Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The alimentary tract is best considered as a muscular tube lined internally by an epithelium that varies in structure according to specialized functions required at particular sites along its length; with a few local variations, the structure of the musculature is similar throughout.
The function of the alimentary tract is to take in raw food material and to fragment it into small portions. These are then acted upon by a series of secretions, mainly enzymes, which convert the large molecules into smaller molecules, thus permitting their absorption into the blood and lymph circulation.
The small molecules are mainly amino acids, small peptides, carbohydrates, sugars and lipids, which are transported by the blood and lymph mainly to the liver, where they are used as the building blocks in the synthesis of essential proteins, carbohydrates and lipids.
At the same time, the alimentary tract is designed to protect against pathogens gaining entry to the body.
The alimentary tract can be divided into three functional components, with an additional auxiliary gland system.
The three functional components are the oral cavity, the simple transport passages and the digestive tract ( Fig. 11.1 ).
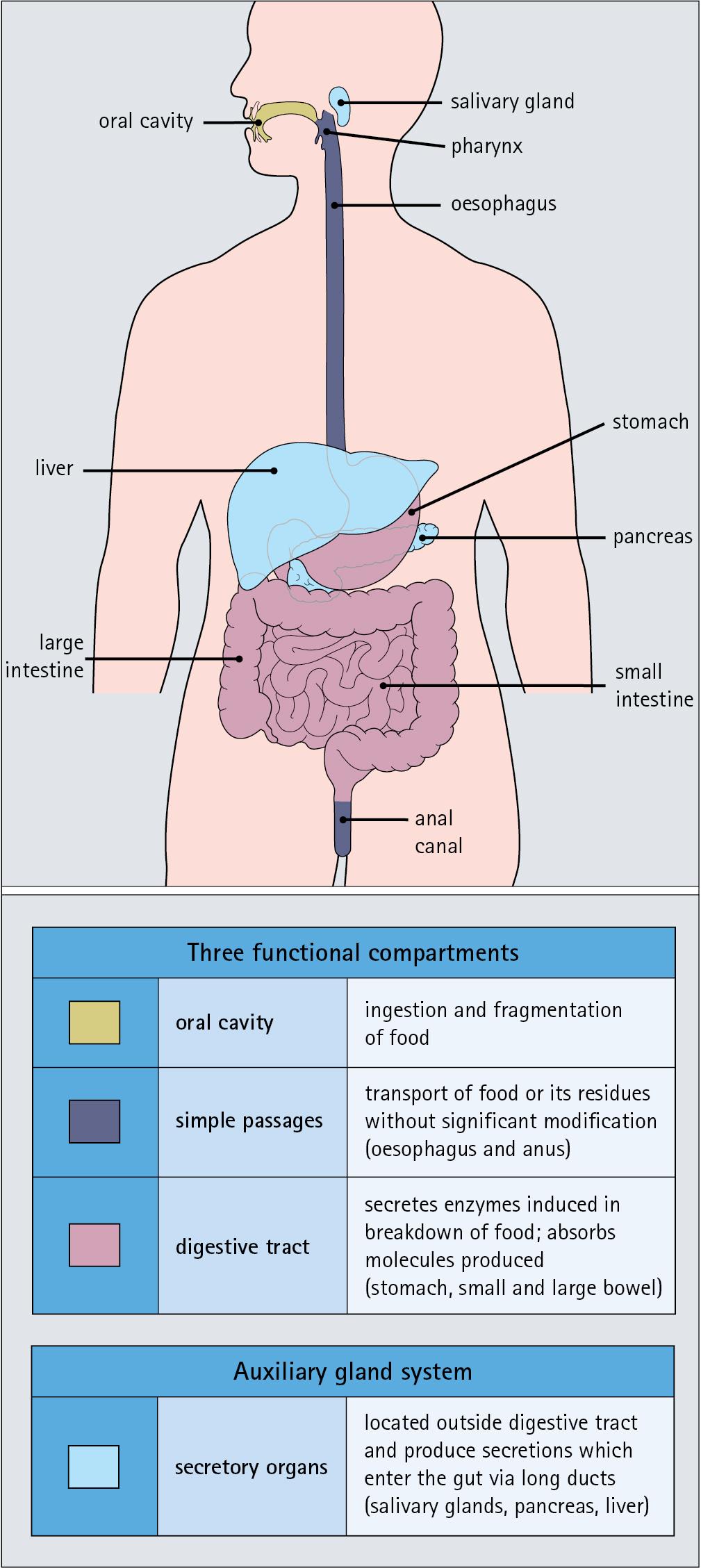
The oral cavity is the area where food is ingested, broken up into smaller fragments by the action of the teeth and softened by secretions in the form of saliva from the salivary gland. The food material is moved around in the oral cavity by movement of the jaws and tongue to facilitate fragmentation. The moistened and fragmented bolus of food is then transferred by deglutition (swallowing) to the first of the simple passages, the oesophagus.
The simple transport passages have no function other than to act as a contractile conduit to pass semisolid material from one area to another. The first simple transport passage is the pharynx, through which food from the mouth passes into the oesophagus, which then transfers the moistened food bolus to the first part of the digestive tract, the stomach, before major digestion begins. The oesophagus is long, and the food bolus is forced along it by smooth muscle action in peristalsis under involuntary nervous control. The other simple transport passage is the anal canal at the end of the digestive tract. This transports semisolid undigested waste material (faeces) from the end of the digestive tract to the exterior. The initial stimulus to transport is involuntary as a result of increasing distension of the rectum, but the timing of the evacuation of faeces can (usually) be controlled by voluntary (skeletal) muscle in an external sphincter. For semisolid material to pass easily through these simple transport passages, it is important that it is lubricated by mucus. For movement through the oesophagus, the mucus is incorporated into the food bolus in the mouth, having been secreted by mucous glands. In the anus, the faeces are lubricated by mucus secreted by the goblet cells in the colonic epithelium.
The digestive tract proper comprises the stomach, the small intestine and large intestine (colon and rectum). The stomach acts as a reservoir where the ingested fragmented food is held up by a sphincter until the acid and enzymatic secretions of the stomach mucosa have broken up the fragmented food into a semiliquid slurry (chyme), which then passes through the sphincter into the small intestine. Here, the process of digestion is continued by a combination of enzymes and other chemicals secreted by the small intestine itself and by the auxiliary glands, the liver and pancreas, the secretions of which enter the small intestine via ducts. In addition to this digestion function, the main function of the small intestine is absorption of the breakdown products of food digestion. The liquid food residue passes from the small intestine into the large intestine, where the majority of the fluid content is reabsorbed until the waste material is converted into semisolid waste material, lubricated for easy passage through the anal canal by mucus secreted by goblet cells in the colon and rectum.
All the components of the alimentary tract are structurally specialized to carry out their specific functions.
The auxiliary gland systems contributing to the function of the alimentary tract are the salivary glands, the liver and the pancreas, all of which pass their secretions into the main alimentary tract through ducts. The salivary glands are discussed on page 208; the pancreas on page 227; and the liver, which has many important functions other than those associated with digestion, merits a chapter of its own (see Chapter 12 ).
The oral cavity is lined throughout by stratified squamous epithelium but contains many highly specialized structures.
The mouth is lined by stratified squamous epithelium, the underlying submucosa containing varying numbers of salivary glands, which can secrete both a serous and a mucous fluid. Skeletal muscle fibres are common in the deeper layers and are responsible for altering the size and shape of the cavity and for moving food; skeletal muscle fibres form the bulk of the tongue and are also numerous and important in the cheeks.
In some areas, the deep tissues of the oral cavity consist of bone, either simple plates of bone (as in the hard palate) or modified bone (teeth). The immovable hard palate produces a rigid structure against which the tongue can move, and the teeth, which are embedded in bony supports (the mandibles and maxillae), are the main tools for fragmenting ingested food.
The lips are covered by squamous epithelium and contain glands and underlying muscle.
The oral orifice is bounded by the lips, the external aspects of which are covered by hair-bearing skin with sebaceous glands and eccrine sweat ducts. Between the hair-bearing outer surface and the moist, fluid-bathed inner surface, there is a transitional zone known as the vermilion because of its pinkish-red appearance. Here, the epithelium is non-keratinizing stratified squamous with a prominent rete ridge system (see Chapter 18 ) and the papillae between the epithelial downgrowths contain prominent blood vessels, which are responsible for the pinkish-red colour.
The inner surface of the lips is lined by a non-keratinizing stratified squamous epithelium with a less well-developed rete ridge system, and small clumps of salivary tissue disgorge their secretions onto its surface through short ducts. In addition, occasional sebaceous glands (Fordyce’s spots), which are particularly common near the angles of the mouth, open directly onto the mucosal surface, rather than into a hair follicle as in the skin.
