, IL-5, and IL-13 enable immunoglobulin E (IgE) production and eosinophilic inflammation, both of which are important for the clearance of helminthic disease and the allergic response. IL-13, in particular, is critical in driving key pathological features of the allergic response, which includes eosinophilic inflammation and airway mucus production, remodeling, and hyperreactivity in asthma. TH2 differentiation is initiated by weak TCR signals coupled with IL-4 receptor signaling and signal transducer and activator of transcription 6 (STAT-6). This results in upregulation of GATA-3 transcription factor, the master regulator of TH2 differentiation. GATA-3 enhances TH2 cytokine production and inhibits TH1 developmental pathways.
TH1 cells specialize in macrophage activation by IFN-γ production and contact-dependent stimulation through the use of a variety of cell-surface costimulatory ligands, therefore playing a major role in intracellular pathogen clearance and delayed-type hypersensitivity. TH1 differentiation is directed by IFNs generated by the innate response to infection, which ultimately leads to upregulation of T-bet, the master regulator of TH1 differentiation. T-bet directs IFN-γ production and IL-12 receptor expression. The presence of IL-12 results in activation of STAT-4, which further enhances IFN-γ production and TH1 effector formation. IFN-γ is an essential contributor to the generation of chronic inflammatory responses characterized by mononuclear cellular infiltration and activated macrophages. In addition to orchestrating the immune response by secreting various cytokines and contributing to cytotoxic T lymphocyte activities, the characteristic inflammatory reaction induced by CD4 + T lymphocytes is the delayed-type hypersensitivity reaction, which is elicited by challenge with antigen in immune-sensitized individuals. The typical example is the cutaneous reaction to challenge with the purified protein derivative of Mycobacterium tuberculosis in those previously infected or vaccinated. Clinically, the delayed-type hypersensitivity reaction is manifested by local erythema and induration 24 to 48 hours after challenge. Microscopically, the lesion shows perivascular accumulations of leukocytes—initially neutrophils, later lymphocytes and activated macrophages—with edema and fibrin deposition. Chronic delayed-type hypersensitivity reactions result in the formation of granulomas, nodular collections of macrophages and lymphocytes and, possibly, fibrosis as a result of the cytokines produced by macrophages, which stimulate fibroblast proliferation and collagen synthesis.
TH17 cells are a subset of T cells that fill an essential gap in our understanding of inflammatory processes because it was unclear how TH1 cells actually mediate inflammation in the tissues by the expression of IFN-γ. TH17 cells secrete IL-17 (or IL-17A), IL-17F, IL-6, TNF-α, and IL-22. Differentiation of naïve effector T cells in the presence of IL-6 and TGF-β results in IL-17 expression through the transcription factors smad2 and smad3, STAT3, and NFκB1. IL-17 is a potent inflammatory cytokine involved in the recruitment and proliferation of neutrophils. It is also known to induce proinflammatory cytokines—such as TNF-α, IL-1β, and IL-6—as well as chemokines CXCL1, 2, and 8, which, together, are hallmarks of acute inflammatory processes. The chemokines mobilize neutrophil recruitment, a characteristic feature of TH17-mediated inflammation. The role of TH17 cells in atopic disease is intricate. Cytokines associated with TH17 have been shown to contribute to the development of various asthma pathologies, including remodeling and increased smooth muscle mass. In patients with AR, an association has been suggested between severity of disease and serum IL-17 levels, but the same relationship has not been duplicated in patients with asthma. The percentage of IL-17–positive cells in the peripheral blood of patients with AR or asthma has also been found to be higher compared with that of healthy controls, but IL-17–positive cells were not found to be increased in bronchoalveolar fluid lavage in patients with asthma compared to controls. On the other hand, exogenously administered IL-17 reduces pulmonary eosinophil recruitment and bronchial hyperreactivity, which suggests a regulatory role for this interleukin. Therefore, the TH17-directed neutrophil infiltration is inversely linked to the TH2-mediated eosinophil direction, much like the inverse relationships between TH1 and TH2 cells. It is therefore currently unclear whether IL-17 is playing a pathogenic or proresolution role in the allergic lung. In the upper airways, IL-17 has been found in nasal polyps and, interestingly, is expressed in polyps from patients in South China who have a neutrophil-predominant infiltration, in contrast to polyps from patients in Belgium who express IL-5 and have an eosinophil-predominant infiltration.
CD4 + T cells can also differentiate into cells characterized by the ability to suppress T cell responses and prevent autoimmunity or Tregs. Natural Tregs (nTregs) develop in the thymus and constitutively express the high-affinity IL-2 receptor, CD25, in accordance with their high dependency on IL-2 for survival. The transcription factor forkhead box P3 (Foxp3) is also stably expressed by nTregs and is required for their suppressive function, and nTregs account for 1% to 10% of the CD4 + T cell population in healthy adult humans; most investigators identify them from the expression of the preceding factors as CD4 + , CD25 + , and Foxp3 + cells. The importance of these cells is underscored by the description of patients who carry rare loss-of-function mutations in the FOXP3 gene, in whom a range of autoimmune and inflammatory disorders develops, referred to as immunodysregulation, polyendocrinopathy, enteropathy, X-linked syndrome . This syndrome includes type 1 diabetes, autoimmune thyroiditis, eczema, bleeding abnormalities, and chronic wasting. These individuals also demonstrate an increased susceptibility to infection and an elevated incidence of allergic-type symptoms. Other Treg populations may develop extrathymically from naïve CD4 + T cells under the influence of transforming growth factor-β (TGF-β) and are referred to as induced Tregs or TGF- β –producing type 3 helper T cells. These cells are Foxp3 positive and are functionally indistinguishable from nTregs. Treg type 1 cells do not express Foxp3 but they do produce high amounts of the inhibitory cytokine IL-10 and develop outside the thymus under the control of IL-10–conditioned dendritic cells. Treg cells are important in the maintenance of immune homeostasis in the airways and their actions seem to be largely mediated by secretion of IL-10 and TGF-β, two antiinflammatory cytokines.
Emerging evidence suggests that early life events are important in programming immune regulatory pathways to maintain a state of airway health and that the emergence of AR and asthma is a consequence of breakdown of these regulatory events (see the discussion of the hygiene hypothesis later in this chapter). Supporting this hypothesis is the positive influence of Tregs on allergic disease. CD4 + and CD25 + T cells isolated from the peripheral blood of patients with AR are found to be less effective in suppressing allergen-specific T cell proliferation and TH2 cytokine production than cells obtained from nonatopic controls. Similar results that suggest poor suppressive function have been shown in Tregs isolated from the peripheral blood or bronchoalveolar lavage of asthmatic children. Finally, as will be discussed later, successful allergen-specific immunotherapy is associated with the development of allergen-specific Treg cells, which help to control allergic immune responses via a pivotal role of IL-10 and TGF-β.
Both CD4 + and CD8 + T cells differentiate into functionally distinct subsets after exposure to antigen. This process is best described for the transition of CD4 + T cells from naïve to effector populations. Resting naïve CD4 + T cells, designated helper T cells (TH), release almost no cytokines. Early after stimulation by antigen and APC, the TH cells begin to produce IL-2 and are designated TH0 cells. As the TH cells continue to respond to the activating signal, they progress toward polar extremes of differentiation, designated TH1 and TH2, depending on the nature of the cytokines present at the site of activation. IL-12 produced by macrophages or NK cells induce differentiation toward TH1, and IL-4 produced by NK1.1-positive T cells or mast cells induces differentiation toward TH2. TH1 cells produce IL-2, IFN-γ, and lymphotoxin, whereas TH2 cells produce IL-4, IL-5, IL-9, IL-10, and IL-13 and GM-CSF. Generally, TH1 cells support cell-mediated immune responses and play a critical role in the defense against intracellular pathogens such as viruses, mycobacteria, and the Listeria bacterium, whereas TH2 cells are critical in the responses induced by extracellular pathogens—antigens such as parasites, bacteria, and allergens.
