Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Conducting airway research is difficult, partly because of the low incidence of truly difficult airways and the ethics of exposing patients to unnecessary risk (e.g., a randomized controlled trial [RCT] in front-of-neck access).
Although a meta-analysis of RCTs may be regarded as the epitome of research (level 1a), there may not be enough RDTs in a certain field to provide meaningful answers.
Surrogates for airway management outcomes are commonly used (time to intubation or percentage of glottic view obtained), and although these are useful scientific tools, they may not tell you if a technique or a device is appropriate for your clinical practice.
Many similar devices are available, from supraglottic airways (SGAs) to video laryngoscopes, but similarities in form and function do not necessarily equal similarities in clinical effectiveness, and each device must be evaluated carefully.
When managing the difficult airway, the performance of any airway device must be considered along with the performance of the individual and the entire team under stress (so-called human factors).
No matter how effective a device has been demonstrated to be in a study, it can only be effective if the user has been trained to use it.
Many national societies have generated guidelines for the management of difficult airways; these have been constructed using different methodologies and must be considered in the way they were constructed.
There is much to be learned from the analysis of near misses, critical incidents, or closed claims even though these may not be hypothesis-driven research in their own right.
Although pediatric research can be difficult practically and ethically, good research in the field of pediatric airway management can be conducted and is important in informing best practice. Just as studies among devices must be extrapolated with care, so must device or procedure efficacy between adults and children and vice versa.
Novel pathogens may force researchers to extrapolate from preexisting knowledge until necessary research can be carried out, as happened with COVID-19 disease.
Airway research is a broad topic, from the physiology of why airways tend to obstruct when consciousness is lost to evaluation of the many different devices available for airway management today. The COVID-19 pandemic brought strategies in airway management to the fore and forced clinicians to combine various strands of research to deliver coordinated patient care. This was not always the case. The paper describing the Macintosh laryngoscope occupied less than one printed page and, although it included a description and picture of the device, it did not include any patients. Similarly, Archie Brain’s initial report of the (classic) Laryngeal Mask Airway (cLMA) in the British Journal of Anaesthesia reported its use in just 23 patients. New devices have spawned new terminology, such as oropharyngeal leak pressure (OLP) and percentage of glottic opening (POGO), and ways to measure them. New devices have also given rise to new strategies for dealing with intubation difficulties. However, while this problem-based or reactionary approach to airway research is appealing, a structured approach is better. There is a graded hierarchy when assessing the evidence provided in a study ( Table 51.1 ). This is separate to the grade assigned to recommendations based on evidence ( Table 51.2 ), as used in some airway guidelines.
| Level of Evidence | Type of Study |
|---|---|
| 1a | Systematic review of RCTs |
| 1b | Single RCT |
| 1c | All-or-none study (i.e., when all patients died before the therapy became available, but some now survive on it; or when some patients died before the therapy became available, but none now die on it) |
| 2a | Systematic review of level 2b cohort studies |
| 2b | Single cohort study or low-quality RCT |
| 2c | Outcomes studies that investigate outcomes of healthcare practices using epidemiology to link outcomes (e.g., quality of care, quality of life) with independent variables such as geography, income, lifestyle, etc. |
| 3a | Systematic review of level 3b studies |
| 3b | Single case-control or historical-control study |
| 4 | Case report or case series |
| 5 | Expert opinion or ideas based on theory, on bench studies, or first principles alone |
| Grade | Level of Evidence Available |
|---|---|
| A |
|
| B |
|
|
|
|
|
| C |
|
|
|
|
|
| D |
|
|
Randomized controlled trials (RCTs) are regarded as the best way to determine whether a given outcome is a result of a specific treatment (cause-effect relationship) or whether it is a random effect. RCTs can be combined to improve the evidence base. A systematic review is an attempt to answer a specific research question by gathering all evidence that meets clearly predefined criteria. Part of that process is an explicit methodology, which would produce the same results if it was reapplied by other researchers. Thus, bias is minimized but it can never be eliminated entirely. Findings of included studies should then be presented. This further reduces the risk of bias or the influence of the authors’ opinions as may be unintentionally present in a narrative review. An ability to adequately assess the methodology of a study is a vital component of deciding how and when to apply results to clinical practice.