In the deeper parts of the lips, bundles of striated muscle fibres (orbicularis oris muscle) are arranged mainly in a concentric manner around the oral orifice. This muscle is responsible for, among other things, opening and closing the oral orifice.
The cheeks are lined internally by squamous epithelium and contain glands and deep muscle.
The cheeks are lined by thick, non-keratinizing squamous epithelium, the cells of which are often rich in glycogen. Areas of keratinization are common, usually as a result of chronic friction from ill-fitting dentures or from persistent cheek biting. The submucosa contains minor salivary glands (buccal glands), and the deep tissues contain the skeletal muscle fibres of the cheek muscles (buccinator).
The palate is rich in salivary gland tissue (palatine salivary glands).
The epithelium of the palate is non-keratinizing, stratified squamous epithelium with, in the hard palate, a prominent rete ridge pattern, which reflects the frictional stresses this area is subjected to during mastication. Beneath the salivary gland tissue, the submucosa is firmly tethered to the periosteum of the palatal bone. The oral surface of the soft palate is covered with non-keratinizing stratified squamous epithelium extending to its posterior edge, where it meets ciliated columnar epithelium of the nasal surface.
The floor of the mouth contains salivary glands.
The floor of the mouth is covered by thin, non-keratinized stratified squamous epithelium, which is continuous with that of the ventral surface of the tongue.
There are many minor salivary glands in the floor of the mouth (minor sublingual glands) and larger glands situated on either side of the midline frenulum of the ventral surface of the tongue (major sublingual glands, see p. 208).
The tongue is muscular and covered in squamous epithelium.
The tongue is a highly muscular organ that protrudes upwards and forwards into the oral cavity from its floor. The ventral surface of the tongue is covered by thin, non-keratinizing stratified squamous epithelium, continuous with that of the floor of the mouth. In contrast, the dorsal surface, which is commonly in contact with the hard palate during feeding, talking and at rest, is covered with a thick, keratinizing stratified squamous epithelium, which shows considerable specialization.
The upper surface of the tongue is divided into two main zones.
The dorsal surface of the tongue is divided into an anterior two-thirds and a posterior one-third. The two parts are separated by a V-shaped line of 6–10 dome-shaped protrusions, the circumvallate papillae ( Fig. 11.2 ). Circumvallate papillae appear as flattened domes, the bases of which are depressed below the dorsal surface.
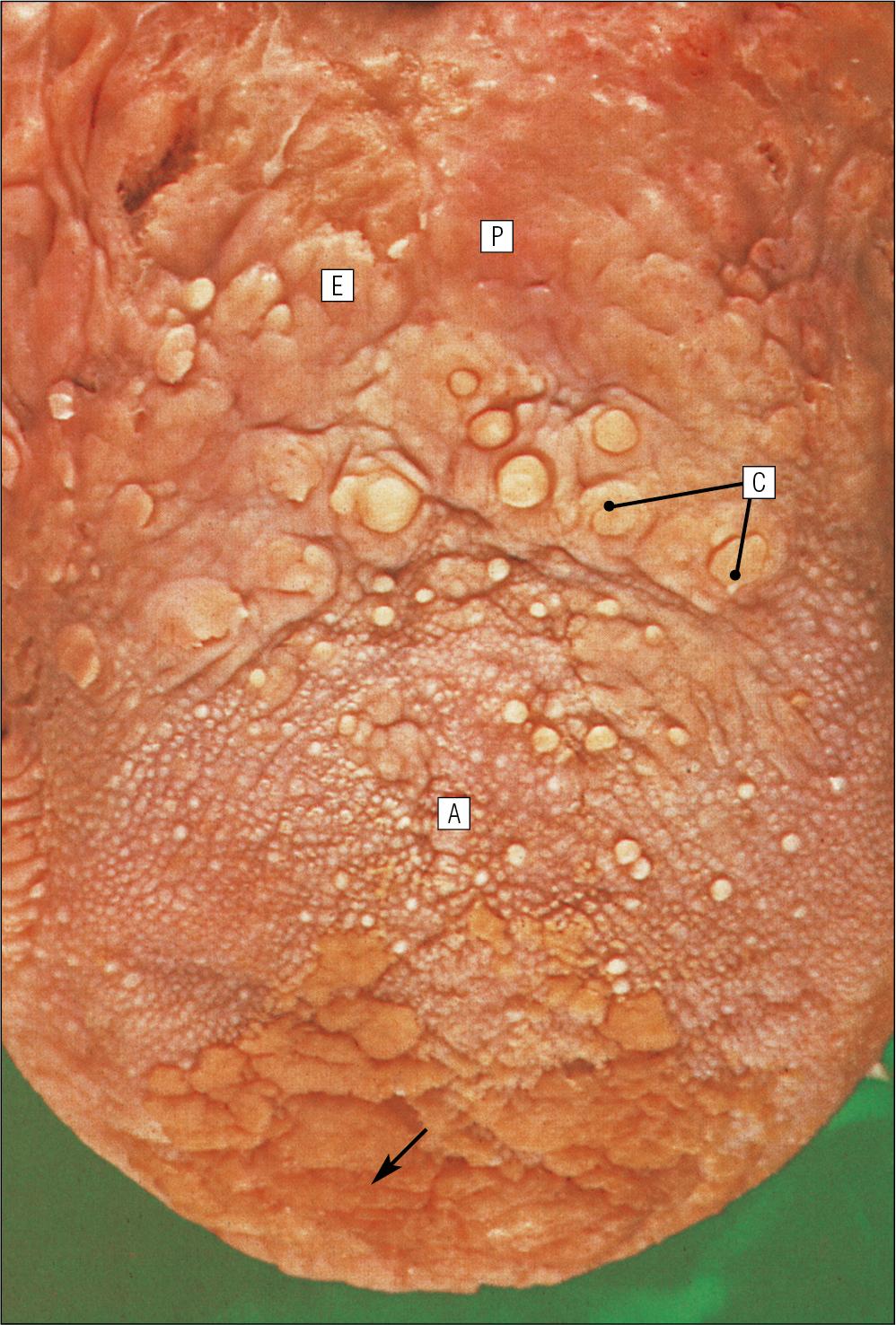
Each circumvallate papilla is surrounded by a narrow, moat-like channel, in the epithelium of which are numerous taste buds. These taste buds are thought to detect bitter taste. Small salivary glands discharge their secretions into the channels ( Fig. 11.3 ).
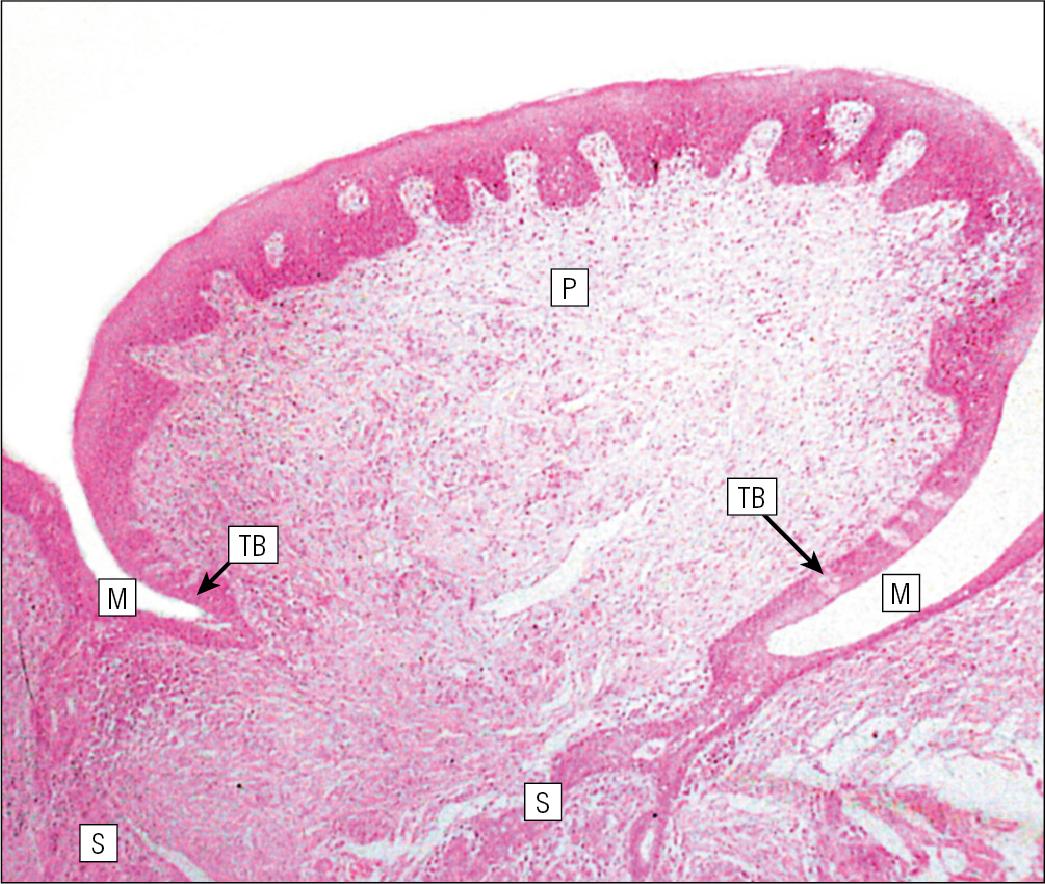
The posterior third of the tongue is characterized by the presence of lymphoid tissue.