More recently, the concept of TH1/TH2 cells has been expanded to include other T-cell effectors such as TH17, TH22, and TH9 as well as the Tregs mentioned above. Evidence also suggests that T cells do not become fully committed to a specific subset after exposure to allergen but maintain the ability to change to a different effector phenotype as dictated by the microenvironmental milieu, a concept referred to as T cell plasticity . A typical example in allergic diseases is the description of a novel subset of TH2-type effector cells able to produce both TH2 and TH17 cytokines and to express the transcription factors for both these phenotypes. These cells are therefore able to promote the recruitment of a more diverse inflammatory complement that includes not only eosinophils but also neutrophils into sites of allergic inflammation. Further evidence of T cell plasticity lies in the finding that in normal subjects, as well as in those with mild to moderate asthma, circulating CD4 cells express either single or multiple transcription factors. This suggests that although TH cells can be defined by a single transcription factor (that corresponds to a specific effector phenotype) during stable disease, they might be able to express more than one of these factors in altered disease situations, such as allergic stimulation or disease exacerbation, and thus change phenotypes. It is believed that STAT proteins, which are instrumental in the development of particular T cell effector subsets, function as sensors of the external cytokine environment and facilitate the acquisition of epigenetic changes that, in turn, alter T cell effector phenotypes. Progress with immunization to different types of adjuvants demonstrates the feasibility of reprogramming allergic TH2-type responses in atopic patients to nonallergic TH1-type responses, thereby potentially altering the course of allergic disease.
B cells constitute approximately 10% of peripheral blood leukocytes. They provide humoral immunity against extracellular pathogens through the production of antibodies that neutralize pathogens and toxins, facilitate opsonization, and activate complement. Primary infection or vaccination results in prolonged production of high-affinity specific antibodies, the basis of adaptive humoral immunity. On the other hand, IgM antibodies are produced in the absence of infection, are of lower affinity, play a role in first-line defense against bacterial infection, and assist in clearance of endogenous cellular debris. Naïve follicular B cells reside in the follicles of secondary lymphoid tissues. Antigen arrives in these lymphoid organs through circulation of soluble molecules or immune complexes or via transportation by dendritic cells. The B cells, via the B cell receptors, process the antigens in the context of MHC class II and then migrate to the T cell–B cell interface, the border between the T cell zone and B cell follicle, where they encounter primed TH cells of cognate specificity. This generates signals from T cell-derived cytokines and triggers binding between CD40 ligand (CD40L, on T cells) and CD40 (on B cells) that sustains B cell activation and promotes immunoglobulin class switching. Signaling through CD40 and its interaction with CD40 ligand on T cells is essential for the induction of isotype switching.
The effector T cell cytokines have various functions: IL-1 and IL-2 promote B cell activation and growth, IL-10 causes switching to IgG1 and IgG3, IL-4 and IL-13 cause switching to IgE, and TGF-β causes switching to IgA. IFN-γ, or some other undefined product of TH1 cells, appears to induce switching to IgG2. Activated B cells either migrate into the follicle and, with continued T cell help, initiate the germinal center reaction or migrate to the marginal zone and differentiate into short-lived plasma cells. These latter cells secrete antibody for 2 to 3 weeks, which provides a rapid but transient source of effector molecules.
The B cells in the germinal center undergo specificity diversification through somatic hypermutation and high-affinity variants are selected by survival advantage, a process termed affinity maturation . The cells that then exit the germinal center reaction give rise to the memory compartment, which consists of affinity-matured memory B cells and long-lived plasma cells. When memory cells encounter antigen again, they divide rapidly and expand their numbers or differentiate into antibody-secreting plasma cells. These long-lived plasma cells are terminally differentiated B cells incapable of further division that home to the bone marrow and secrete high-affinity class-switched antibody. These B cell responses are orchestrated with the help of T cells and their cytokines and are termed T cell–dependent B cell responses.
In addition to their key function in the production of immunoglobulins, a subset of B cells has an immunosuppressive function and appears to play a regulatory role in inflammatory conditions. Although immunosuppressive B cells have been hypothesized to exist since the 1970s, these cells, known as regulatory B cells or Bregs, are now beginning to be better understood. Their immunosuppressive capacity is mediated by the secretion of IL-10, TGF-β, and IL-35. Support for the existence of allergen-specific human Breg cells comes from the detection of their increase during the course of allergen-specific immunotherapy, suggesting they play a role in the allergen-tolerance response in immunotherapy.
Innate lymphoid cells (ILCs) are a fairly recently described subset of immune cells that have lymphoid morphology but are distinguished by their lack of antigen receptors. These cells are stimulated by local production of cytokines, cell-surface receptors, and lipid mediators produced by other cells. ILCs respond by producing cytokines, thereby shaping the subsequent immune response. There are three described groups of ILCs, each group resembling a subset of TH cells based on their cytokine production profile. Group 1 ILCs produce IFN-γ, which is typical of TH1-type inflammation. NK cells fall under this category. Group 2 ILCs produce cytokines more consistent with the TH2 inflammatory response, including IL-4, IL-5, IL-9, and IL-13. Group 3 ILCs resemble TH17 cells and produce IL-17A and IL-22.
ILCs, particularly group 2 ILCs, have been implicated in allergic airway inflammation. ILC2 activation has been shown to induce asthma-like airway inflammation independent of other immune system components of the acquired immune system such as B and T cells and IgE. One recent study demonstrated that ILC2 numbers increased significantly in the nasal mucosa and decrease in the peripheral blood of patients with aspirin-exacerbated respiratory disease (AERD) after COX-1 ingestion, suggesting that ILC2s are recruited to the nasal mucosa and likely play a key role in the pathophysiology of AERD.
Monocytes and macrophages arise from colony-forming unit–granulocyte-macrophage (CFU-GM) progenitors, which differentiate into monoblasts, promonocytes, and monocytes. Monocytes account for approximately 10% of circulating leukocytes. Mature monocytes leave the bone marrow and circulate in the bloodstream until they enter tissues, where they develop into macrophages. These cells include Langerhans cells in the epidermis, Kupffer cells in the liver, microglial cells in the central nervous system, and the broad class of dendritic cells present in most tissues of the body. Dendritic cells appear to be the most potent APCs, but macrophages, Langerhans cells, and Kupffer cells are also prominent.
Like neutrophils, monocytes and macrophages are also highly phagocytic for microbes and particles that have been marked for clearance by binding immunoglobulin or complement, or both. Phagocytosis is facilitated by opsonization, which coats foreign material with antibodies. After phagocytosis, an intracellular vacuole forms around the foreign material and lysosomal enzymes released into the vacuole destroy the foreign invader. These cells appear to be mobilized shortly after neutrophils and they persist for long periods at sites of chronic inflammation and infection. They use production of nitric oxide as a major mechanism for killing microbial pathogens and produce large amounts of cytokines, such as IL-12 and INF-γ, which gives them a regulatory role in adaptive immune responses.
Dendritic cells are present at airway mucosal surfaces and serve as innate sensors of foreign antigens and then transmit the information to the immune system. The close association of dendritic cells with epithelial cells in the airway mucosa suggests a role for dendritic-epithelial cell interactions in modulating inflammatory responses to allergens. Multiple proteins are secreted by epithelial and dendritic cells that help to modulate each other's functions and promote inflammatory cytokine secretion and recruitment of inflammatory cells.