Meta-analysis is a statistical procedure that integrates the results of several independent studies deemed combinable to provide a more objective assessment of the available evidence. It reports an effect between two groups (e.g., intervention and control), which will have both magnitude and direction. Poorly conducted meta-analyses will produce misleading results, particularly if study designs, within-study biases, variation across studies, and reporting biases are not considered.
Once eligible studies have been identified, a measure of the treatment effect with its 95% confidence intervals (CI) of each individual study is made (odds ratios [ORs] or relative risks [RRs] are usually included), then an overall effect as a weighted average is calculated. Greater emphasis is given to more informative studies by using a weighting factor (inverse of the variance). This means larger studies, with smaller standard errors, have a greater impact on the overall results than smaller studies.
Results of a meta-analysis are presented in a forest plot ( Fig 51.1 ). The squares on the horizontal lines are the component studies, with their confidence intervals, plotted on either side of a line of no effect depending on the individual study result. The overall estimate is presented at the bottom of the plot in the shape of a diamond. The center of the diamond represents the pooled point estimate, and the horizontal extremes represent the confidence interval. A long, thin diamond represents a wide confidence interval with the potential for a great deal of uncertainty, whereas a broad, short diamond shows that the evidence for effect is strong. Regardless of shape, if the diamond crosses the line of equality/no effect, then there is good evidence that there is no observed difference (absence of effect).
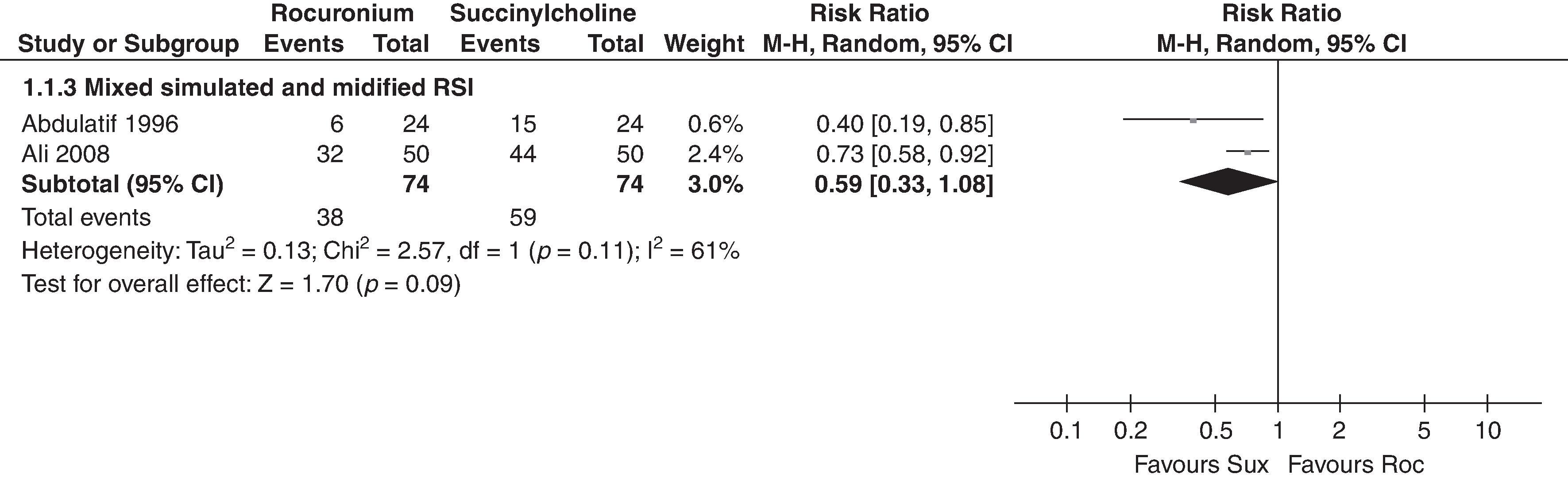
Meta-analyses should also include an assessment of heterogeneity, which asks whether it is reasonable to pool the data from separate studies. If the CI for the results of individual studies (the horizontal lines) have poor overlap, this generally indicates the presence of statistical heterogeneity. This is a clinical judgment, and although it is possible to report a statistical test for heterogeneity ( p < 0.05 may indicate a problem), most meta-analyses are not sufficiently powered to allow its detection. Meta-analyses are not perfect, and misleading conclusions can be generated. Various tools exist to help readers assess systematic reviews in a methodical way.
