Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
By the end of this chapter the reader should:
Understand the genetic and environmental factors in the aetiology of allergic disorders
Know the scientific basis of allergic disorders
Understand the rationale for investigation and management of allergic disorders
The immune system primarily protects the host from microbial attack without causing harm to that host. It has two interacting components: the innate and the acquired systems, summarized in Table 16.1 .
| Feature | Innate | Acquired |
|---|---|---|
| Recognition | Pattern recognition molecules reacting to pathogen-associated molecular patterns (PAMPs), e.g. lipopolysaccharide | Large range of specific molecules (or fragments of molecules) |
| Speed of response | Immediate | Fast, as cellular migration and interaction required |
| Memory | None | Efficient memory function |
| Humoral components | Complement | Antibodies |
| Cellular components | Neutrophils, eosinophils, basophils, mast cells, epithelial cells, macrophage, innate-responding lymphocytes | B and T lymphocytes |
| NB: Some cells (e.g. mast cells, eosinophils) which traditionally have been classified as innate cells are now known to be involved in both innate and acquired immune responses. Similarly, some lymphocytes are now known to be involved in innate immune responses. | ||
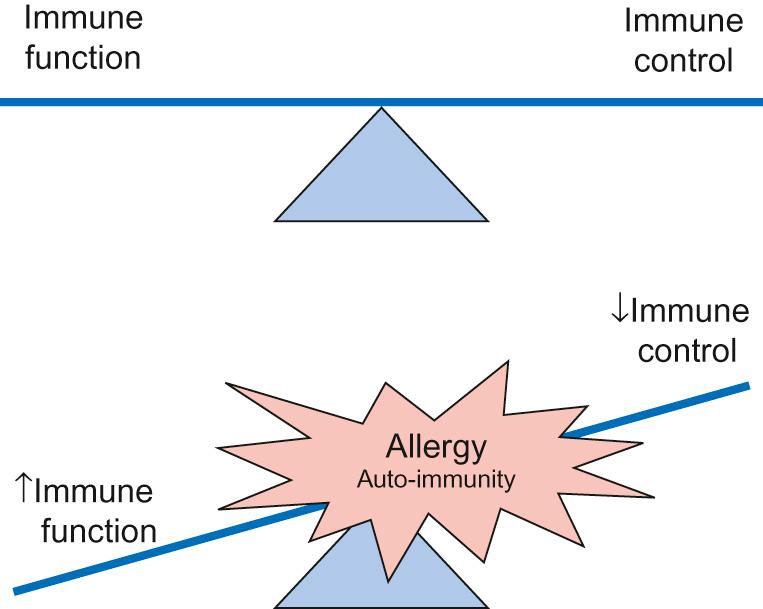
The immune system is tightly regulated – too little immunity can result in immunodeficiency while inappropriate/excess immune function will cause harm to the host. Allergy can be considered a consequence of an inappropriate immune response ( Fig. 16.1 ): the immune system ‘reacts’ to an otherwise innocuous substance (allergen), e.g. pollen grain. ‘Hypersensitivity’ reactions to these substances are usually to protein or protein fragments, recognized by the immune system, which results in a clinical reaction. However, the immune mechanism is no different to an appropriate immune response to a parasite.
There are four main mechanisms (originally characterized by Gell and Coombs) by which a hypersensitivity reaction can occur ( Table 16.2 ). Most classical allergic conditions involve a type 1 hypersensitivity reaction, and require an initial ‘sensitization’ step in which antigen-specific IgE antibody is produced by B cells and plasma cells ( Fig. 16.2 ). IgE subsequently binds to receptors on mast cells and basophils. Upon subsequent re-exposure to the allergen, IgE on the surface of mast cells recognizes and binds to the allergen.
* N.B. Each mechanism also plays an important role in the host response to pathogens.