For example, thymic stromal lymphopoietin (TSLP) is an epithelium-derived cytokine, the epithelial expression of which is increased in asthmatic patients and correlates with asthma severity. TSLP has been shown to induce dendritic cells to drive the differentiation of naïve CD4 + T cells into inflammatory TH2 cells, and it also stimulates dendritic cells to synthesize high concentrations of TH2 cell attractants. TSLP also activates dendritic cells to secrete IL-8 and eotaxin 2, which, in turn, leads to the recruitment of neutrophils and eosinophils. This illustrates the close cooperation between epithelial cells and dendritic cells in the promotion of allergic inflammation.
Finally, dendritic cells influence whether the response to an inhaled antigen will entail the induction of tolerance to that antigen or allergic inflammation. This is nicely demonstrated by an allergic murine asthma model, in which mice deficient in CD103 + dendritic cells were actually refractory to the development of allergic sensitization through the airway.
Neutrophils arise from CFU-GM progenitor cells that give rise to myeloblasts, which differentiate into promyelocytes, myelocytes and, finally, into mature neutrophils. After maturation in the bone marrow, neutrophils circulate in the peripheral blood, where they account for 60% to 65% of leukocytes. Neutrophils produce large quantities of reactive oxygen species cytotoxic to bacterial pathogens and enzymes that appear to participate in tissue remodeling and repair after injury. They accumulate in large quantities at sites of bacterial infection and tissue injury and possess prominent phagocytic capabilities that permit them to sequester microbes and particulate antigens internally, where they can be destroyed and degraded; thus neutrophils play a major role in the clearance of microbial pathogens and repair of tissue injury.
Eosinophils are derived from colony-forming unit–eosinophil (CFU-Eo), a progenitor that differentiates into an eosinophilic myeloblast, promyelocyte, myelocyte and, finally, a mature eosinophil. Eosinophils constitute 2% to 5% of circulating leukocytes in normal individuals and are readily recognized from their prominent cytoplasmic granules, which contain toxic molecules and enzymes that are particularly active against helminths and other parasites. The production of eosinophils from the bone marrow and the survival of eosinophils in peripheral tissues are enhanced by the cytokine IL-5, which maintains the viability of eosinophils through inhibition of apoptosis.
Eosinophils are prominent cells in most allergic responses. They possess several surface markers and receptors involved in differentiation, recruitment into tissues, activation, synthesis, and release of their multiple mediators. Mature eosinophils express functional heterodimeric receptors for the three cytokines—GM-CSF, IL-3, and IL-5—that promote eosinophilopoiesis and stimulate the functioning of mature eosinophils.
The eosinophils’ cationic granule proteins include major basic protein (MBP), eosinophil peroxidase (EPO), eosinophil cationic protein (ECP), and eosinophil-derived neurotoxin. Another prominent protein of the eosinophil is the Charcot-Leyden crystal protein, which constitutes an estimated 7% to 10% of total cellular protein, possesses lysophospholipase activity and forms the distinctive hexagonal bipyramidal crystals that are the hallmark of eosinophil-associated inflammation. MBP is a potent cytotoxin and helminth toxin in vitro. It can kill bacteria and many types of normal and neoplastic mammalian cells, stimulate histamine release from basophils and mast cells, activate neutrophils and platelets, and augment superoxide generation by alveolar macrophages. It can also induce bronchoconstriction and transient airway hyperreactivity when instilled into the monkey trachea. ECP, like MBP, has marked toxicity for helminth parasites, blood hemoflagellates, bacteria, and mammalian cells and tissues and has been shown in several studies to produce respiratory epithelial damage similar to that seen in severe asthma. As with MBP and ECP, EPO is highly cationic and exerts some cytotoxic effects on parasites and mammalian cells in the absence of hydrogen peroxide; however, EPO is highly effective in combination with hydrogen peroxide and a halide cofactor (iodide, bromide, or chloride), from which EPO catalyzes the production of the toxic hypohalous acid. In the presence of these compounds, EPO is highly toxic to various unicellular, multicellular, and other targets that include viruses, mycoplasma and other bacteria, fungi, and parasites. Eosinophil-derived neurotoxin is a poor cationic toxin with only limited toxicity for helminths and mammalian cells, but it induces significant neurologic damage when injected intrathecally or intracerebrally into rabbits or guinea pigs. In allergic conditions, eosinophils may play a dual role. They can suppress the local tissue response to inflammatory mediators involved in IgE-mediated hypersensitivity reactions by inactivating histamine, platelet-activating factor, and heparin. On the other hand, eosinophils can augment destruction through the toxic effects of the products they release upon degranulation. The balance between these two seemingly contradictory functions of eosinophils in IgE-mediated reactions is still under investigation.
Basophils mature from a progenitor colony-forming unit–basophil mast cell (CFU-BM) into basophilic myeloblasts, promyelocytes, myelocytes, and then mature basophils. Mast cells are thought to develop from the same progenitor, but less is known about their specific stages of development. Basophils and mast cells are morphologically similar, and both exhibit cell-surface expression of high-affinity receptors for IgE (FcεRI), which makes them key initiators of immediate hypersensitivity responses and the host response to helminthic parasites. These cells release histamine and other preformed mediators from their granules and produce important quantities of lipid mediators that stimulate tissue inflammation, edema, and smooth muscle contraction. Studies have shown that in addition to this role, mast cells play prominent roles in the host response to bacterial infections.
In addition to their well-recognized role in thrombosis and hemostasis, platelets also contribute to inflammation. They contain high concentrations of 5-hydroxytryptamine (serotonin) in their dense granules and several observations support increased levels of this vasoactive amine in patients with asthma. Evidence supporting the role of platelets in allergic inflammation centers around the following observations: (1) platelets can express IgE receptors; (2) they undergo migration into lung tissues after allergen exposure in both mice and humans; (3) elevated serotonin levels, presumably originating from platelets, have been measured in bronchoalveolar lavage of individuals with allergic asthma after allergen challenge; (4) platelets play an important role in allergen-induced leukocyte recruitment to the lungs after allergen challenge in mice; (5) they prime dendritic cells via serotonin; and (6) temporary platelet depletion (>95%) at the time of allergen sensitization leads to a suppression of IgE and IL-4 synthesis, and a decrease in the pulmonary recruitment of eosinophils, macrophages, and lymphocytes after subsequent allergen exposure.
Follicular dendritic cells, specialized APCs in the B cell areas of lymph nodes and the spleen, are important in the generation and maintenance of memory B cells, which they achieve by trapping antigen-antibody complexes. Peripheral tissue dendritic cells engulf and process antigen, leave the tissues and then go home to T cell areas in draining lymph nodes or the spleen. In the lymph nodes, these APCs can directly present processed antigens to resting T cells to induce the latter's proliferation and differentiation. Monocytes/macrophages exist as monocytes in blood and as macrophages, a more differentiated form, in various tissues such as the lungs, liver, and brain. In addition to phagocytic and cytotoxic functions, these cells have receptors for various cytokines (IL-4, IFN-γ), which can regulate their function. Activated macrophages are also a major source of several cytokines (IFN, IL-1, TNF), complement proteins, and prostaglandins. All APCs have MHC class II surface molecules.
Foreign or self proteins undergo hydrolytic cleavage within the APCs and become oligopeptides, which are then loaded onto antigen-binding grooves of MHC molecules before expression at the cell surface ( Fig. 35.3 ). Class I molecules usually bind peptides that are 8 to 10 amino acids long and are derived from proteins synthesized intracellularly (e.g., tumor antigens and viruses), whereas class II molecules bind peptides that are 14 to 22 amino acids long and are derived from proteins synthesized extracellularly (e.g., nonreplicating vaccines and extracellular bacteria). Lipids and lipid derivatives are processed much like extracellular proteins in endosomes: they are combined with CD1, an MHC-like molecule, and are presented to double-negative T cells that frequently bear γδ-receptors.