Low, smooth, dome-shaped elevations of the covering epithelium of the posterior third of the tongue are the result of lymphoid tissue (the lingual tonsillar tissue) in the submucosa ( Fig. 11.4 ). This lymphoid tissue is part of the mucosa-associated lymphoid tissue (MALT, see p. 154) system protecting (with the palatine tonsils and the pharyngeal adenoids) the oral portal of entry.
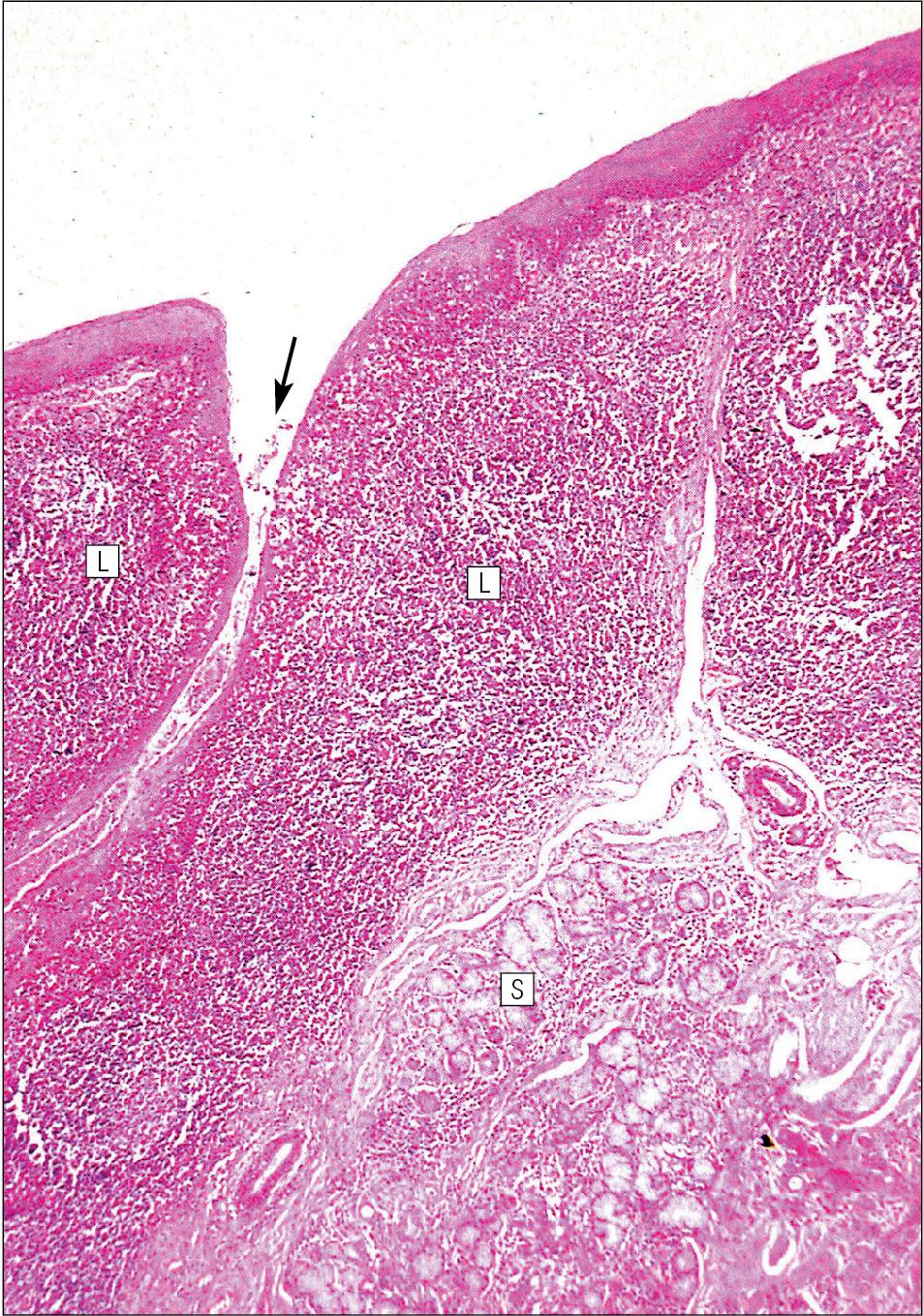
Lymphocytes are numerous within the overlying, non-keratinizing stratified squamous epithelium, which extends down into the lymphoid tissue as narrow clefts. Small salivary glands open into the bottom of the narrow clefts, becoming more prominent and numerous near the line of circumvallate papillae.
The surface epithelium of the anterior two-thirds of the tongue is raised in a series of elevations called papillae.
The three types of papillae in man are circumvallate (described earlier), filiform and fungiform.
Filiform papillae are the most numerous papillae and are found all over the dorsum of the anterior two-thirds of the tongue. They are tall, narrow and pointed ( Fig. 11.5 ) and are keratinized, particularly at their tips. Filiform papillae contain no identifiable taste buds.
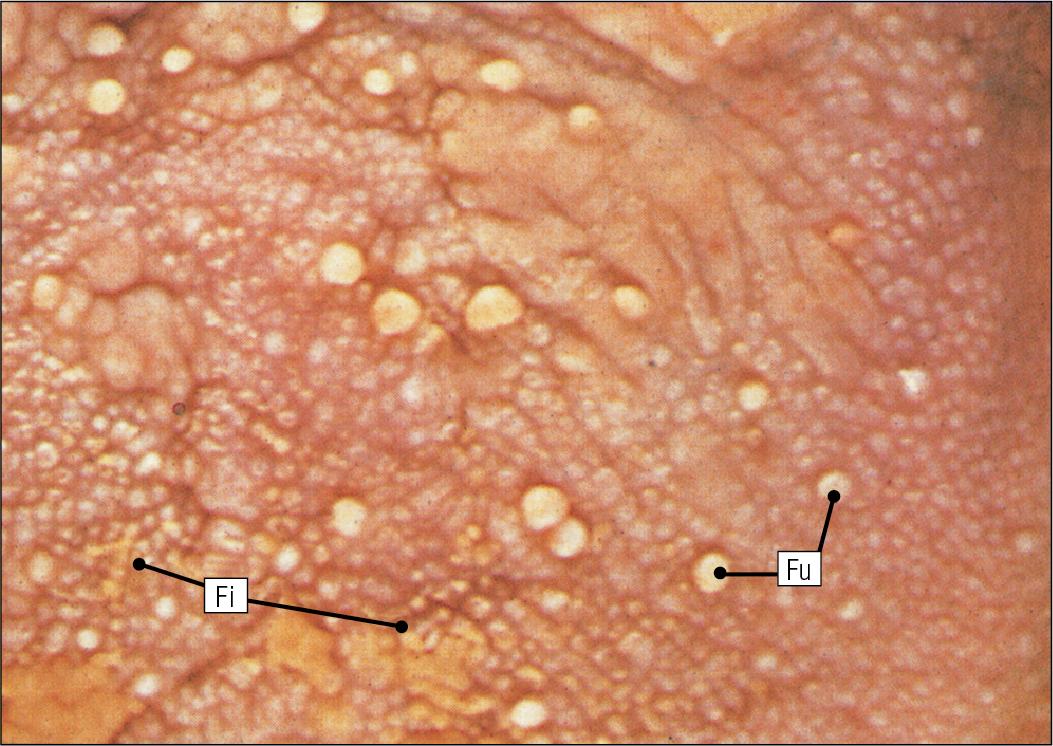
Fungiform papillae (see Fig. 11.5 ) are scattered apparently randomly among the filiform papillae on the dorsal surface of the tongue and have a mushroom shape. Taste buds are present in their covering epithelium, with those at the anterior tip of the tongue detecting sweet taste and those just behind the tip and some way along the lateral borders detecting salty taste.
Taste buds are specialized sensory organs located in the epithelium of the tongue.
