Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Alagille syndrome (ALGS) is a rare multisystem disorder and one of the most frequent inherited causes of cholestatic liver disease in children. Traditionally, ALGS has been characterized by the presence of at least three of the following five principal clinical features: bile duct paucity and/or cholestasis, cardiac involvement (typically peripheral pulmonary artery stenosis), skeletal anomalies (often butterfly vertebrae), ophthalmological involvement (usually posterior embryotoxon), and/or characteristic facies. However, since 2000, the phenotypic spectrum has expanded and now includes both renal and vascular anomalies as disease-defining characteristics. The multisystem nature of ALGS stems from heterozygous mutations in one of two genes in the Notch signaling pathway, which is fundamental in early development; JAGGED1 (JAG1) mutations are reported in up to 94% of cases, and mutations in the NOTCH2 receptor are found in 1% to 2%. De novo mutations account for approximately 60% of ALGS cases.
There is considerable variation in the clinical course and ultimate prognosis of liver disease in ALGS. ALGS typically manifests as jaundice, high gamma-glutamyltransferase cholestasis, and failure to thrive in infancy. Remarkably, many of these patients achieve spontaneous resolution of their cholestasis during the first 5 years of life; others develop progressively worsening cholestatic liver disease and eventually biliary cirrhosis, necessitating liver transplantation (LT). There are no known genotypic predictors of hepatic outcome in ALGS. There have been some attempts to identify clinical parameters, present in early childhood, to predict eventual hepatic outcomes in children with ALGS. In a multicenter international study of 144 ALGS patients, Mouzaki et al. identified a serum total bilirubin cutoff of 3.8 mg/dL (65 mmol/L) between 12 and 24 months of life as a threshold to distinguish between children with good and poor hepatic outcomes later in life, where a poor outcome was defined by the need for biliary diversion or LT.
Based on historical data from heterogenous cohorts of ALGS patients, LT is indicated in 20% to 50% of pediatric cases. More recent data suggest that up to 70% of children presenting with severe cholestasis require LT during childhood. Indications for transplantation in children with ALGS can be categorized into two groups: (1) those with one or a combination of complications secondary to chronic cholestasis, including refractory pruritus and disfiguring xanthomas, failure to thrive, fat-soluble vitamin deficiency, and/or recurrent pathological fractures; and (2) those with biliary cirrhosis and/or evidence of portal hypertension. The former represent the most common indication for LT in ALGS. Transplantation for pruritus as the primary indication in early childhood remains somewhat controversial because symptoms can resolve or stabilize in select children by school age.
Determining the optimal timing of referral and LT listing in ALGS is another challenge for clinicians, and several factors should be taken into consideration, particularly the extent and degree of systemic disease involvement. In this chapter, we will provide an overview of challenges and management issues associated with LT in children with ALGS.
Growth impairment and nutritional deficiencies are an ongoing and significant issue in infants and children with ALGS, with a reported prevalence of 50% to 87%. The underlying causes of growth deficits in ALGS are multifactorial and stem from a combination of increased energy expenditure, decreased absorption of fats and fat-soluble vitamins, and the presence of congenital cardiac or renal disease.
Attention to the nutritional and growth status of ALGS transplantation candidates is critical and has significant prognostic implications. Data from the Studies of Pediatric Liver Transplantation (SPLIT) registry showed that more than half of ALGS transplant candidates had a documented height (66%) and weight deficit (63%) at the time of transplantation listing in comparison with only 22% and 21% of children with biliary atresia (BA), respectively. Surprisingly, only 24% of ALGS patients received perioperative nutrition support. These data highlight a need for more aggressive nutritional management in ALGS patients during the pre-transplantation evaluation and waiting list period.