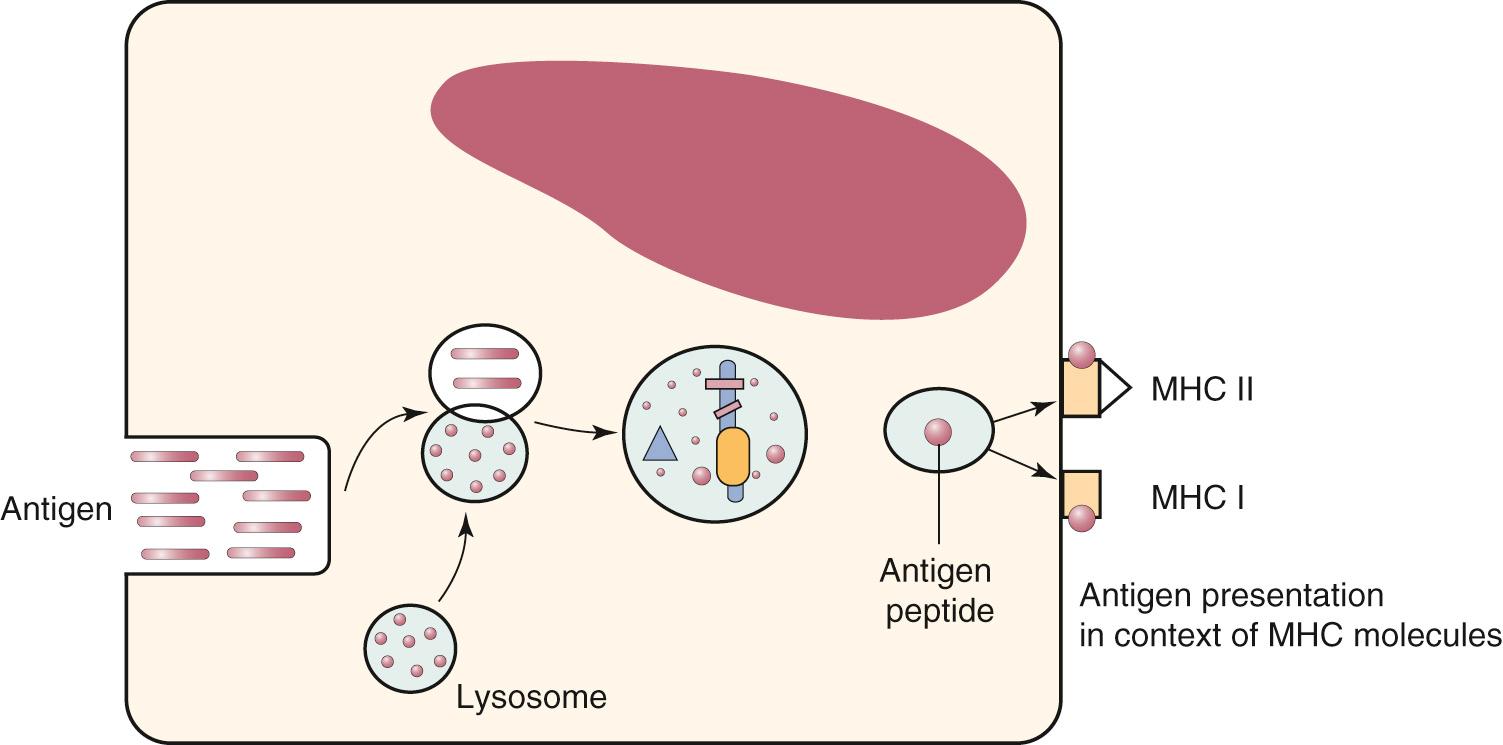
In addition to the mechanism of presentation of oligopeptide antigens to lymphocytes via MHC molecules, T cells can recognize haptens, which are covalently or noncovalently complexed with peptides that reside in the MHC-binding groove. Another exception is the presentation of superantigens, which are proteins of approximately 30 kDa produced by a broad spectrum of microbes that range from retroviruses to bacteria. These antigens do not undergo processing to oligopeptides but bind intact to MHC class II molecules and the TCR outside the antigen-binding grooves; they can activate more T cells than conventional peptide antigens can.
Humoral immunity consists of responses that are T cell dependent and T cell independent. Follicular B cells present MHC-restricted protein antigens and receive T cell help that promotes immunoglobulin class switching, affinity maturation, and memory differentiation as discussed previously. These antigen responses are referred to as T cell–dependent responses . T cell–independent B cell responses occur with large antigens that have repeating antigenic determinants, such as carbohydrates, which constitute the capsule and cell wall components of bacteria and cannot be presented in MHC class II molecules. This B cell response represents a major protective role against bacterial pathogens such as S. pneumoniae . These pathogens bridge immunoglobulins on the B cell surface and cause activation and the subsequent secretion of antibodies, primarily of the IgM class. Antibodies to S. pneumoniae mediate the opsonization of bacteria by binding to the bacterial cell surface, thereby targeting them for destruction by Fc receptor–bearing macrophages. Young children and the elderly, who generally show poor response to T cell–independent antigens, are at increased risk for these bacterial infections. Protective immunity against Haemophilus influenzae and meningococcal infections is also mediated by T cell–independent B cell responses.
Antibody-dependent cellular cytotoxity (ADCC) can lead to the destruction of invading foreign organisms (bacteria and helminths), virus-infected cells, or tumor cells. In the process of destruction of invading organisms, ADCC involves the targeting of effector cells to these organisms by antibodies. The antibody's variable regions provide specificity for the organism, whereas the antibody's constant region focuses effector cells to the site via various Fc receptors. In ADCC directed against altered self cells, the process involves the reaction of antibodies with cell-surface receptors that produce antibody-coated target cells for reaction with NK cells that secondarily destroy these altered self cells. The process occurs via binding of the antibody to FcγRIII receptor (CD16) on NK cells.
The immunoglobulins (antibodies) secreted by activated B cells are glycoproteins composed of polypeptide (82% to 96%) and carbohydrate (4% to 18%). Immunoglobulin molecules are composed of two identical 50-kDa heavy chains and two identical 25-kDa κ or λ light chains ( Fig. 35.4 ). The amino-terminal portions of the heavy and light chains vary in amino acid sequence from one antibody molecule to another. These variable portions are designated V H and V κ or V λ , respectively. The juxtaposition of one V H segment and one V κ or V λ segment creates the antigen-binding portion of the immunoglobulin molecule, and each immunoglobulin molecule has two identical antigen-binding sites. The carboxyl-terminal portions of the heavy and light chains are constant in each subclass of antibody. The heavy-chain constant regions pair to form the Fc domain of the molecule that is responsible for most of the effector functions of the immunoglobulin molecule, including binding to Fc receptors and activating complement.