Each taste bud occupies the full thickness of the epithelium and comprises pale-staining, spindle-shaped cells in an oval cluster ( Fig. 11.6 ). The luminal surfaces of the cells open into a small defect in the epithelium, the taste pore, and each cell bears a number of microvilli. Ultrastructurally, some of the spindle-shaped cells have synaptic vesicles and are associated with small afferent nerve fibres; these are the taste receptor cells. Other cells with more electron-dense cytoplasm and scanty secretory granules are supporting sustentacular cells. There are also cells resembling the taste receptor cells but lacking the synaptic vesicles and afferent nerve connections. The turnover of these cells is rapid (every 10–14 days), so there is a population of stem cells at the base of each taste bud.
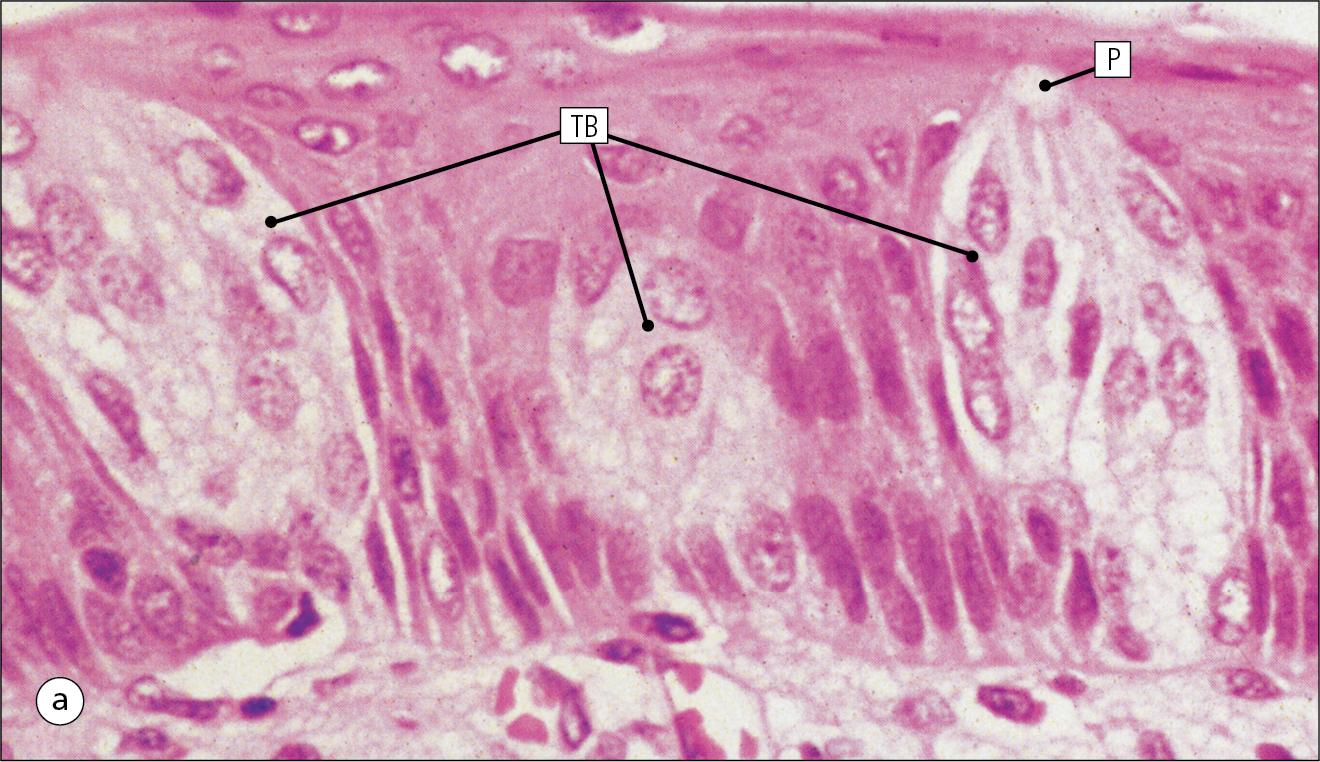
The taste buds of the dorsal tongue detect acid, sweet, bitter and salty and provide early warning that food may be unpalatable.
Covered by stratified squamous epithelium, thin on ventral surface, thick and papilliform on dorsal surface
Filiform, fungiform and circumvallate papillae on anterior two-thirds; the latter demarcate anterior and posterior parts of the tongue
Taste buds are present on circumvallate and fungiform papillae
Consists of striated muscle with a small amount of adipose tissue
Salivary tissue in submucosa, mainly near circumvallate papillae
Covered by smooth, non-papillate stratified squamous epithelium
Submucosa contains lymphoid aggregates (MALT), forming part of Waldeyer’s ring
Bulk is muscle and adipose tissue, with some salivary tissue in submucosa and muscle, mainly near circumvallate papillae
The musculature of the tongue comprises a complex pattern of skeletal muscle fibres. These fibres run in bands longitudinally, vertically, transversely and obliquely, with a variable amount of adipose tissue in between ( Fig. 11.7 ). This arrangement gives the tongue great mobility to manipulate food in the mouth and for swallowing; it also provides the fine control of tongue movement for speech.
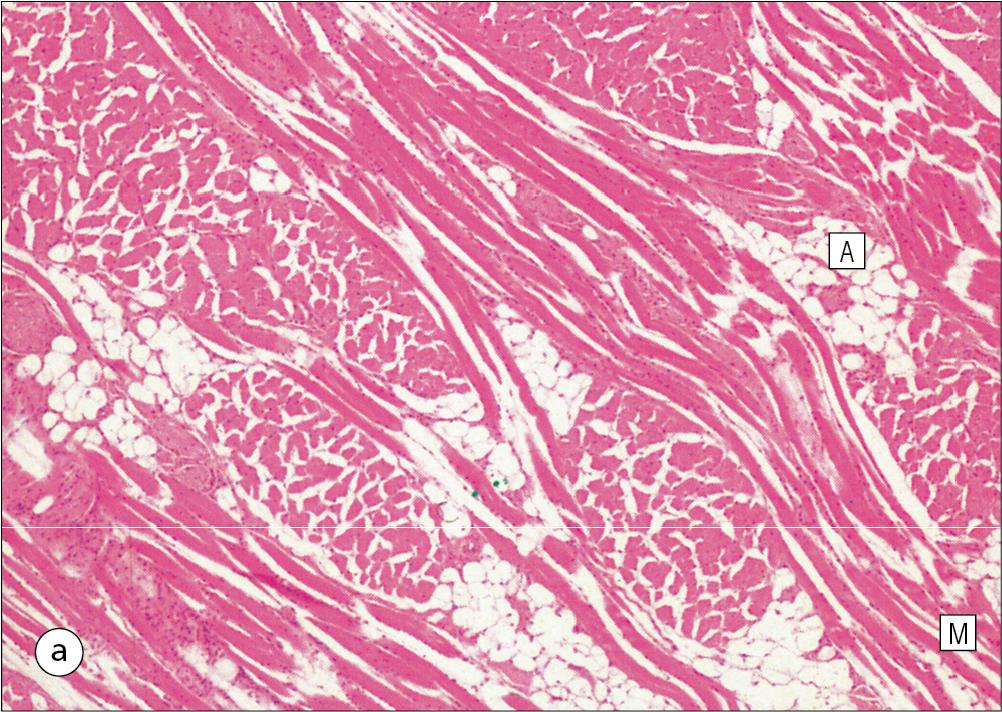
Islands of salivary tissue are present in the submucosa between the muscle and surface epithelium at the junction between the posterior one-third and the anterior two-thirds. Some of the deeper salivary gland extends down into the superficial muscular zone.
The main task of the oral cavity, the fragmentation of large food masses, is performed by the teeth, which are hard, heavily mineralized structures embedded in the raised alveolar ridges of the maxilla and mandible.
Teeth are arranged so that the free surface of those embedded in the mandible (lower teeth) oppose and contact those in the maxilla (upper teeth), allowing food material to be trapped between them.
The anterior teeth (incisor and canine teeth) have narrow, chisel-shaped or pointed free edges for chopping food into medium-sized pieces, whereas the posterior teeth (premolars and molars) have broader, flatter, free surfaces for grinding medium-sized food pieces into smaller fragments.
The mandible is joined to the body of the skull by the temporomandibular joint, which permits the mandible to slide backwards and forwards and from side to side, thus aiding the grinding of food between the broad surfaces of the opposing molar teeth.