Systematic reviews sit at the top of the evidence-based hierarchy but are not guaranteed to provide clear answers. For example, the meta-analysis of the Pentax AWS showed no clinical benefit when using this video laryngoscope despite including over 1800 patients. This is contrary to the demonstration of its effectiveness in 293 patients with previously recorded or likely-to-be difficult airways.
Similarly, a meta-analysis can only be conducted if there is more than one sufficiently sized RCT randomized controlled trial to allow meaningful conclusions to be drawn. One review assessing “the safety and effectiveness of a flexible intubation scope (FIS) used for tracheal intubation in obese patients (body mass index [BMI] >30) with other methods of intubation” concluded that “More primary research is needed.” Another Cochrane Review sought to establish whether succinylcholine or rocuronium provided better intubating conditions. It “found no statistical difference in intubation conditions when succinylcholine was compared with 1.2 mg/kg rocuronium; however, succinylcholine was clinically superior, as it has a shorter duration of action.” The review clearly stated that it did not consider sugammadex, giving it a clear methodology but making it more difficult to apply to clinical practice and studies comparing the intubating conditions provided by the two agents continue.
Cochrane Reviews are systematic reviews, made available in the Cochrane Library ( www.cochranelibrary.com ). The Cochrane Library is the world’s largest independent provider of systematic reviews. Searching the library with the Medical Subject Headings (MeSH) keywords “Airway Management” revealed 199 reviews (date of search: March 21, 2021). Cochrane produces a variety of resources, including a workbook that acts as the official guide for the preparation of a Cochrane Review.
A comprehensive literature review is a common place to start any research investigation; however, all of the available databases, some of which are outlined here, are subtly different and the benefit of expert advice from a qualified medical librarian cannot be underestimated.
Medline is the National Library of Medicine journal citation database started in the 1960s, with records going back to 1946. It currently indexes over 5200 journal titles. PubMed ( https://pubmed.ncbi.nlm.nih.gov ) has been available since 1996. It contains over 31 million references and includes the Medline database with additional entries. EMBASE is a database maintained by Elsevier, which indexes over 8500 journals from over 95 countries, including all Medline titles. There are other databases—for example, EBSCO (Elton B. Stephens Company) and Continuing Education Module Tutorial (CINAHL; cumulative index to nursing and allied health literature). Different institutions and libraries will have permissions to access different databases. PubMed is freely accessible through the Internet, complete with a variety of online tutorials and videos to improve search results. The TRIP database (originally T urning R esearch I nto P ractice, https://www.tripdatabase.com ) claims to be a clinical search engine delivering research evidence to support practice and care. However, to derive most benefit from these search engines, users must understand how the algorithm scores and weights articles as this will influence the order in which search findings are presented.
Other free academic search engines (e.g., Google Scholar at http://scholar.google.com ) offer a wide variety of features to both researcher and author, including citation alerts and export options to bibliography management software. A study into the ranking algorithm suggested that the citation count was the most important feature, meaning that highly cited articles appear earlier in search results than those cited less frequently.
Open-access journals are separate from individual articles, which are made open access by a specific journal. Authors may be required to pay an article processing charge, which covers the cost of the review and editing process, and the article is then free to download for all users. These journals may or may not be listed in all bibliographic databases, but there is now a directory of open-access journals ( http://doaj.org ), which had indexed 3599 journals under its Medicine heading as of March 2021. Readers should always check how open-access articles are selected for publication by different journal titles because the quality of peer review can vary.
