Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The last 25 years has seen enormous growth in our knowledge of skin function, with new subspecialties of cutaneous biology emerging during that time, not least of which is cutaneous neuroendocrinology. The position of the skin, our largest organ by weight (≈12% of total body weight) and extent, and as a sensor of the periphery has prompted some researchers to describe skin as our “brain on the outside.” Although now over a decade old, we think that the best single discussion on the function of skin can be found in the multiauthor discussion review, “What is the ‘true’ function of skin?” From an anatomic and physiologic perspectives alone, it is clear that skin is truly a biologic universe in that it incorporates all the body's major support systems—blood, muscle, and innervation, and including immunocompetence, psychoemotional reactivity, ultraviolet radiation sensing, and endocrine function. These functions participate in the homeostasis not just of skin and its appendages but also of the entire mammalian body. Although this view was initially polemic to some, particularly many in the endocrinology community, it now appears self-evident given that the skin occupies such a strategic location between the noxious external and biochemically active internal environments. Thus, skin can rightfully be expected to be critical in preserving the constancy of our body's internal environment. Despite exquisite adaptations driven from a raft of key evolutionary selective pressures for life on an ultraviolet radiation (UVR)–drenched terrestrial planet, still skin conditions still rank as the fourth leading cause of nonfatal disease burden, with this burden rising still further as we age.
It may be impossible to describe the true function of skin, but rather we should ask “Is there anything that the skin can't contribute to?” Research on the skin's remarkable stress sensing, much of which is transduced via its equivalent of the hypothalamic-pituitary-adrenal and thyroid axes, provides us with an opportunity to assess how age may affect these key axes in terms of skin physiology. Well-nourished and UVR-protected skin and associated integumental adnexa exhibit truly remarkable resilience to chronologic (or intrinsic) aging. In this chapter, we will examine the structural changes to the skin as a consequence not only of this type of aging, but will also examine the contributors to so-called extrinsic aging (e.g., UVR, trauma, chemical) and how both types of aging present challenges to skin integrity.
The two main global giveaways of our lost youth are most readily detected by changes to our skin, including so-called wrinkling and changes to the skin's principal appendage, the hair follicle, especially canities or common graying and hair thinning and baldness. Increasingly, we appear to be less and less keen to sport this universally recognized aging phenotype. Our expectations for the extension of optimal functioning continue to grow well into our 70s and beyond. This is not unreasonable because life expectancy in the West is expected to be 100 years of age in the next decade, with further extensions to 120 years in the decades beyond 2025. The implications of this demographic change for skin aging, which has no precedent in human history, has even more significant implications for women because they will spend up to half of their lives postmenopause, during which falling estrogen levels adversely affect skin integrity and function. Aspirations for healthy and functional aging continue to drive a rapidly expanding skin and hair care market that brings increasingly sophisticated cosmetics and cosmeceuticals, pharmaceuticals, and surgeries to the palette of options to assuage our vanities, but also to aid our increasingly dry and itchy, infection-prone, immune-unstable skin, with its vascular complications and increasing risk of cutaneous malignancy.
Given its strategic interface position on the body, the skin is uniquely subject to a wide range of aging drivers, not only to intrinsic (chronologic) aging, which are generally under genetic and hormonal influences, but also to extrinsic aging caused by environmental factors, principally including UVR, smoking, diet, chemicals, and trauma. UVR-induced aging is so powerful that it has been designated separately by the term photoaging . The sheer differential impact of the latter can be seen when comparing sun-protected buttocks skin with sun-exposed hand or facial skin in an older, but active, white adult. Both types of aging have their distinct morphologic and histologic features, with only some overlapping biologic, biochemical, and molecular mechanisms. Interestingly, analyses of composite facial images created from women who were considered to look young or old for their age have reported that changes to the structure of subcutaneous tissue were also partly responsible for this perceived effect. Moreover, when the heritability of these appearance traits (e.g., perceived age, pigmented age spots, skin wrinkles, sun damage) was analyzed, it was reported that these features were more or less equally influenced by genetic and environmental factors.
Finally, we will focus here on reevaluating some older accepted data of skin aging, including its “yin-yang” relationship to the sun, but also will see how cell, molecular biologic, and other discoveries may help develop approaches to maintain this evolutionarily, highly selected for organ at optimum function during our ever-increasing longevity.
The very slow process of intrinsic aging varies among populations, between individuals of the same ethnicity, and between different sites on the same individual. This type of aging is essentially only visible at old age and is characterized by unblemished, smooth, pale(r), drier, less elastic skin, with fine wrinkles and somewhat exaggerated expression lines (reflecting additional subcutaneous changes). The process of intrinsic aging falls into two categories—one engendered within the tissue itself, including reductions in dermal mast cells, fibroblasts, and collagen production, flattening of the dermal-epidermal junction, and loss of rete ridges, and one caused by the influence of aging in other organs (e.g., age-related hormonal changes). Flattening of the epidermis is perhaps the most striking feature of intrinsically aged skin. This is caused by a loss of reciprocal interdigitation of capillary-rich dermal papillae, a likely consequence of reduced nutrient support by the vascularized dermis to the avascular epidermis. Together these are thought to contribute to the increase fragility of intrinsically aged skin in the very old. Intrinsically aged epidermis is also controlled by progressive telomere shortening, compounded by low-grade oxidative damage to telomeres and other cellular constituents. A study of normal human epidermis has established that progressive telomere shortening associated with aging is characterized by tissue-specific loss rates.