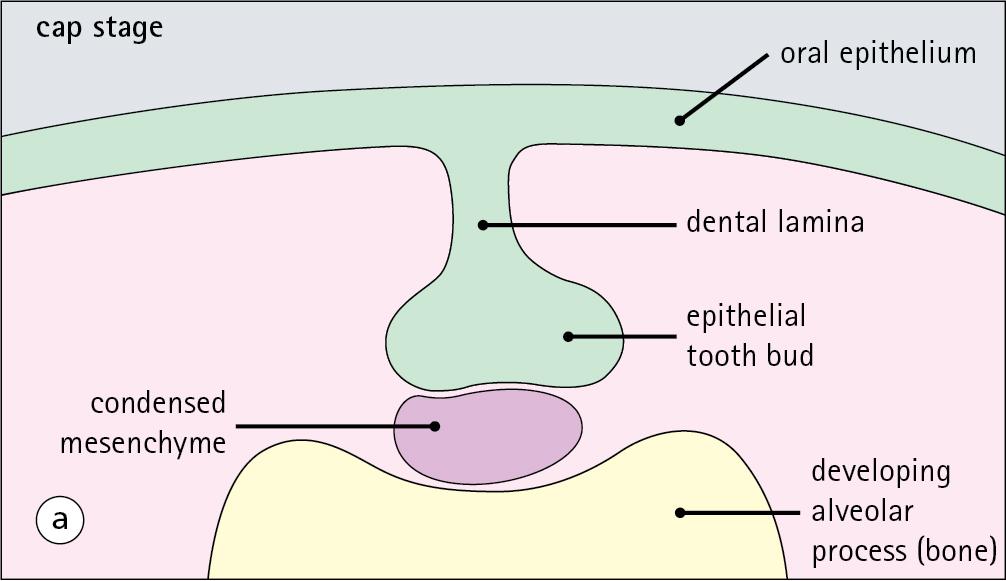
Each tooth can be divided into two anatomical components: the crown and the root.
The tooth is divided into two structural regions:
The crown protrudes into the oral cavity.
The root is embedded in the bone of the mandible or maxilla.
The junction between the crown and the root is called the neck. The mature tooth has five components: the central pulp cavity, dentine, enamel, cementum and periodontal ligament ( Fig. 11.8 ).
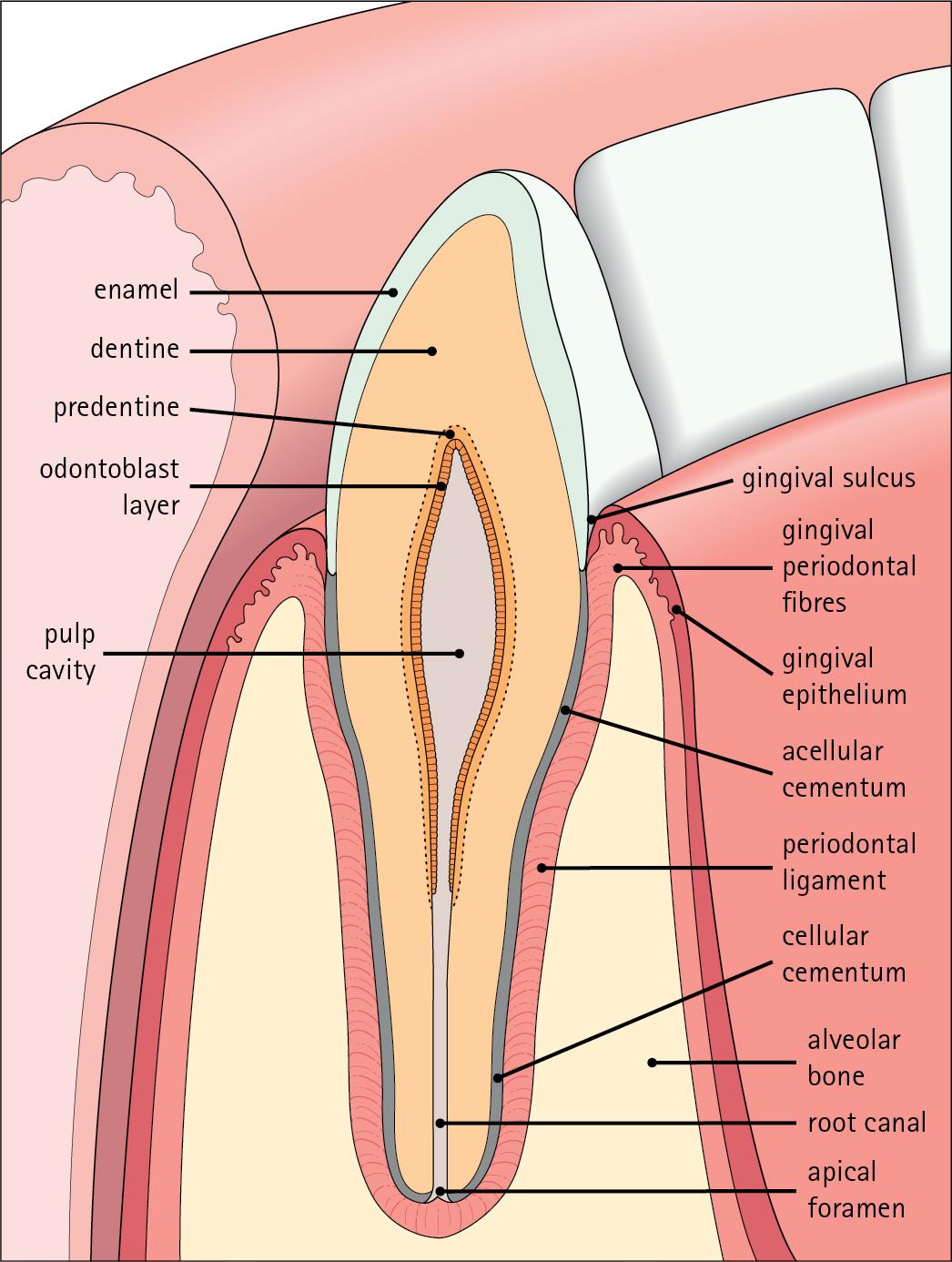
The centre of each tooth comprises the pulp, which contains nutrient vessels and nerves.
The pulp cavity is the soft central core of the tooth and contains collagen and fibroblasts embedded in an acellular matrix. The cavity approximates in shape that of the tooth as a whole, and its acellular matrix is composed of glycosaminoglycans.
Through the pulp cavity run the blood vessels that nourish the odontoblasts (see later) and the nerve twigs that provide dental sensation. These vessels and nerves enter and leave through a small apical foramen at the tip of the root.
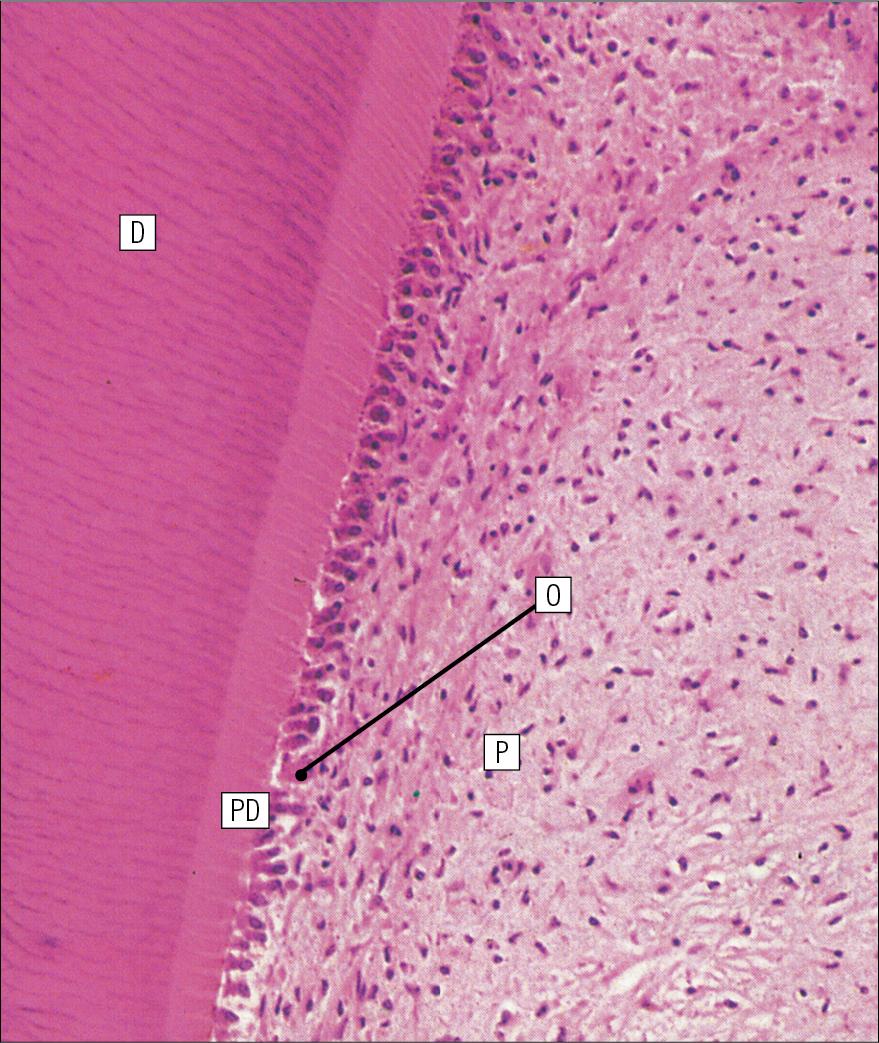
The pulp cavity is narrow throughout most of the root (the root canal ) but is expanded in the neck and crown (the pulp chamber ). Its outer surface is lined by odontoblasts, which continually produce dentine. As dentine is progressively laid down, the pulp cavity diminishes in size.