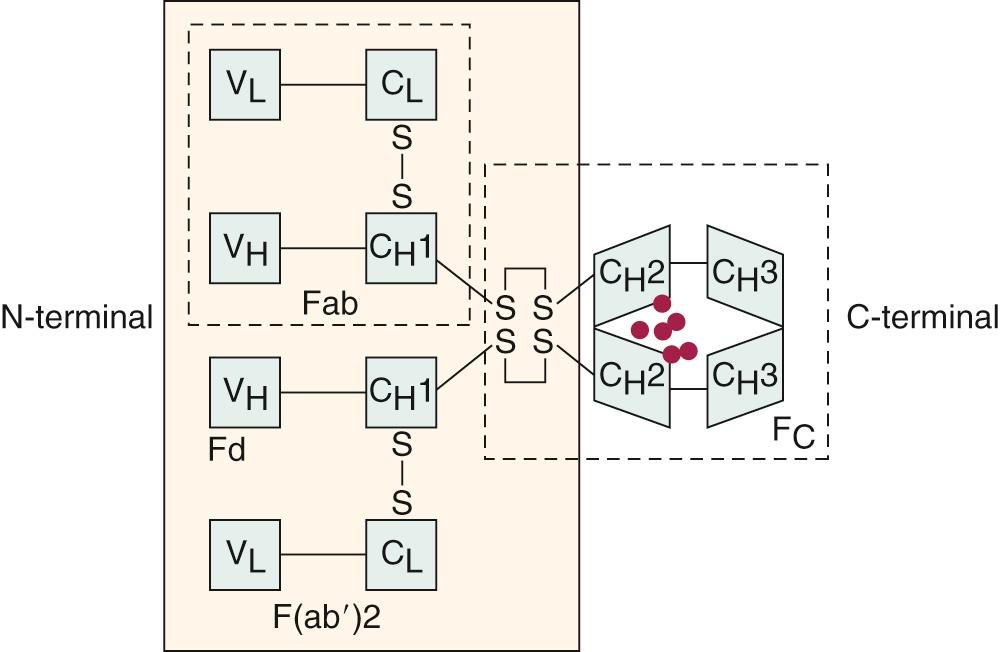
The isotype of a heavy or light chain is defined by the constant region antigenic determinants, which, in turn, are defined by the particular constant region gene of that isotype. All isotypes are present in a healthy person. Because there are nine known separate heavy-chain constant region genes, nine heavy-chain isotypes define the class and subclass of the antibody molecule. These are designated g1, g2, g3, g4, m, a1, a2, d, and e, and the corresponding immunoglobulin isotypes (class or subclass) are G1, G2, G3, G4, M, A1, A2, D, and E. Monomers consist of a single immunoglobulin molecule (e.g., IgG), whereas polymers have multiple basic units (e.g., IgM has five basic units and dimeric IgA has two units). The polymerization of these immunoglobulins is facilitated by the presence of a small glycopeptide with an unusually high content of aspartic acid, known as the J chain.
Immunoglobulin G (IgG) constitutes approximately 75% of the total serum immunoglobulins and consists of four subclasses: IgG1, IgG2, IgG3, and IgG4. IgG is a monomer with a molecular weight of 150,000 Da. Specific Fc receptors for IgG are present on monocytes, macrophages, and neutrophils. IgG is usually bound to the Fc receptor before binding with antigen and IgG functions as an opsonin that facilitates phagocytosis. In general, the IgG antibody response to soluble protein antigens involves the IgG1 and IgG3 subclasses, whereas polysaccharide antigens elicit primarily IgG2 antibodies. IgG is the immunoglobulin primarily involved in secondary or recall immune responses and is the only immunoglobulin that can cross the placenta and protect the neonate. IgG can fix complement, which leads to neutralization, opsonization, bacteriolysis, agglutination, and hemolysis.
Immunoglobulin M (IgM) constitutes approximately 10% of serum immunoglobulins. It normally exists as a pentamer of five IgM subunits linked by disulfide bonds and J chains with a molecular weight of 900,000 Da. Along with IgD, it is the major immunoglobulin expressed on the surface of B cells. Membrane IgM functions as the earliest antigen receptor on B cells. Antigen binding results in B-cell activation and differentiation, which leads to pentameric IgM secretion. IgM predominates in the early humoral response and levels then decline rapidly; the IgM is replaced by IgG of the same specificity. IgM is the most efficient complement-fixing antibody, a feature that increases its array of biologic activities, as with IgG.
Immunoglobulin A (IgA) constitutes 15% of the total serum immunoglobulins and exists in both monomeric and polymeric forms (the IgA dimer is a single J chain joined to two IgA monomeric subunits). Each immunoglobulin molecule has a molecular weight of 160,000 Da. Humans produce more IgA than any other immunoglobulin class and the major role of this molecule is mucosal immunity. Monomeric IgA is synthesized by plasma cells located in the interstitial spaces of exocrine glands. These monomers combine with the J chain, which is also synthesized by IgA plasma cells, to form IgA2-J dimers. These dimers are too large to cross the tight junctions of the exocrine gland epithelium so they are transported across the epithelium by an active secretory component–dependent mechanism. During transport, IgA dimer covalently links to the secretory component by a disulfide bond. The newly formed complex of IgA dimer and secretory component, referred to as secretory IgA, is subsequently released into exocrine secretions by exocytosis. Secretory IgA appears to be derived from locally produced IgA, not intravascular IgA.
IgA is the predominant immunoglobulin in external secretions and provides the primary defense mechanism against local mucosal infections. The main function of IgA is to neutralize foreign substances and prevent their systemic access.
IgE accounts for only 0.004% of the total serum immunoglobulins and normally exists as a monomer with a molecular weight of 200,000 Da. High-affinity IgE-specific Fc receptors exist on mast cells and basophils, whereas low-affinity IgE receptors exist on neutrophils, eosinophils, macrophages, and platelets. Cross-bridging of IgE molecules on the surface of mast cells and basophils by antigen triggers the release of mediators from mast cells and basophils, a hallmark of allergic reactions. In addition to mediating immediate hypersensitivity reactions, IgE can mediate antibody-mediated cellular cytotoxicity.
IgE at allergic reaction sites can come from different sources that include the bone marrow, the regional lymph nodes, and the local mucosa. In the bone marrow, IgE is produced by plasma cells that are allergen specific and have been shown to persist in the bone marrow and spleen after allergen exposure has stopped. Evidence also suggests that bone marrow transplantation in patients can transfer atopy, which supports the bone marrow as a source of specific IgE. IgE-containing lymphoid follicles have been detected in human tonsils and adenoids as well as in cervical lymph nodes, which suggests that IgE is produced in regional lymph nodes and is probably subsequently transported to the respiratory mucosa by migratory mast cells. The suspicion that IgE could be produced locally at the mucosal level originated from early studies that demonstrated allergen-specific IgE in nasal secretions of patients with AR irrespective of skin-prick test response. Subsequent work demonstrated IgE-producing cells and IgE-encoding messenger RNA (mRNA) in nasal mucosa and lavage in both patients with AR and in healthy controls, although levels were higher in the allergic patients. This and other similar evidence suggest that allergen-specific IgE-positive cells and plasma cells accumulate in the respiratory mucosa of atopic subjects. This capability of IgE to be produced locally could explain why certain patients have a clinical picture consistent with the diagnosis of AR but have no evidence of positive skin testing and no increase in serum-specific IgE.
Immunoglobulin D (IgD) constitutes 0.2% of the total serum immunoglobulins and exists as a monomer with a molecular weight of 180,000 Da. The primary function of IgD appears to be that of a membrane-bound antigen receptor on the B cell surface.
Human cells are covered in molecules composed of linked sugar moieties, known as glycans. The field of glycoimmunology is a growing area of research focusing on the recognition of glycans by specific glycan binding proteins (GBPs) leading to a variety of proinflammatory and anti-inflammatory cellular responses. The most studied GBPs belong to two families: sialic acid-binding immunoglobulin-like lectins or Siglecs and selectins. Siglecs have been described in humans and mice and human Siglecs are expressed on a variety of immune cells including basophils, CD8 cells, dendritic cells, eosinophils, mast cells, monocytes, and NK cells. They have been shown to have different effects including inhibition of basophil and mast cell activation, induction of eosinophil cell death, control of B and T cell activation, which are all potentially important functions in allergic disease. In a salient example of their importance in allergic diseases, mice deficient in Siglec-F (the murine equivalent of human Siglec-8) display exaggerated allergic eosinophilic pulmonary inflammation. Moreover, in murine models of allergic lung eosinophilia, anti-Siglec-F antibodies decrease blood and tissue eosinophil numbers and blunt tissue remodeling.