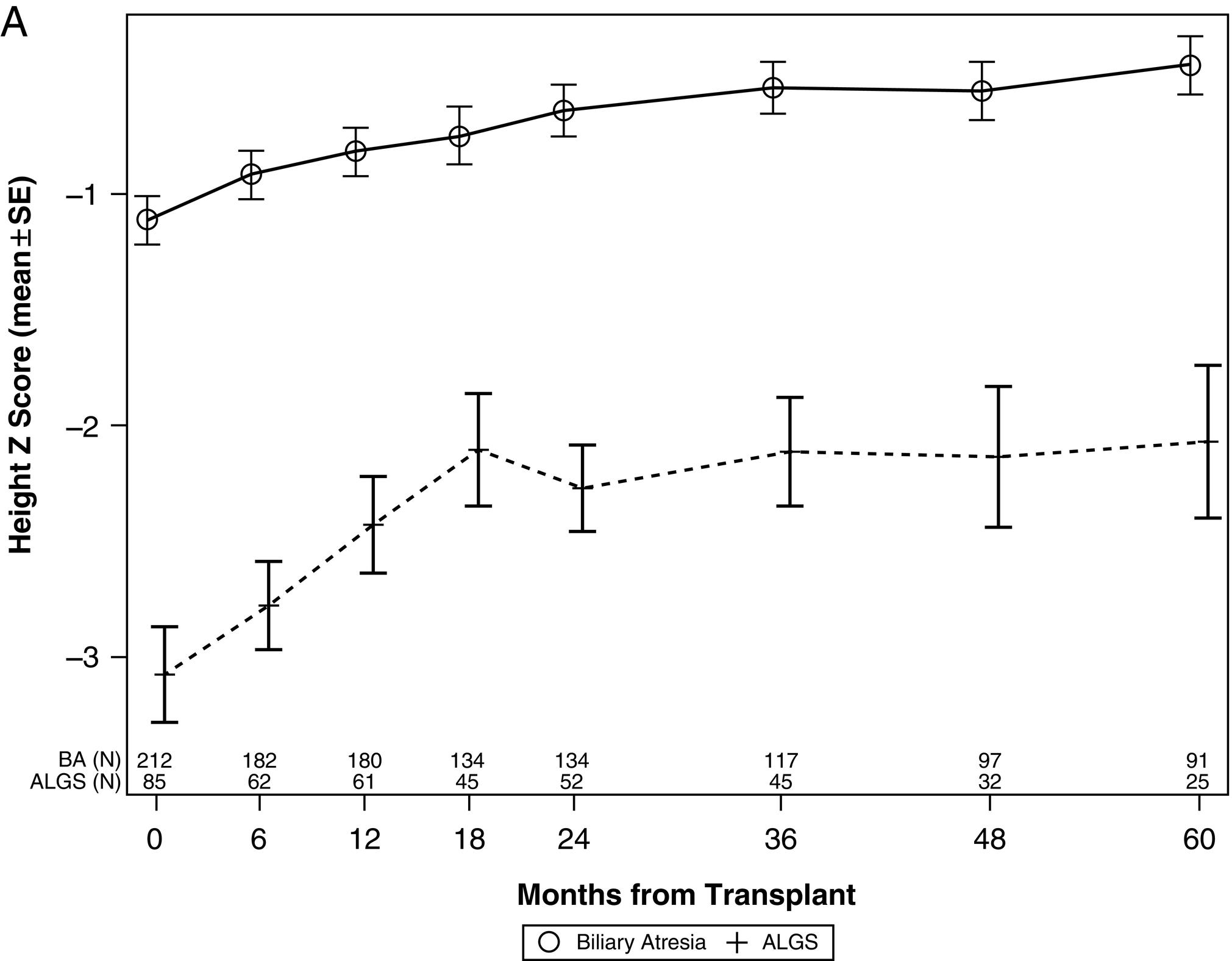
Children with ALGS exhibit catch-up growth following successful LT; however, pre-transplantation growth faltering is not completely reversed. The SPLIT study of LT in ALGS demonstrated that in comparison with children with BA, a significant deficit in linear growth persisted in the ALGS group throughout the 5-year follow-up period. This may reflect an intrinsic limitation of growth potential in ALGS. However, this study also showed that ALGS patients experience greater growth velocity than age-matched BA counterparts in the first 24 months post-LT ( Fig. 35.1 ). These data suggest that growth failure is not an inevitable feature of ALGS and can certainly be ameliorated in those with liver disease, also implying that growth failure be considered an important indication for LT in ALGS.
Cardiac anomalies are a hallmark feature of ALGS, with a reported prevalence of 94%. The extent and pattern of cardiac involvement is highly variable, ranging from an asymptomatic murmur, or peripheral pulmonary stenosis (PPS), to more complex intracardiac anomalies, such as tetralogy of Fallot, with or without pulmonary atresia. PPS accounts for approximately 73% of reported cardiac anomalies in ALGS and can lead to pressure or volume overload of the right ventricle (RV), RV hypertrophy, and, in severe cases, right-sided heart failure.
Evaluation of prospective LT candidates with ALGS requires careful assessment of the cardiovascular system, with special emphasis on the functional capacity of the RV. Elevated right heart pressures and hypertrophy impair the heart’s ability to respond to the marked preload changes when the inferior vena cava is clamped during transplantation surgery. To address this, the King’s College Group suggested a cardiac catheterization and dynamic stress test with dobutamine to simulate perioperative conditions in the LT evaluation of ALGS patients. A dynamic stress exercise (DSE) induces peripheral vasodilation and increases cardiac output, thereby mirroring the conditions of graft reperfusion. If the transplant candidate achieves a cardiac output of more than 40% during DSE, the patient’s cardiac reserve is considered sufficient for transplantation. This approach is clearly physiologically relevant but may not be required for all ALGS LT candidates and can be targeted toward those considered at risk of increased RV pressures based on echocardiography and expert pediatric cardiology opinion.
Stenosis and/or hypoplasia of the pulmonary arteries may contribute to post-reperfusion hemodynamic instability and adverse post-transplantation outcomes in ALGS patients. Again, expert pediatric cardiology input is crucial to manage cardiac anomalies before LT. Cardiac interventions reported in ALGS patients before LT include balloon valvuloplasty and stenting, as well as more invasive cardiac surgery. In the presence of complex intracardiac anomalies, the risk-benefit ratio of LT must be carefully weighed, and detailed pre-operative screening should be undertaken by an experienced multidisciplinary team. In the setting of very complex cardiac involvement and advanced liver disease in ALGS, especially in infancy, palliation is not unreasonable. Combined heart-LT has been reported in only a few ALGS cases.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here