The specialized mineralized matrix of the tooth is called dentine and is secreted by odontoblasts.
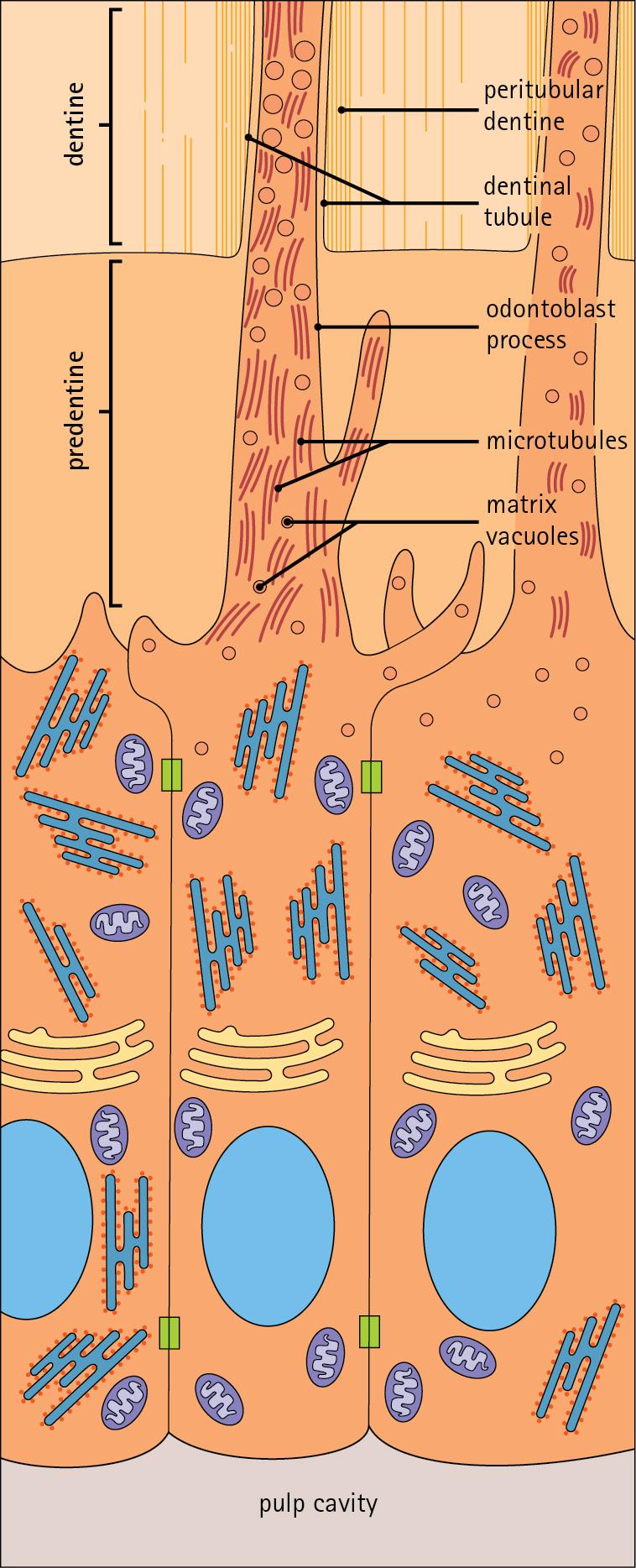
Dentine is composed of mineral salts and organic material. The mineral salts are calcium salts in the form of crystalline hydroxyapatite (about 70%–80%) arranged as long, hollow parallel tubules, the dentinal tubules . Within these run the organic material in the form of fine cytoplasmic processes of the odontoblasts and the type I collagen fibres and glycosaminoglycans that they produce (about 20%–30%).
Dentine is initially laid down by odontoblasts as a glycosaminoglycan matrix in which collagen fibres are linearly arranged. This non-mineralized predentine is synthesized by odontoblasts located at the outer limits of the pulp cavity ( Fig. 11.9 ).
In humans, the odontoblast cytoplasmic processes extend only 25%–50% of the full length of the dentinal tubule; thus dentine close to the dentine–enamel border appears to contain empty tubules. In life, these empty tubules may contain fluid, which is lost during tissue processing.
As odontoblasts progressively synthesize new predentine at the inner surface of the pulp cavity ( Figs. 11.9 and 11.10 ), the cavity slowly decreases in size throughout life.
Dentinogenesis commences with the formation of predentine by odontoblasts.
In predentine, randomly scattered collagen fibres (type I collagen) produced by the odontoblasts are embedded in an extracellular matrix of phosphoprotein and glycosaminoglycans (mainly chondroitin-6-sulfate).
Mineralization is initiated by the discharge of the matrix vacuoles from the odontoblast process and its branches running through the predentine layer.
Sometime after its formation, predentine becomes mineralized at its border with previously mineralized dentine; close to this predentine–dentine border the collagen fibres of predentine become more numerous and tightly packed. The dentine lining the dentinal tubules (peritubular dentine) is particularly compact and heavily mineralized ( Fig. 11.11 ).
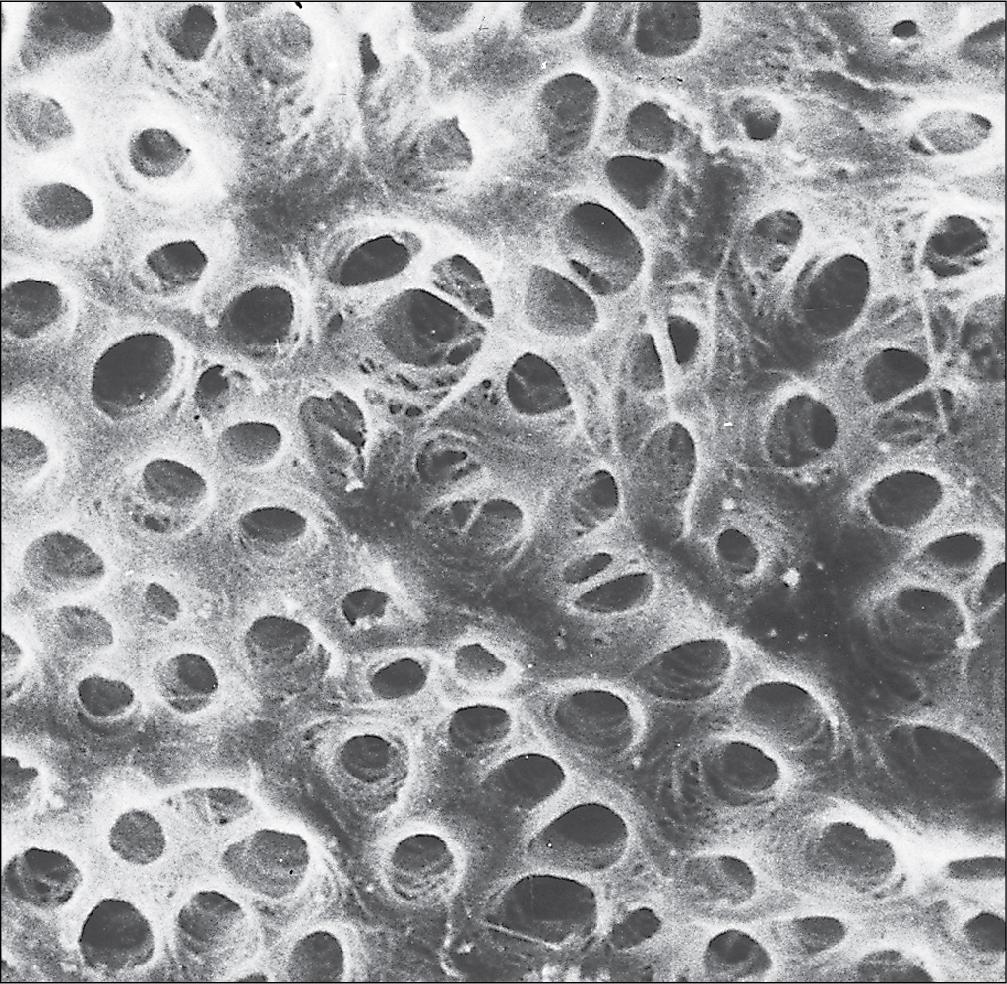
Enamel is the hardest material in the body.