Many allergic responses, particularly those mediated by IgE, consist of two acute phases:
The early phase response occurs rapidly, often within minutes of allergen exposure, and is caused by the release of histamine and other vasoactive mediators from tissue-resident mast cells. This response resolves spontaneously within 1–2 hours
A late phase response (LPR) in around 50% of reactions – well characterized in the upper or lower airways, but less so in skin or food allergy – there is a late phase response which is slow to peak (4–6 hours) and slow to resolve. In the nasal airway, this causes sustained nasal congestion; in the lower airways, LPR manifests as cough and wheezing, which persists for at least 24 hours and can cause increased bronchial hyper-responsiveness for 1–2 weeks. It is associated with initial neutrophil influx and, later, more persistent eosinophilic inflammation.
LPR should be distinguished from chronic allergic inflammation. Continued exposure to the allergen induces a state of chronic inflammation. This is characterized by the development of allergen hyper-responsiveness, where the tissues (for example, lower airways) become more sensitive and responsive to any trigger (both allergen itself, and non-specific mediator release) causing an amplification of allergic reactions.
Up to 40% of children in the UK suffer from some form of allergic disease, including atopic eczema, food allergy, allergic rhino-conjunctivitis, asthma, insect sting hypersensitivity and drug allergy.
Allergic diseases have become the commonest chronic conditions affecting children and young adults. They mostly begin in early life and pose a major health economic burden, which has increased dramatically in developed countries over the last 30–50 years and is now also impacting on developing countries. The conditions cause considerable morbidity and occasional mortality, and are the consequence of complex gene–environmental interactions. Most are partially controllable but not curable, which emphasizes the need to understand the mechanisms leading to the development of allergy, as this could identify potential targets for future prevention.
Increases in allergic diseases were initially seen in affluent Western world countries, most notably the UK, Australia, New Zealand, USA and Canada. This trend was first noted in respiratory allergy (asthma and hay fever), and in some countries, prevalence now appears to have plateaued. The increase in the prevalence of eczema and particularly food allergy has occurred more recently, which suggests that perhaps there are different mechanisms involved in the development of sensitization to aeroallergens compared with foods.
It has long been known that allergic conditions run in families. This has led to the use of the term atopy as a descriptor of genetic susceptibility to develop allergic sensitization and disease. With the spectacular developments in genomics, it has been possible to identify a host of genes associated with susceptibility to developing allergic sensitization and/or allergic disease. However, genetic susceptibility must be assessed both in relation to the interaction with environment and to understand that independent genetic factors are associated with susceptibility to allergic sensitization, compared with those associated with susceptibility to specific allergic conditions such as asthma or eczema.
Initially, genetic studies identified potential candidate genes based on an understanding of function in relation to immune responses. For example, a number of associations with allergic sensitization have been identified close to the genes for IL-4 and IL-13, important cytokines for switching B-cell production from IgM to IgE. More recently, genome-wide association studies (GWAS) have reversed the process, by mapping the whole genome of individuals and identifying differences in those with a particular disease compared with those without. GWAS have identified many polymorphisms associated with allergic disease which fall outside the conventional mechanistic understanding of allergic sensitization, and provide evidence for different mechanisms associated with the onset of allergic disease such as asthma or eczema. The most well-known example are polymorphisms in the gene for the protein, filaggrin (expressed in skin epithelial cells), which result in excessive leakiness of the skin making it susceptible to water loss, skin drying and penetration by irritants, infectious agents and allergens. There are strong associations with eczema and food allergy. This has led to the concept that food allergy may result from a primary exposure to food allergens through the skin, rather than the gastrointestinal route. The strongest association with asthma is with polymorphisms in the gene ORM-DL3 , expressed in airway epithelium.
Some genetic polymorphisms are only associated with increased risks of a specific allergic manifestation in relation to particular environmental exposures. Thus, patients with polymorphisms in glutathione methyl-transferase genes only have a higher risk of asthma if they either live in a high pollution environment or are exposed to environmental tobacco smoke. In the absence of these exposures, the genetic polymorphism is not associated with an increased risk of asthma.