Observational or longitudinal studies that look at the incidence of infrequent events can be conducted, either by looking at the incidence of device failures (e.g., a supraglottic airway [SGA] or video-assisted laryngoscope [VAL] ) or the incidence of a rare condition in its own right. These types of investigations have advantages and disadvantages. De Jong’s study looked at 11,035 patients in the operating room and 1400 in critical care. In patients with obesity, the incidence of difficult intubation (DI) was twice as frequent in the intensive care unit (ICU) as in the operating room (16.3% vs 8.2%). However, the study defined obesity as being a BMI >30 (giving an incidence of 20%), and difficulty was defined as three or more laryngoscopic attempts to place the endotracheal tube, as lasting longer than 10 minutes using conventional laryngoscopy, or both. Other cohort studies report different findings based on their inclusion criteria. The incidence of anticipated airway difficulty was 2.2% in the Fourth National Audit Project (NAP4) of the Royal College of Anaesthetists and the Difficult Airway Society study. Nørskov’s study suggested an incidence of difficult tracheal intubation of 1.86%. The vast majority of these were unanticipated, whereas there were a high number of false-positive DIs. Again, without considering the methodology, results can be confusing.
With a relatively low incidence of absolute difficulty, studies must include very large numbers of people to ensure the inclusion of enough patients with genuinely difficult airways. This problem is compounded when considering patients with predicted difficult airways. Most patients anticipated to be difficult were not (75%, 700/929). Therefore, when using airway assessment as an indicator of difficulty, a large number of patients who are not difficult to intubate will also be included; this naturally skews the results of any investigation.
Difficulty in intubation may be overcome (VAL or flexible scope intubation [FSI]) or circumvented (waking the patient up). Difficult face mask ventilation (FMV) presents an anesthetist with a much more immediate problem. Kheterpal’s group looked at factors predicting difficult mask ventilation and difficult laryngoscopy. They found several factors (age ≥46, BMI >30, male sex, Mallampati III or IV, neck mass or radiation, limited thyromental distance, sleep apnea, presence of teeth or beard, thick neck, limited cervical spine mobility, limited jaw protrusion) to be independent risk factors for difficult FMV and difficult laryngoscopy (actual incidence 0.4%). This is a useful finding in and of itself, but it is featured here because to achieve this the group considered 492,239 cases across four institutions, taking 6 years. Such effort is a salutary warning to anyone who wishes to conduct meaningful airway research.
Many observational studies of SGAs (including Brain’s in 1983) included relatively small numbers of patients. , , Pandit, however, recently reiterated the risks of concluding that a device was effective based on a small study, suggesting that when considering an SGA, we should accept an upper 95% CI for failure of around 2.5% (based on the failure rate of the cLMA of <1%). , He described how to see at least one failure of a device using these parameters a trial must include around 250 observations/device insertions. Requiring this many observations of a device to define its likely success makes any such observational trial more difficult to perform, but it must be considered when reviewing the available evidence for a device.
This does not mean that small studies are not of value. Any preliminary investigation should feature small numbers so that larger studies can be developed, effectiveness and safety ascertained. This small initial study approach can also be seen in the development of the videolaryngoscope, where the use of the GlideScope was reported in just one case, or in apneic oxygenation using high-flow nasal oxygen (HFNO) where the initial cohort study considered its use in 25 patients.
Although the ideal design for any trial is an RCT, the fundamental tenets of such a study, including a clearly defined question, two nearly identical groups, control of everything except the variable under investigation, and researchers blinded to the intervention, can be virtually impossible to create in the field of airway management. Blinding operators in equipment trials is virtually impossible. That knowledge may (subconsciously) influence their behavior with it. Similarly, many studies of equipment are, at least in some way, funded by the equipment manufacturers (e.g., the provision of cheap or at-cost airway devices). This again may (unintentionally) influence an investigator’s opinions and induce reporting bias. Even the absolute benefit of an RCT given its cost versus the benefit derived has been questioned.