Enamel is composed almost entirely of the mineral hydroxyapatite [Ca 10 (PO 4 ) 6 (OH) 2 ], which is arranged in tightly packed hexagonal enamel rods or prisms ( Fig. 11.12 ) about 4 μm in diameter, although some may measure up to 8 μm.
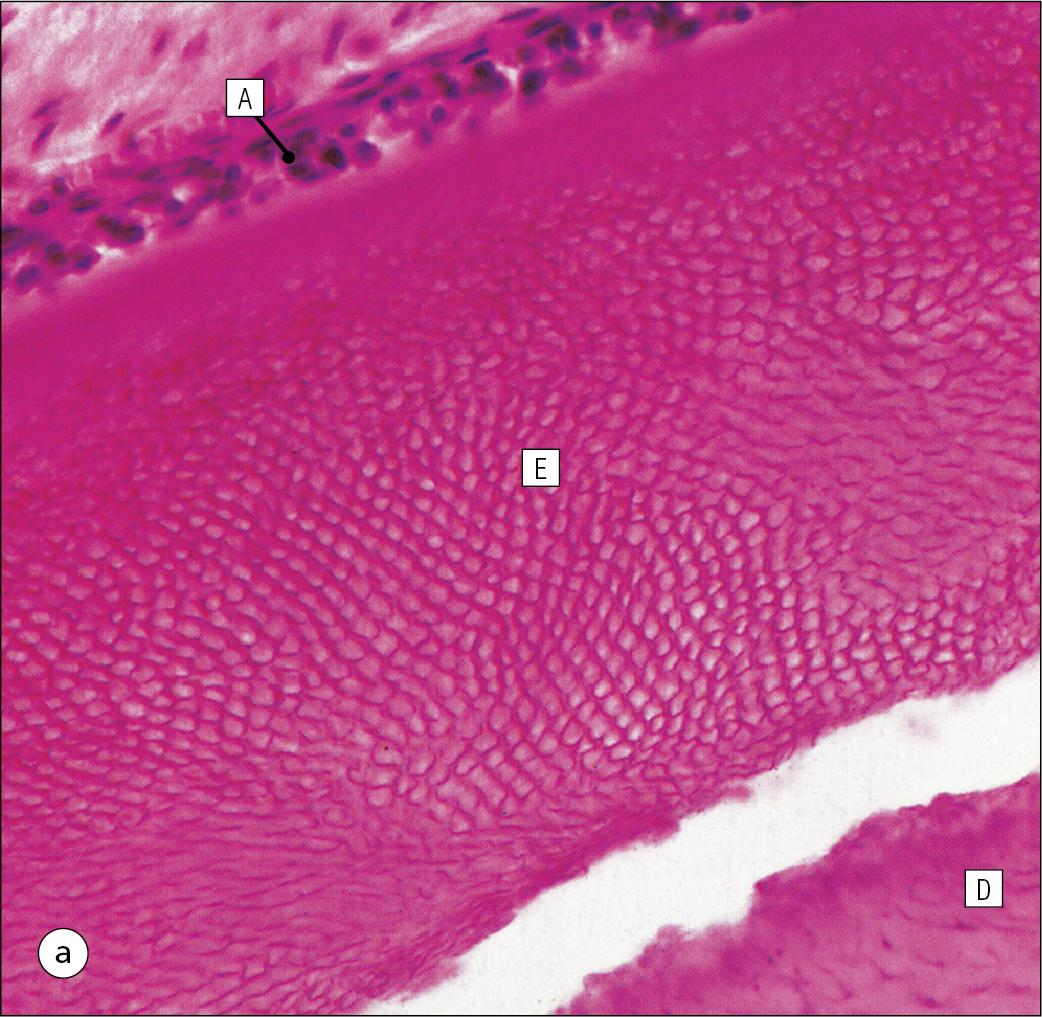
Each enamel rod extends through the full thickness of the enamel. The small interstices between adjacent rods are occupied by hydroxyapatite crystals. A small amount of organic matrix (protein and polysaccharide) represents the remnants of the matrix synthesized and excreted by the enamel-producing cells, the ameloblasts, before mineralization of the enamel.
Enamel is formed during tooth development by the ameloblasts.
Ameloblasts degenerate when the tooth erupts, after which time the enamel cannot be replaced by new synthesis. In the developing tooth (see Fig. 11.17 ), the functioning ameloblast is a tall, narrow cell, with its base attached to the cells of the stratum intermedium ( Fig. 11.13 ). The nucleus is located basally, and basal cytoplasm contains abundant mitochondria. The supranuclear cytoplasm contains a large, active Golgi complex and abundant rough endoplasmic reticulum, together with microtubules, which are predominantly longitudinally arranged, and secretory vacuoles which become larger and more numerous near the upper pole.
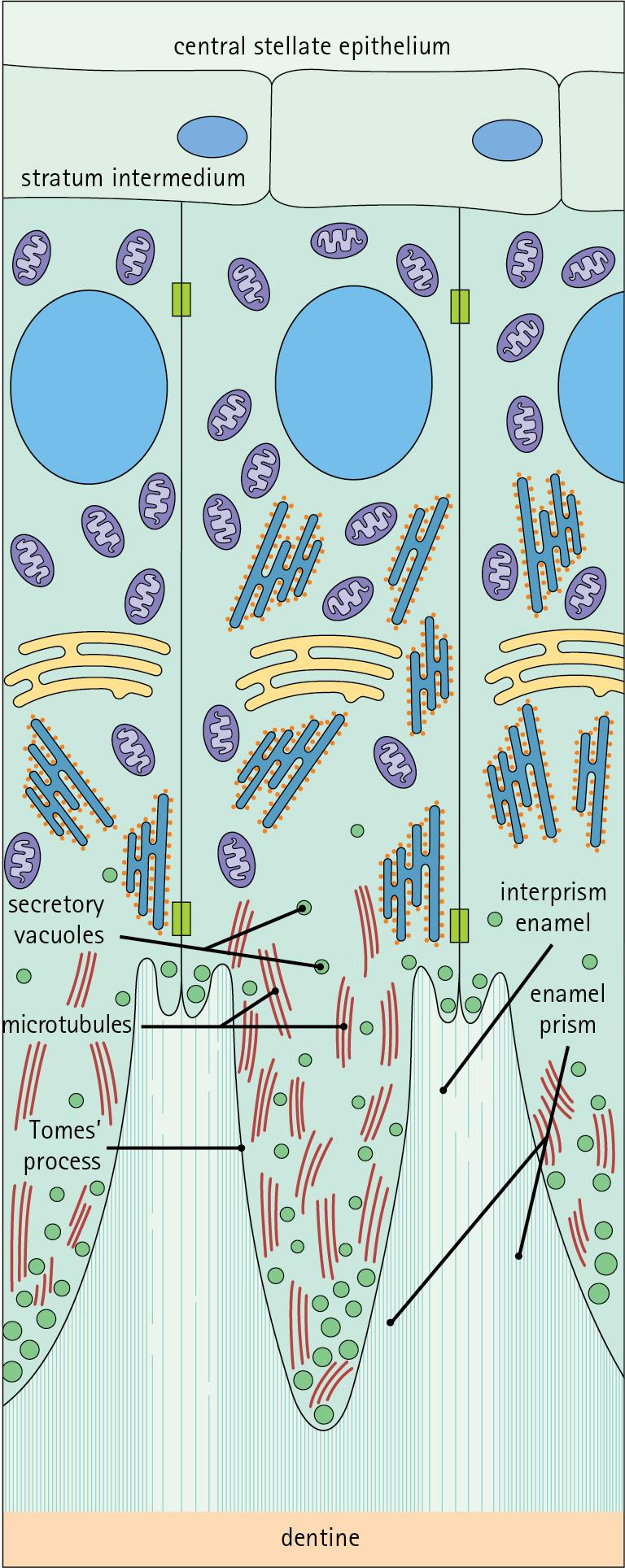
At the upper pole, the cell elongates into a single large Tomes’s process and forms a fringe of smaller processes around its neck. The Tomes’ process contains numerous microtubules and large numbers of secretory vacuoles.
The rough endoplasmic reticulum synthesizes various proteins and glycoproteins (including amelogenin and enamelin), which form the organic matrix of enamel (pre-enamel) and are packaged by the Golgi complex into secretory vacuoles. These then move into the Tomes’ process and the small neck processes, discharging their contents onto the surface.
Mineralization of the matrix proteins by hydroxyapatite occurs almost instantaneously, producing small enamel crystallites and, with progressive mineralization, the enamel rods or prisms.