Given the very rapid increases in prevalence of allergic diseases in less than one generation, changes in DNA sequences are clearly not the explanation. Factors associated with an evolving affluent environment are likely to be critical and include diet, allergen, pollutant and microbial exposure.
There are subtle differences in the immune responses of neonates who subsequently develop allergic disease. Having an allergic mother confers a higher likelihood of allergy in the offspring than if the father is allergic. Thus, the immunological environment generated by the mother impacts on the fetus's evolving immune responses, committing them towards an allergic phenotype. Maternal nutrition in pregnancy has been identified as an important factor; strong associations have been identified between some allergic manifestations and lower omega-3 to omega-6 polyunsaturated fatty acid ratios, reduced fresh fruit and vegetable intake, and vitamin D deficiency. To date, these epidemiological associations have not translated into favourable outcomes from intervention studies of fish oils in pregnancy, modifications of fresh fruit and vegetable intake, and vitamin D supplementation.
Allergen avoidance during pregnancy and in early infancy would seem to be a reasonable approach to prevention, but outcomes from many trials have been spectacularly disappointing. House dust mite avoidance during pregnancy may have a small effect in reducing early infant wheeze, but has had no impact on subsequent development of atopic asthma. Mothers were previously advised to avoid allergens such as nuts in pregnancy and in the infant's diet. The concern originally arose from the observation that some children appeared to react to food allergen at first exposure, implying that the initial exposure (causing sensitization) must have occurred prior to birth, i.e. in utero. However, epidemiological evidence indicates that, if anything, avoidance may be associated with an increased risk of allergy. Rather than a linear relationship between the level of allergen exposure and sensitization, there appears to be a bell-shaped curve, where very low and high exposures are associated with a lower risk. High exposure may result in the induction of immunological tolerance and may be a more appropriate approach to allergy prevention in the future.
In the postnatal period, the focus has been on early infant feeding. Exclusive breastfeeding (for at least four months) has been associated with a reduced risk of eczema and food allergy in some but not all studies. The impact on later development of asthma is less clear. In non-breastfed infants, there is limited evidence that the use of extensively hydrolysed milk formula results in a reduced risk of eczema, but not asthma. Paradoxically, delaying the introduction of allergenic foods during weaning may be associated with an increased risk of food allergy. Intervention trials assessing the optimal time for introduction of potentially allergenic foods are currently underway, to establish whether this is a cause-and-effect relationship.
The demographic trends in reduction in infectious diseases over the last half century have been mirrored by a progressive increase in non-communicable diseases, including allergy and autoimmune conditions. Allergic diseases occur more commonly in firstborn than second and subsequent children in a family. This led to the formulation of the hygiene hypothesis, which states that early exposure to infections (such as occurs in later-born children exposed to their older siblings) reduces the risk of allergic sensitization and disease. This hypothesis has been substantiated by a number of other epidemiological associations, including high use of antibiotics during pregnancy and the postnatal period, which increases the risks of asthma, while babies born into Bavarian farming families exposed to high levels of endotoxin have a lower risk of allergy and allergic disease. It has been suggested that the latter may be due to ingestion of unpasteurized milk by mothers and by their infants during weaning. Unpasteurized milk contains a range of bacteria, which could have a probiotic effect. The use of probiotics during pregnancy and in infants to prevent the onset of allergic disease has been studied, but there is significant heterogeneity in results and there is still no convincing evidence that this approach will alter the development of allergic disease. Recent studies utilizing prebiotic oligosaccharides (which occur in human breast milk) suggest this approach might reduce the risk of allergic sensitization and eczema, but more trials are needed to confirm this.