Cytokines are a diverse group of small, secreted protein mediators that mediate interactions among different effector cells. Each cytokine may have multiple activities on different cell types and several cytokines often have related functions. They can also have synergistic or antagonistic activities and can inhibit or induce the synthesis of other cytokines. Cytokines can be divided into several groups: the ILs, IFNs, tumor necrosis factor (TNF) and related molecules, transforming growth factors (TGFs), and hematopoietic growth factors. Each of these different cytokines, their principal cell sources, and their main effects in humans are summarized in Table 35.1 .
| Cytokine | Cell Sources | Predominant Effects |
|---|---|---|
| I nterleukins | ||
| IL-1α, IL-1β functionally equivalent isoforms | Macrophages, neutrophils, epithelial and endothelial cells, monocytes, lymphocytes, keratinocytes | Fever, local inflammation, T-cell and macrophage activation; principal mediator(s) of septic shock; differentiation of TH17 cells |
| IL-2 | Activated T cells, DCs, NK cells | Proliferation of effector T and B cells, development of Treg cells, differentiation and proliferation of NK cells |
| IL-3 | CD4 + T cells, thymic epithelial cells, mast cells, eosinophils, macrophages, NK cells | Synergistic action in early hematopoiesis; activates and promotes recruitment of basophils and eosinophils in late allergic reactions |
| IL-4 | TH2 cells, mast cells, basophils, NK T cells, eosinophils, γ/δ–T cells | Differentiation and growth of TH2 subset, B-cell activation, survival factor for T and B cells, isotype switch to IgE and IgG1, suppression of TH1 cells, growth factor for mast cells |
| IL-5 | Activated TH2 cells and mast cells, NK cells, NK T cells, eosinophils | Eosinophil survival, differentiation, and chemotaxis, differentiation and function of myeloid cells, remodeling and wound healing |
| IL-6 | T cells, mononuclear phagocytes, vascular endothelial cells, fibroblasts | T- and B-cell growth and differentiation, acute-phase protein production by the liver |
| IL-7 | Stromal cells in bone marrow and thymus, B cells, monocytes, macrophages, epithelial cells, keratinocytes, DCs | Growth of pre-B cells and pre-T cells, synthesis induction of inflammatory mediators in monocytes |
| IL-8, CXC chemokine | Activated mononuclear phagocytes, fibroblasts and endothelial cells | Chemoattractant for neutrophils, NK cells, T cells, basophils, eosinophils; promotes neutrophil inflammatory responses, mobilization of hematopoietic stem cells |
| IL-9 | TH2 cells, TH9 cells, mast cells, eosinophils | T cell and mast cell growth factor, inhibition of TH1 cytokines, proliferation of CD8 + T cells and mast cells, IgE production, chemokine and mucus production in bronchial epithelial cells |
| IL-10 | T cells, B cells, monocytes, macrophages, DCs | Important in innate and adaptive immunity, immune suppression |
| IL-11 | Stromal cells: fibroblasts, epithelial cells, endothelial cells, vascular smooth muscle cells, synoviocytes, osteoblasts | Growth factor for myeloid, erythroid, and megakaryocyte progenitors; bone remodeling; protects epithelial cells and connective tissue; induction of acute-phase protein; inhibition of macrophage activity; promotion of neuronal development |
| IL-12 | Macrophages, monocytes, neutrophils, DCs, B cells, microglia | Induces TH1 differentiation and cytotoxicity |
| IL-13 | T cells, NK T cells and mast cells, basophils, eosinophils | Switching to IgG4 and IgE, upregulation of CD23, MHC-II on B cells, induction of CD11b, CD11c, CD18, CD29, CD23, and MHC-II on monocytes; activation of eosinophils and mast cells, recruitment and survival of eosinophils, defense against parasitic infections |
| IL-14 | Activated T cells | Growth factor for B cells |
| IL-15 | Monocytes, activated CD4 + T cells, keratinocytes, skeletal muscle cells | Stimulates growth and development of NK cells; stimulates activation and proliferation of T and B cells |
| IL-16 | T cells, mast cells, eosinophils, monocytes, DCs, fibroblasts, airway epithelial cells | Chemotaxis, modulation of T-cell response |
| IL-17A | TH17 cells, CD8 + T cells, NK cells, NK T cells, γ/δ–T cells, neutrophils | Induces proinflammatory cytokine and chemokine production by epithelial and endothelial cells and fibroblasts; recruitment of neutrophils |
| IL-17B | Neuronal cells, chondrocytes | Induces proinflammatory cytokine and chemokine production Chondrogenesis and osteogenesis |
| IL-17C | Immune cells under certain conditions | Induces proinflammatory cytokine and chemokine production |
| IL-17D | Resting B and T cells | Induces proinflammatory cytokine and chemokine production |
| IL-17F | TH17 cells, CD8 + T cells, NK cells, NK T cells, γ/δ–T cells, neutrophils | Induces proinflammatory cytokine and chemokine production; recruitment of neutrophils |
| IL-18 | Macrophages, Kupffer cells, keratinocytes, osteoblasts, astrocytes, DCs | Induces IFN-γ production by T cells and NK cells; promotes TH1 or TH2 cell responses depending on cytokine milieu |
| IL-19 | Monocytes, keratinocytes, airway epithelial cells, B cells | Unknown |
| IL-20 | Monocytes, keratinocytes, epithelial and endothelial cells | Appears to have a role in skin development and is suspected of regulating inflammation in the skin |
| IL-21 | T cells (predominantly TH17), NK T cells | Regulates proliferation, differentiation, apoptosis, antibody isotype balance, cytotoxic activity |
| IL-22 | Activated T cells (predominantly TH17), NK T cells | Pathogen defense, wound healing, tissue reorganization |
| IL-23 | Macrophages and activated dendritic cells | Stimulates production of proinflammatory IL-17 and promotes memory |
| IL-24 | Melanocytes, T cells, monocytes | Tumor suppression |
| IL-25 | TH2 cells, mast and epithelial cells, eosinophils and basophils from atopic individuals | Enhances allergic responses and promotes TH2 inflammation |
| IL-26 | Activated T cells (predominantly TH17), NK T cells | Activates and regulates epithelial cells |
| IL-27 | Activated DCs, macrophages, epithelial cells | Promotes development along the TH1 phenotype, suppresses the TH2 phenotype, inhibits TH17 response |
| IL-28 and IL-29 | Monocyte-derived DCs | Induces an antiviral state in infected cells |
| IL-30 (p28 subunit of IL-27) | DCs, macrophages | Induces IL-12–mediated liver injury |
| IL-31 | Activated CD4 + T cells (mainly TH2) and CD8 + T cells | Induces IL-6, IL-8, CXCL1, CXCL8, CC chemokine ligand 2, and CC chemokine ligand 8 production in eosinophils; upregulates chemokine mRNA expression in keratinocytes, expression of growth factors and chemokines in epithelial cells, inhibition of proliferation, and apoptosis in epithelial cells |
| IL-32 | Monocytes, macrophages, NK cells, T cells, epithelial cells | Induces TNF-α, IL-8, and IL-6 apoptosis |
| IL-33 | Necrotic cells | Induces TH2 inflammation |
| IL-34 | Heart, brain, spleen, liver, kidney, thymus, testes, ovary, small intestine, prostate, colon | Proliferation |
| IL-35 | Treg cells | Proliferation of Treg cells and inhibition of TH17-cell function, suppression of inflammatory responses |