The Difficult Airway Society (DAS, United Kingdom) devised the ADEPT process (airway device evaluation project team). The authors sought to address the key questions regarding the purchasing of an airway device based on evidence. The group concluded that “All airway-related equipment under consideration must fulfil the minimum criterion, that there exists for it at least one source of level 3b trial evidence concerning its use, published in peer-reviewed scientific literature.” This is completely different standard than that required by regulatory bodies to approve the sale of airway devices.
European devices are governed by European Union (EU) law and, as such, must all carry a Conformité Européenne (CE) mark. For medical devices this means that it meets the standards of EU Directive 93/42/ EEC.
In the United States, this governing role falls to the US Food and Drug Administration’s Center for Devices and Radiological Health (CDRH), and in Australia it is within the remit of the Therapeutic Goods Administration within the Department of Health.
Despite the ADEPT recommendations for level 3b evidence, many clinicians have found observational studies of both SGAs and VALs beneficial in terms of determining whether or not a device should be used in clinical practice. Noble as the ADEPT standards are, at the time of writing there have been no published studies using this methodology.
A well-crafted research question does not however lead to an easy-to-conduct airway study. Considering the technique of an FSI illustrates the problems that can be faced when conducting airway research. An FSI is a recognized way of facilitating tracheal intubation in a patient known or anticipated to be a DI, and guidelines about its conduct have recently been released. However very few of the recommendations were derived from the highest levels of evidence (see Table 51.2 ); most were recommendations at Grade D (Expert opinion, inconclusive studies). However, constructing the study as to how FSI might be most effectively conducted or even taught is difficult. Fig. 51.2 shows the various aspects that must be considered when performing an awake FSI. In an ideal study, all parameters in Fig. 51.2 would be standardized, barring the one under investigation. However, the number of features to be standardized is high, making the perfect study difficult to design and conduct.
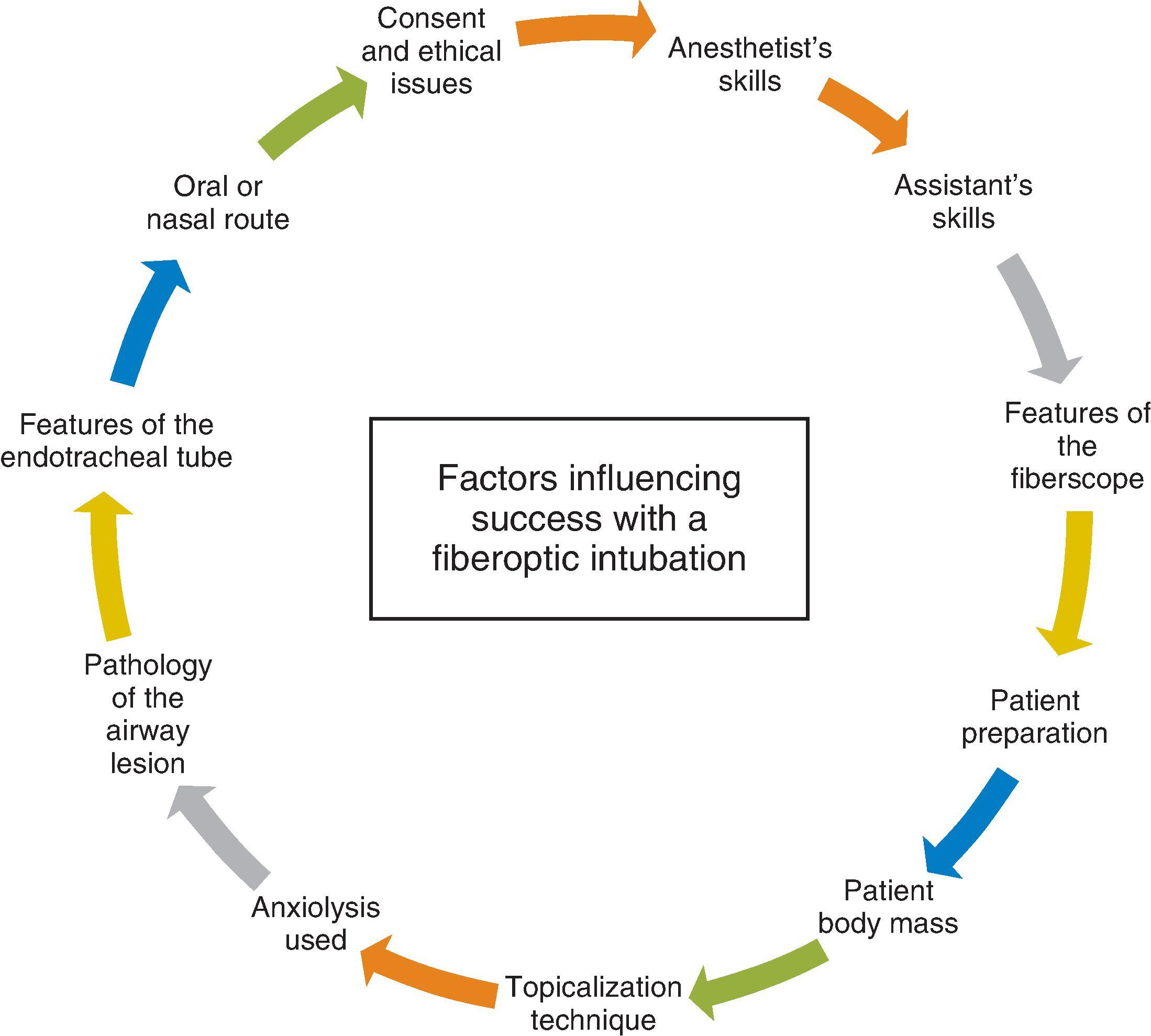
The number of awake FSIs that should be performed to make an individual competent varies among investigators. However, skilled FIS manipulation requires many more FSIs than those required to make the laryngoscopist just competent. The immediate assistance available to the laryngoscopist will also have a role in the success of the procedure, and although this can be standardized (e.g., the same assistant used in every case or a standardized operating procedure for the assistant), it is almost impossible to control for all such external factors that will potentially impact the study findings.