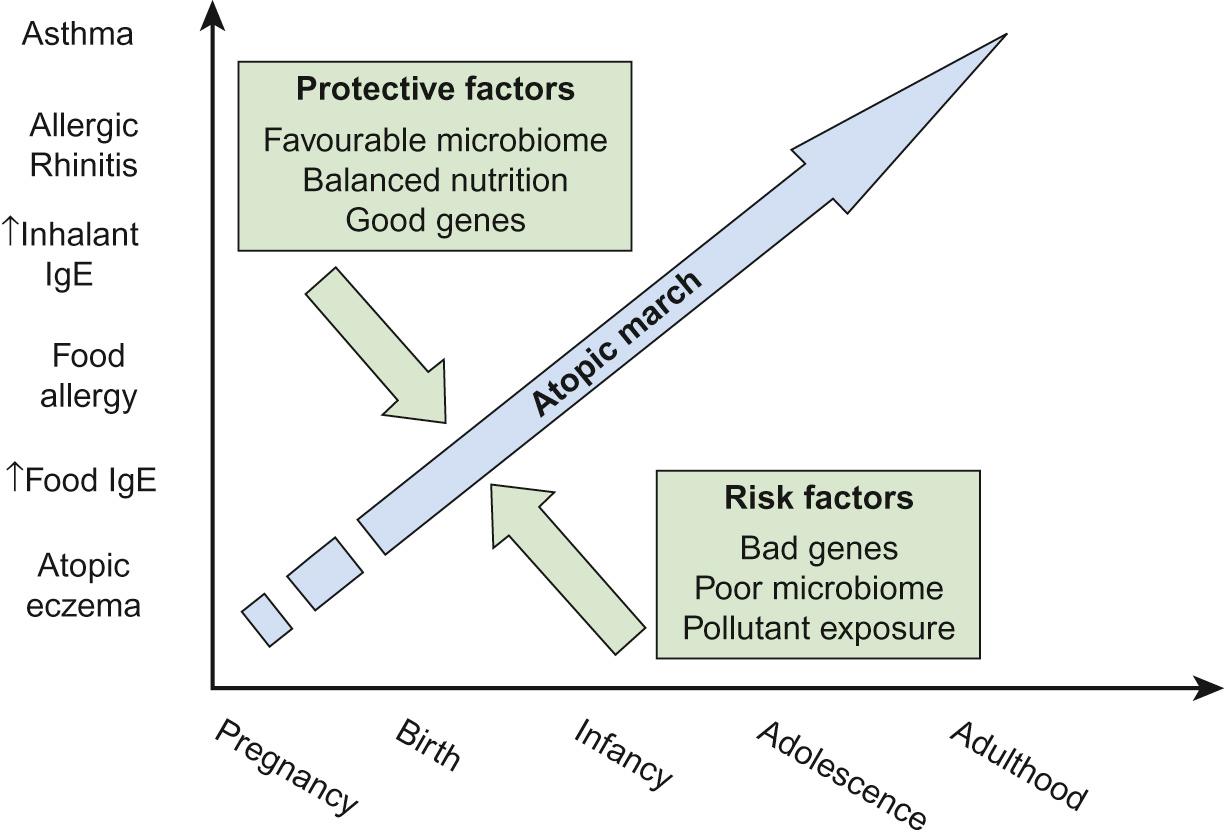
Many infants initially presenting with food allergy and eczema subsequently develop allergic rhinitis and/or asthma. This has been termed the allergic or atopic march ( Fig. 16.3 ). However, there are other subjects who only ever have early eczema, sometimes with food allergy, and never develop subsequent allergic problems, while others with allergic asthma have never had preceding eczema. Nevertheless, there is some mechanistic credibility to the concept of the allergic march. Filaggrin gene polymorphisms, leading to excessive skin permeability, are not only associated with increased risk of food allergy and eczema but also with an increased risk of subsequent asthma. The mechanistic concept would be that sensitization to aeroallergens has occurred through the skin with subsequent homing of sensitized lymphocytes to the airway.