| IL-37 | Monocytes, tonsil plasma cells, breast carcinoma cells | Suppresses proinflammatory cytokines and inhibits DC activation |
| IL-38 | Basal cells of skin, spleen, placenta, fetal liver, thymus, proliferating B cells of tonsils | Because of its homology with other IL-1 family members, IL-38 is thought to have similar biological activity, namely stimulation of the expression of genes associated with inflammation |
| TSLP | Epithelial cells | Promotes T-cell proliferation and differentiation, activates DCs to prime for TH2 cell differentiation |
| I nterferons | ||
| IFN-γ | T cells, NK and NK T cells, macrophages, TH1 cells, B cells | Macrophage activation, increased expression of MHC molecules and antigen-presenting components, Ig class switching, suppression of TH2 response, antiviral properties, promotes cytotoxic activity |
| IFN-α | Leukocytes | Antiviral, increases MHC class I expression |
| IFN-β | Fibroblasts | Antiviral, increases MHC class I expression |
| T umor N ecrosis F actor and R elated M olecules | ||
| TNF | Activated monocytes/macrophages and T, B, and NK cells | Major inflammatory mediator induced by the presence of gram-negative bacteria and their components; potent immunoregulatory, cytotoxic, antiviral, and procoagulatory activities |
| LTα (previously called TNF-β) | Activated TH1, B, and NK cells | Promotes inflammation, has antiviral activity, and kills tumor cells by apoptosis |
| LTαβ | Activated TH1, B, and NK cells | Has a specialized role in secondary lymphoid organ development |
| OPGL | Osteoblasts, bone marrow stromal cells, activated T cells | Stimulates osteoclasts and bone resorption |
| BAFF | Monocytes, macrophages, dendritic cells | Survival factor required for the maturation of B cells |
| T ransforming G rowth F actor | ||
| TGF-β | Chondrocytes, monocytes, T cells | Antiinflammatory; inhibits cell growth; induces IgA secretion by B cells; plays a role in adhesion, proliferation, differentiation, transformation, chemotaxis, and immunoregulation |
| H ematopoietic G rowth F actors | ||
| SCF | Stromal cells in the fetal liver, bone marrow, and thymus; in the central nervous system; and in the gut mucosa | Supports the survival and growth of the earliest hematopoietic precursors in vivo |
| GM-CSF | Activated T cells, macrophages, stromal cells, and endothelial cells | Stimulates growth and differentiation of myelomonocytic lineage cells, particularly dendritic cells and inflammatory leukocytes |
| G-CSF | Activated T cells, endothelial cells, fibroblasts, and mononuclear phagocytes | Stimulates neutrophil development and differentiation |
| M-CSF | Endothelial cells, fibroblasts, mononuclear phagocytes | Influences CFU-GM cells to differentiate into monocytes and macrophages in vitro |
The chemokines are a superfamily of low-molecular-weight, secreted heparin-binding molecules that serve as potent chemoattractants for cells of the immune system. More than 50 chemokines are currently recognized, and they are characterized by the presence of three or four conserved cysteine residues. Table 35.2 lists the new systematic names of these chemokines as well as their common names and their target cells.
| Systematic Name | Common Name(s)/Ligand(s) | Target Cell(s) |
|---|---|---|
| CXC C hemokines | ||
| CXCL1 | GROα/MGSAα | Neutrophil |
| CXCL2 | GROβ/MGSAβ | Neutrophil |
| CXCL3 | GROδ/MGSAδ | Neutrophil |
| CXCL4 | Platelet factor-4 | Fibroblast |
| CXCL5 | Epithelial neutrophil-activating peptide 78 | Neutrophil |
| CXCL6 | Granulocyte chemotactic protein 2 | Neutrophil |
| CXCL7 | Neutrophil-activating peptide 2 | Neutrophil |
| CXCL8 | IL-8 | Neutrophil, basophil, T cell |
| CXCL9 | Monokine induced by IFN-γ | Activated T cell |
| CXCL10 | IFN-γ–inducible protein 10 | Activated T cell |
| CXCL11 | IFN-inducible T-cell α−chemoattractant | Activated T cell |
| CXCL12 | Stromal cell–derived factor 1a/b | CD34 + bone marrow cell, T cell, dendritic cell, B cell, activated CD4 cell |
| CXCL13 | B-cell–attracting chemokine1 | Naïve B cell, activated CD4 cell |
| CXCL14 | Breast and kidney–expressed chemokine | Monocytes, dendritic cells, NK T cells |
| CXCL15 | Lungkine | Neutrophils |
| CXCL16 | Small inducible cytokine B6 | T cell, NK T cell |
| CC C hemokines | ||
| CCL1 | I-309 (a human chemokine) | Neutrophil, T cell |
| CCL2 | MCP-1/monocyte chemotactic and activating factor/tumor-derived chemotactic factor | T cell, monocyte, basophil |
| CCL3 | MIP-1α | Monocyte, macrophage, T cell, NK cell, basophil |
| CCL3L1 | LD78β | |
| CCL4 | MIP-1β | Monocyte, macrophage, T cell, NK cell, basophil |
| CCL5 | Regulated upon activation, normal T cell–expressed and secreted (RANTES) | Monocyte, macrophage, T cell, NK cell, basophil, eosinophil, dendritic cell |
| CCL6 | C-10/MRP-2 | |
| CCL7 | MCP-3 | T cell, monocyte, eosinophil, basophil, dendritic cell |
| CCL8 | MCP-2 | T cell, monocyte, eosinophil, basophil |
| CCL9/10 | MIP-1γ | |
| CCL11 | Eotaxin | Eosinophil |
| CCL12 | MCP-5 | Monocytes |
| CCL13 | MCP-4 | T cell, monocyte, eosinophil, basophil, dendritic cell |
| CCL14 | HCC-1 | Monocyte |
| CCL15 | HCC-2/leukotactin 1/MIP-1δ | T cell, monocyte, dendritic cell |
| CCL16 | HCC-4/liver-expressed chemokine | Monocyte |
| CCL17 | Thymus- and activation-regulated chemokine | T cell, immature dendritic cell, T cell, thymocyte |
| CCL18 | MIP-4, dendritic cell–derived CC chemokine/pulmonary and activation-regulated chemokine/activation-induced, chemokine-related molecule 1 | Naïve T cell, T cell |
| CCL19 | MIP-3β/exodus 3 | Naïve T cell, mature dendritic cell, B cell |
| CCL20 | MIP-3α/liver- and activation-regulated chemokine/exodus 1 | T cell, bone marrow dendritic cell |
| CCL21 | 6Ckine/secondary lymphoid tissue chemokine/exodus 2 | Naïve T cell, B cell |
| CCL22 | Macrophage-derived chemokine-stimulated T-cell chemoattractant protein 1 | Immature dendritic cell, T cell |
| CCL23 | MPIF-1/CK 8/CK 8-1 | Monocyte, T cell |
| CCL24 | Eotaxin 2/MPIF-2 | Eosinophil, basophil |
| CCL25 | Thymus-expressed chemokine | Macrophage, thymocyte, dendritic cell |
| CCL26 | Eotaxin 3 | |
| CCL27 | Cutaneous T-cell–activating chemokine/IL-11 receptor α-locus chemokine | T cell |
| CCL28 | MEC/Mucosa-associated epithelial chemokine | T cell, eosinophil |
| C C hemokines | ||
| XCL1 | Lymphotactin/SCM-1β/activation-induced, chemokine-related molecule | T cell, NK cell |
| XCL2 | SCM-1β | |
| CXC3C C hemokine | ||
| CXC3CL1 | Fractalkine | T cell, monocyte |
In the defense against infections, antibodies are operative against bacteria or bacterial products; cell-mediated immunity operates primarily against viral, fungal, and mycotic infections. The killing effects of immune reactions are extremely efficient and, when specifically directed, can eliminate organisms quickly; however, these same immune mechanisms may cause host-tissue destruction and, therefore, lead to disease states. In some situations, this destructive effect of immune reactions is termed allergy or hypersensitivity and is considered an immunopathological reaction.