The BMI or lean body mass of the patient will determine the total safe dose of local anesthetic that can be used, but this may limit the topicalization techniques available to use for some patients. Does this mean that study protocols must accommodate these different techniques? If so, are all the investigators equally trained in each technique? Even the scope chosen may have some bearing on the success of the procedure. The nature of the tube used will impact the success of an awake FSI, but at least some part of tube selection may be influenced by the intended surgical procedure. The underlying pathology will also determine both route chosen and difficulty of the procedure. A new technique for topicalizing the airway will fail if all the patients in the intervention group have complex airway lesions that take time and skill to negotiate. Given that it is unlikely that anyone’s practice will be able to standardize for the nature of the airway lesion, it is easy to see how the study of an awake flexible technique will be compromised by the impossibility of a controlled study design. One option might be to investigate its use in an anesthetized patient; however, the practicalities of FSI in anesthetized patients are different than that in awake subjects. This makes extrapolating findings from a study on asleep patients directly to an effective awake technique very difficult.
It is also worth considering the effective outcome measures. In terms of adequacy of topicalization, outcome measures such as success of the procedure (ETT through the cords) must be coupled with measures of outcome such as patient tolerance and compliance. This means a standardized premedication/sedation regimen must be employed; however, this may be influenced by the underlying pathologic condition. Similarly, to give unbiased assessment of the technique studied, awake patients’ anxiety levels also must be recorded and included in any study analysis. One option might be to test and assess these factors in patients with known normal airways. Although this has been done on medically qualified subjects wishing to learn the technique, , there are complications associated with it, making ethical approval and subject recruitment more difficult.
The perfect airway study is, therefore, immensely difficult to design. Given the actual low incidence of difficult airways, many studies rely initially on making extrapolations from patients with normal airways or the use of surrogate endpoints for procedure effectiveness.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here