Given that the regulation of intrinsic aging is largely beyond our influence (e.g., short of hormone supplementation, albeit with associated health implications), significant consideration is being directed toward the prevention and treatment of extrinsic aging-associated changes to skin structure and appearance. The greatest source of extrinsic aging comes from accumulated sun (unprotected) exposure called photoaging and so is largely confined to the face, neck, and hands and less so to the lower arms and legs. It has been estimated that over 80% of aging of the face is due to chronic UVR exposure, whereas acute UVR exposure of the skin will cause sunburn, tanning, inflammation, immunosuppression, and damage to the connective tissue of the dermis. It should be noted that the impact of environmental factors on so-called extrinsic aging cannot be completely separated from how the skin will respond to chronologic aging, given the significant impact of exogenous factors on how skin physiology is regulated (e.g., pro-oxidant and antioxidant influences on cell turnover via neuroendocrine and immune biologic response modifiers). The characteristics of extrinsically aged skin include coarse wrinkling, rough texture, sallow complexion with mottled pigmentation, and loss of skin elasticity. Much of this change can be ascribed to the effects of UVR-induced photoaging.
Photoaging is caused by solar irradiation. At the earth's surface, sunlight consists mostly of infrared light, with 44% visible light and only 3% UVR (when it is cloudless and the sun is directly overhead). The earth's atmosphere blocks the vast majority of the sun's UVR (100 to 400 nm). UVR reaching our planet's surface (and so potentially our skin and eyes) consists of more than 95% UVA (315 to 400 nm) and about 5% UVB (280 to 315 nm). Germicidal UVC (100- to 280-nm) radiation is extremely hazardous to skin but is completely absorbed by the ozone layer and atmosphere, fortunately. Another important consideration is the ratio of UVA to UVB reaching our skin, which depends on the latitude (and thus the height of the sun), season, and time of day. More UVB is present is midday sun during summer than at other times of the day or year. Most studies in the literature have used solar-simulated radiation with a spectrum (UVA/UVB ratio < 18, and often much lower) as a proxy for the noon summer sun on a clear day, although a more representative real-world UVA/UVB ratio is 25.
Although researchers believe that the deeply penetrating UVA damages connective tissue in the dermis and also increases risk for skin cancer, UVB only penetrates as far as the epidermis, where it can cause sunburn, tanning, and photocarcinogenesis. UVB is the major cause for direct DNA damage and induces inflammation and immunosuppression. UVA is thought to play a greater role in skin photoaging given its greater abundance in sunlight and the greater average depth of penetration into the skin's dermis and epidermis. In pale-skinned whites, the first signs of extrinsic aging on exposed sites are already apparent by 15 years of age, whereas on nonexposed sites, they are not apparent until age 30 years. Worryingly, the pursuit of a tan remains a high priority in Western culture, associated as it is with ever-rising rates of skin cancer and prematurely aged skin. Moreover, the increasing use of sun protection, such as topical sunscreen cream with so-called sun protection factor (SPF) ratings, has not come without problems. For example, stated protection levels can require the topical application of an unrealistic (i.e., cosmetically unacceptable) amount of cream, and users are often misguided in thinking that a single suboptimal application of a nonwaterproof sunscreen permits them to increase their time in the sun significantly, including with intervening swims. It has recently been proposed that even when applied correctly, sunscreen use will result in suberythemal exposure, and we have still to learn more about the ideal ratio of UVB/UVA protection needed to improve long-term photoprotection outcomes.
In addition to the negative effects of exposure to UVA and UVB (e.g., induction of melanoma and nonmelanoma skin cancers, cataract formation, systemic immunosuppression that may reactivate latent viral infection, skin aging), it should be remembered that exposure to UVB radiation also has positive effects. These include suppression of autoimmune reactivity, mood enhancement via endorphin production, and vitamin D synthesis to aid calcium homeostasis. There is increasing concern about the rising incidence of vitamin D deficiency or at least its insufficiency.
Clinically, photoaged skin is characterized by deep wrinkles, laxity, roughness, a sallow or yellow color, increased fragility, purpura formation, mottled pigmentary changes, telangiectasia, impaired wound healing, and benign and malignant growths. The degree of accumulated sun exposure determines the magnitude of the associated skin changes. The mechanisms through which UVR induces accelerated aging are discussed later in this chapter. The second most powerful inducer of extrinsic aging is cigarette smoking.