These reactions are divided into four types. Type I reactions, mast cell–mediated reactions, use the release of mast cell or basophil mediators to create responses to sensitizing allergens and can be IgE dependent (anaphylaxis) or IgE independent (sensitivity to iodide contrast media). Type II reactions, cytotoxic antibody-mediated reactions (IgG- or IgM-mediated), involve the binding of IgG and IgM antibodies to antigens on the surface of target cells (e.g., erythrocytes, neutrophils, platelets, and epithelial cells of glandular or mucosal surfaces) or to antigens on tissues such as basement membranes. This interaction between IgG and IgM antibodies and cell-surface antigens leads to destruction of these cells by opsonization, complement activation, or cell lysis and ADCC. These mechanisms can damage self-antigens in various tissues. One clinical example is known as penicillin-induced autoimmune hemolytic anemia . Type III reactions, immune complex–mediated reactions (IgG- or IgM-complex–mediated), involve antibody-mediated inflammation, whereby the antibody and its antigen form low-solubility immune complexes, deposited in normal tissues; activate complement; and set off an inflammatory response characterized primarily by neutrophil influx, which inflicts tissue injury. Knowledge of this immunologic disease became widespread in the early 1900s, when physicians began using immune animal sera, usually equine sera, to treat bacterial infections, a practice that led to serious illness and even death of the treated subjects from a disease known as serum sickness. Immune complex vasculitis in the skin can also occur in a series of clinical conditions, such as systemic lupus erythematosus, rheumatoid arthritis, drug reactions, and infections. Type IV reactions, delayed-type hypersensitivity reactions (T-cell–mediated), are caused by antibody-independent mechanisms that involve T cells or NK cells. These reactions are the pathological variants of a normal T cell–mediated immune response, in which the T cell response to an environmental antigen becomes exaggerated. A clinical example of such a reaction, as previously described, is the cutaneous reaction to challenge with the purified protein derivative of M. tuberculosis in previously infected or vaccinated patients.
There is a well-recognized familial component to AR, with some studies estimating the heritability of AR to be as high as 80%. The genetic contributions to AR are believed to be complex and involve a number of different genes. Genome wide association studies (GWASs) have identified several single nucleotide polymorphisms (SNPs) in genes that are thought to be associated with AR, such as those that encode human leukocyte antigen (HLA) subtypes, TLRs, and cytokines including interleukins.
In addition to genetic mutations, epigenetic phenomena may contribute to the familial patterns seen in AR. For example, DNA methylation may be affected by environmental factors such as maternal smoking and air pollution, and methylation profiles have been shown to be important in AR.
Epidemiological data provide strong evidence of a steady rise in the incidence of allergic diseases (asthma, rhinitis, atopic dermatitis) and autoimmune diseases (multiple sclerosis, insulin-dependent diabetes mellitus, Crohn disease ) in developed countries since the beginning of the 1970s. Concomitantly, the incidence of many infectious diseases in developed countries has decreased as a result of antibiotics, vaccination, and improved hygiene. A hypothesis therefore emerged that the decrease in infectious diseases is causally linked to the increase in the incidence of allergic disease, the so-called “hygiene hypothesis.” This phenomenon has also been referred to as the “biodiversity hypothesis,” pointing to the potential protective effect of colonization of mucosa with environmental microflora. Some of the evidence for and against the biodiversity hypothesis at it relates to allergic disease is summarized in Table 35.3 .
| Theory or Observation | Number and Type of Studies | Aggregate Grade of Evidence | Endpoints/Comparisons | Summary of Findings |
|---|---|---|---|---|
| Farm living | 1 SR/meta-analysis (including 26 cross-sectional studies, 3 longitudinal, meta-analysis of 8 studies) 1 additional case-control study 2 additional cross-sectional studies |
B | Association of farm exposure (in utero, early childhood, and adulthood) with development of AR | Early childhood farm exposure associated with decreased risk of allergy. Inconsistent association between allergy and exposure in adulthood. Effect strongest with farm animals and stables |
| Animal dander exposure | 13 birth cohort studies (level 2b) 25 additional studies (level 3b) |
B | Association of animal dander exposure (prenatal and early life) with development of AR | Mixed and conflicting results with multiple studies showing borderline protective effect and many other studies showing no effect. Several level 3b studies demonstrated exposure to be a risk factor for development of AR |
| Number of siblings | 1 SR/meta-analysis (53 studies, meta-analysis of 33 studies) 1 additional cross-sectional study |
B | Risk of development of AR in families with 3 siblings vs no siblings | Inverse association between number of siblings and AR. Effect strongest in more affluent countries |
| Bacterial endotoxin | 1 review of 16 studies (6 rural and 10 urban) 1 case-control study |
C | Association of endotoxin exposure and allergen sensitization | Results inconsistent; some studies suggest a protective effect with early childhood exposure to endotoxins |
| Probiotics in infancy to protect against AR | 1 meta-analysis of 29 RCTs | A | Association of probiotic supplementation in pregnancy and infancy with development of AR in early childhood | No association identified |
Also associated with the hygiene/biodiversity hypothesis is the consideration of alterations in the human microbiome and the effect that these alterations may have on the development of allergy. The human body interfaces with trillions of microbes throughout the skin and gastrointestinal (GI) tract. The composition of the microbes colonizing a given individual is known as the “microbiome.” The Human Microbiome Project began in 2007, and since that time there has been an increasing recognition of the relationship between the microbiome and human disease. The interaction of the human host with the microbiome begins in the first days of life as the host begins to be colonized. Studies have shown a link between early life microbiome composition and allergy development. Animal studies support this idea. For example, germ-free mice have an exaggerated susceptibility to allergy. This susceptibility can be reversed with colonization early in life before allergen sensitization, suggesting a critical window early in childhood. This phenomenon is believed to be, at least in part, because of the key role that microbiota play in immune-system regulation and development early in life. Many of the theories and observations in Table 35.3 that are related to hygiene or biodiversity concepts may also be associated with alterations of the human microbiome caused by environmental exposures.
The incidence of allergic disease decreases from north to south in the Northern Hemisphere and reciprocally from south to north in the Southern Hemisphere. Underdiagnosis of allergic and autoimmune diseases in underdeveloped countries could explain some of these geographic differences, but environment appears to be important as well. For example, this phenomenon has been linked to sun exposure and levels of vitamin D. In addition to its traditional role in the endocrine system, vitamin D diminishes the risk of many chronic illnesses including cancer, autoimmunity, infection, and cardiovascular disease. The possible mechanisms by which vitamin D might affect diseases characterized by chronic inflammation could be related to its immunologic effects—including those on T cells, dendritic cells, and macrophages —as well as the ability to promote IL-10 production by Tregs. Additionally, some infections have been found to be distributed according to a south-north gradient in European countries that mirrors the gradient for autoimmune diseases. Low socioeconomic levels and high temperatures, two common features of southern countries, may predispose to infections in a number of ways: less stringent control of microbial contamination of water and food, an increased risk of bacterial proliferation with higher ambient temperatures, and poorer housing conditions may all affect the risk of contamination.
Climate may be a contributing factor to the geographic variability of allergic disease. A recent study in the United States found that climate factors such as humidity, drought index, UV index, and pollen levels were correlated with prevalence of hay fever.
In developed and industrialized countries, a higher prevalence of atopy and asthma is observed compared to undeveloped and less affluent countries. In fact, a positive correlation has been demonstrated between gross national product and the incidence of asthma, diabetes, and multiple sclerosis in Europe. Persons who have immigrated to countries in which allergies are prevalent have higher rates of allergies and asthma than persons who remain in their country of origin. This difference probably involves exposure to a new set of pollutants and allergens and several socioeconomic and cultural issues such as housing conditions, diet, and accessibility to medical services, all of which are likely to affect migrants’ health. The increase in allergy and asthma is usually not related to ethnicity but in certain populations this may play an important role. Studies of migrants support the notion that lifestyle and environmental factors in Western industrialized countries facilitate atopy and asthma.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here