CJ was a full-term normal delivery of high birth weight. He developed facial and flexural eczema at three months of age, having been exclusively breastfed during that time. It became apparent to his mother that when she drank milk or ate eggs in the two hours prior to a breastfeed, his eczema flared. CJ had positive skin prick tests, demonstrating the presence of IgE antibodies to milk, egg proteins and house dust mite at six months of age. By two years, the milk allergy had completely resolved. His egg allergy had, however, persisted. At 24 months he presented with a paroxysmal nocturnal cough inducing vomiting, which had persisted for ten days during an upper respiratory infection. Subsequently he was heard to have wheezing when upset, occasionally in association with a cough at night. On further questioning, his parents had noted that he had experienced excessive sneezing and rhinorrhoea for several months before the onset of the cough and wheeze.
Asthma was well controlled with low-dose inhaled steroids and occasional use of beta-agonists. At 12 years of age, he presented with an acute episode of stridor, wheeze, generalized urticaria and angioedema five minutes after eating a Brazil nut. On assessment in A&E, he was noted to be hypotensive but had a very rapid improvement after an intramuscular injection of 0.3 mg of intramuscular adrenaline. Allergy skin tests were negative to Brazil nut. On further questioning, the Brazil nut had been picked out of a bowl of mixed nuts, and he was actually allergic to peanut.
Many allergic reactions are local, with symptoms only at the location of allergen exposure. Where symptoms are seen at a location remote to the site of allergen exposure, this is termed a ‘generalized’ or systemic reaction; if those symptoms are potentially life-threatening, e.g. involve the respiratory and/or cardiovascular system, the reaction is termed anaphylaxis. Anaphylaxis represents the most severe manifestation of an IgE-mediated reaction. It can be defined as ‘a severe, potentially fatal generalized or systemic hypersensitivity reaction’. The most common triggers of anaphylaxis are food in children and adolescents, and insect venom or medications in adults.
The precise mechanism of anaphylaxis remains unclear. Antihistamines do not prevent anaphylaxis, so other mediators (e.g. other mast cell proteases, platelet activating factor) must be involved. Fortunately, there is good anecdotal evidence that adrenaline (epinephrine) can be very effective in halting an anaphylactic reaction; it is for this reason that children at risk of anaphylactic reactions are commonly prescribed adrenaline auto-injector devices for use in the community. The mechanisms by which adrenaline acts are likely to include:
Alpha-adrenergic effects resulting in vasoconstriction, increasing blood pressure and reducing plasma extravasation
Beta-adrenergic effects, causing broncho-dilatation in the airways
Mast cell stabilization, preventing further degranulation and release of vasoactive mediators.
Food allergy affects up to 10% of preschool children worldwide, and continues to increase in prevalence in many countries. Current UK estimates for the prevalence of peanut allergy are between 2 and 4% in school-aged children.
Food allergy involves an immune response to an otherwise innocuous food protein and must be distinguished from food intolerance, now better termed non-allergic food hypersensitivity ( Fig. 16.4 ). While many genuine food allergies are associated with specific IgE antibody, other immune mechanisms may sometimes be involved. Non-allergic food hypersensitivity reactions are typically transient and often result from the gastrointestinal tract being unable to metabolize non-protein food constituents. A classic example is secondary lactose intolerance resulting from a lack of the enzyme lactase (e.g. following gastroenteritis), which results in accumulation of lactose sugar in the gut causing osmotic fluid retention leading to abdominal discomfort and diarrhoea. The crucial difference is that the immune system is not involved, and thus food intolerances (while potentially debilitating) do not result in life-threatening, immune-mediated reactions. While food allergy can occur on exposure to tiny doses, in general intolerance symptoms only occur with a large-dose exposure.

Food allergy and intolerances should be distinguished from psychological food intolerance, which is common and may follow genuine food allergy which has resolved. Adverse reactions to food may also mimic food allergy, for example the response to scombroid food poisoning (which occurs with badly-stored fish such as tuna and is due to a high histamine content) and food contaminated with staphylococcal endotoxin, which results in acute vomiting.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here