The compact structured enamel prisms are probably derived from the surface of the main Tomes’ process, whereas the small amount of less compact interprism enamel, which has a larger organic matrix component, is probably derived from the small neck processes.
Enamel covers the dentine only in the region of the exposed crown ( Fig. 11.14 ); in the root, the dentine is covered by cementum (see Figs. 11.8 and 11.15 ).

Cementum is a bone-like tissue; it is calcified and contains collagen.
A thin layer of cementum covers the root of the tooth, being thin, compact and acellular (acellular cementum) over the upper region, but thicker and containing lacunae and cementocytes (cellular cementum) lower down (see Fig. 11.15 ).
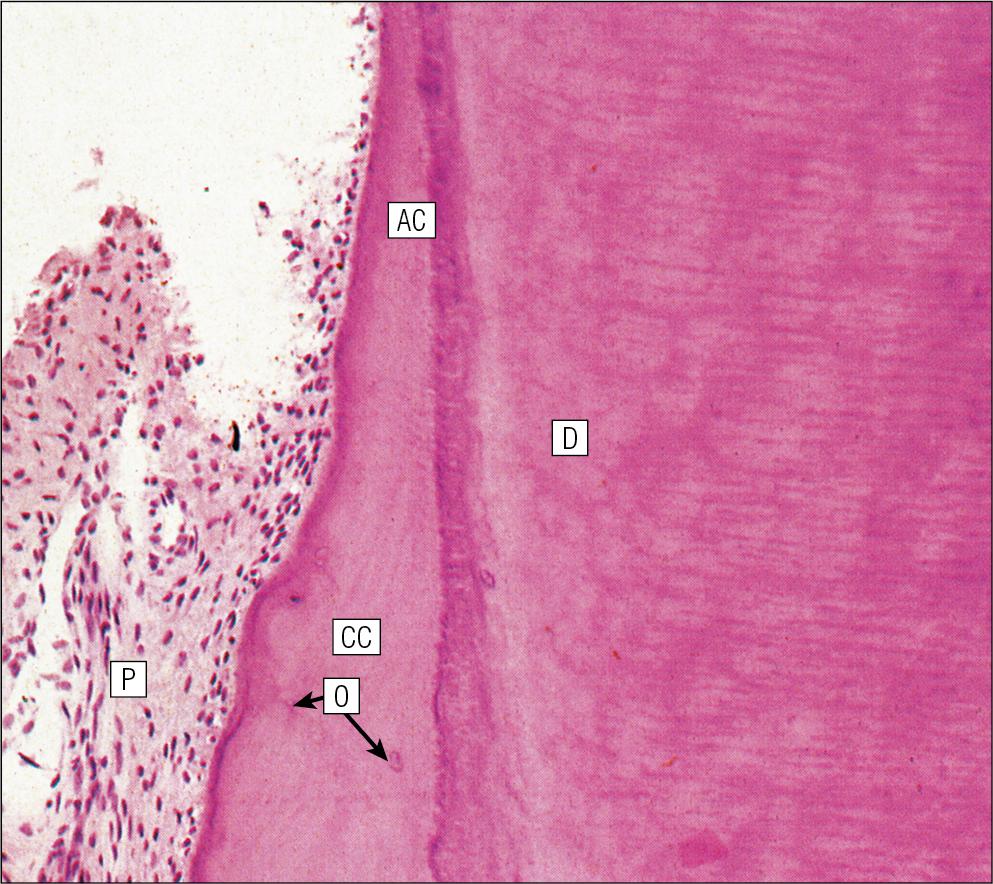
Cementocytes resemble osteocytes (see Chapter 13 ) and remain viable throughout life, being nourished through canaliculi which link the lacunae. They can become activated to produce new cementum when required.
In addition to the cementocytes, which are scattered throughout the cellular cementum, there is a layer of cells called cementoblasts, which are similar to the actively synthetic osteoblasts of bone (see Fig. 13.17 ).
Cementoblasts lie against the surface of the periodontal ligament and probably produce most new cementum by appositional deposition.
The periodontal ligament is a suspensory ligament tethering the tooth in the bony alveolar socket of the mandible or maxilla.
The periodontal ligament is composed of dense collagen and fibrocytes, with the fibres running across the gap between the cementum of the tooth and the bone of the alveolar socket ( Fig. 11.16 ). As the collagen fibres are embedded in a ground substance, this ligament also acts as a shock absorber, in addition to permitting limited movement of the tooth within the bony socket.
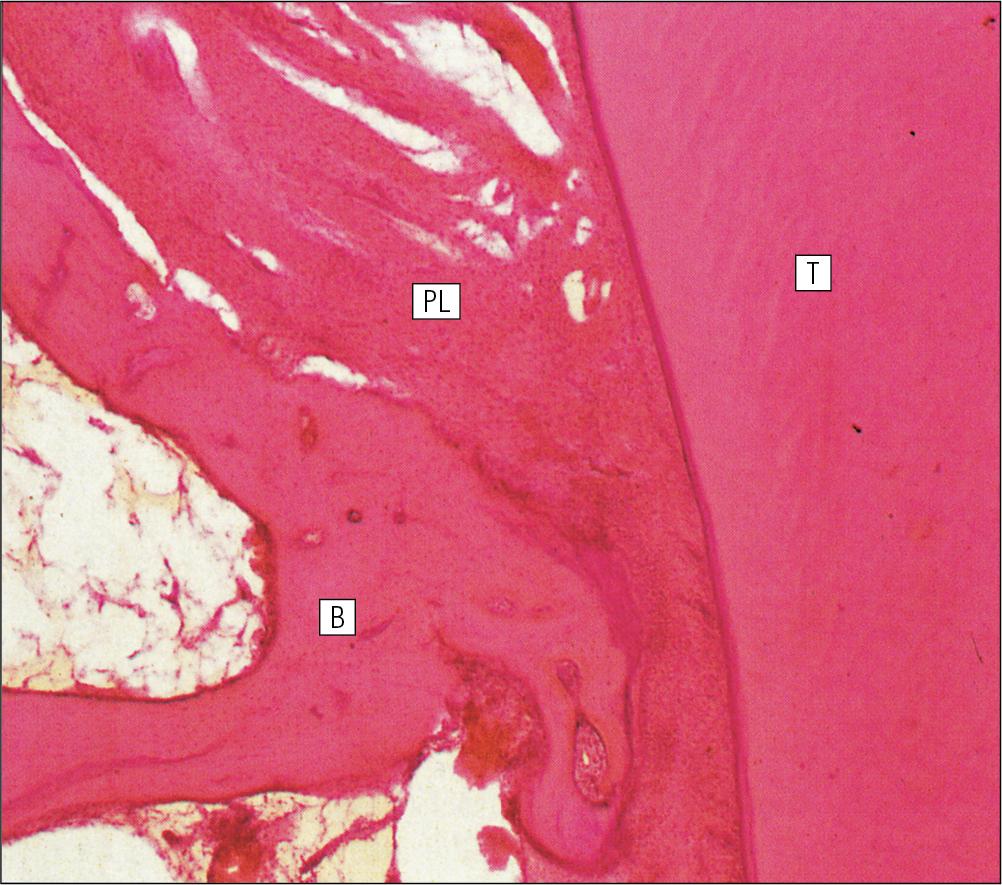
At its cemental and alveolar limits, some of the collagen fibres are inserted into the cementum and bone. The alveolar bone at the inner margin of the socket is composed of woven bone rather than compact lamellar bone. Above the upper limit of the alveolar bone, the fibres of the periodontal ligament become the gingival periodontal fibres and blend with the submucosa of the gingivae.
The most common disorders of teeth are:
Abnormal tooth eruption, leading to malalignment of teeth
Dental caries
Periodontal disease
Dental caries results when bacterial plaque produces acid, which dissolves the calcium hydroxyapatite of the enamel. Such focal decalcification may progress until the erosion involves the deeper dentine, from where the acids and bacteria can advance more rapidly down the dentinal tubules to the pulp cavity, causing tooth pain.
Continued bacterial damage can produce a tooth abscess in the soft pulp.
Destruction of the pulp cavity and its contained blood vessels leads to death of the odontoblast layer and eventual death of the tooth.
Periodontal disease is caused by the accumulation of bacterial plaque (calcified food and bacterial debris) in the gingival sulcus. This excites inflammation in the adjacent gum, which gradually forces the gingiva away from the tooth, widening and damaging the gingival sulcus; deep periodontal pockets form in which food particles and bacteria become trapped. Bacterial proliferation then leads to further inflammation of the gum (gingivitis) and the periodontal ligament (periodontitis).
Persistent periodontitis destroys the periodontal ligament and the tooth becomes loose in its socket.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here