Smoking is an independent risk factor for premature facial wrinkling after controlling for sun exposure, age, gender, and skin pigmentation. The relative risk of moderate to severe wrinkling for current smokers was found to be 2.3 for men and 3.1 for women. There is a clear dose-response relationship, with facial wrinkling increasing in individuals who smoke longer and with increasing numbers of cigarettes daily. When smoking and excessive sun exposure combine, the effect on wrinkling multiplies in that the risk of developing wrinkles increases to 11.4 times that in a normal age-controlled population. The exact mechanism for the aging effects of smoking is poorly understood. The effects may be topical, due to the drying or irritating effect of smoke on the skin; systemic, with induction of matrixmetalloproteinase-1 (MMP-1) ; or by negatively affecting cutaneous microvasculature. Specifically, the dermal microvasculature is constricted by acute and long-term smoking, the severity of which is independently related to duration and intensity of exposure to smoking.
Pigmentation of the skin is protective against the cumulative effects of photoaging. When populations with Fitzpatrick classification types I to VI (ranging from always burn never tan to always tan and never burns) were compared, it was found that those with skin type VI (black) show little difference between exposed and unexposed sites. Moreover, the much higher rates of skin cancer rates among whites compared with African Americans reflects the significant protection from UVR damage that pigmentation provides (up to 500-fold). The appearance of photodamaged skin differs for those with skin types I and II (red hair, freckles, burns easily) and those with skin types III and IV (darker skin, tans easily). The former tend to show atrophic skin changes, but with fewer wrinkles, and focal depigmentation (guttate hypomelanosis) and dysplastic changes, such as actinic keratoses and epidermal malignancies. In contrast, those with types III and IV skin develop hypertrophic responses, such as deep wrinkling, coarseness, a leather-like appearance, and lentigines. Basal cell carcinoma and squamous cell carcinoma occur almost exclusively on sun-exposed skin of light-skinned people. A large and statistically robust study evaluated skin thickness in chronologic aging and photoaging conditions; it was reported that although increases and decreases in skin thickness can be seen in different body sites, there was no general relationship between skin thickness and age.
Thus, it appears that the epidermis thins with age at some body sites, such as the upper inner arm and back of the upper arm, but remains constant at others, such as the buttocks, dorsal forearm, and shoulder. This variation is clearly not accounted for by sun or environmental exposure alone. Differences in study method, population, and body site likely account for different results reported in different studies. Although epidermal thickness appears to remain largely constant with advancing age, there is some variability in keratinocyte shape and size with age, specifically that these cells become shorter and flatter in contrast to an increase in corneocyte size, potentially as a result of decreased epidermal cell turnover with age. Wrinkling in Asian skin has been documented to occur later and with less severity than in white skin.
The epidermis is composed of an outer nonviable layer called the stratum corneum, and the bulk of the viable epidermis consists primarily of keratinocytes (90% to 95% of cells), with smaller populations of Langerhans cells (2%, or 1 for every 53 keratinocytes), melanocytes (3%, or 1 for every 36 with viable keratinocytes, the so-called epidermal melanin unit), and Merkel cells (0.5%).
The stratum corneum is the body's principal barrier to the environment and also plays a major role in determining the level of cutaneous hydration. Its structure is often described by the bricks and mortar model, consisting of protein-rich corneocytes, which are embedded in a matrix of ceramides, cholesterol, and fatty acids. These lipids form multilamellar sheets amid the intercellular spaces of the stratum corneum and are critical to its mechanical and cohesive properties, enabling it to function as an effective water barrier. There is general agreement that the thickness of the stratum corneum does not change with age, and that barrier function does not alter significantly. However, certain features of aging skin do indicate an abnormal skin barrier—namely, the extreme skin dryness (xerosis) and increased susceptibility to irritant dermatitis that accompanies old age. Furthermore, there is evidence of altered permeability to chemical substances and reduced transepidermal water flux in aged skin. It seems that baseline skin barrier function is relatively unaffected by age. This is perhaps counterintuitive, but substances recoverable from the skin surface (e.g., sebum, sweat, components of natural moisturizing factor, corneocyte debris) were not significantly affected by age or ethnicity and gender.
If the skin is subjected to sequential tape stripping, the barrier function in aged skin (>80 years) is much more readily disrupted than in young skin (20 to 30 years). In addition, the same study found that after tape stripping, barrier recovery was greatly disturbed in the older age group. The reason for this abnormality is not entirely understood; however, it appears that there is a global reduction in stratum corneum lipids, which affects what binds the corneocytes. Studies have confirmed that in moderately aged individuals (50 to 80 years), abnormal stratum corneum acidification results in delayed lipid processing, delayed permeability barrier recovery, and abnormal stratum corneum integrity. Not only does the rise in stratum corneum pH interfere with lipid production, it also accelerates the degradation of intercorneocyte connections, the corneodesmosomes. The abnormal acidification is linked to decreased membrane Na + /H + transport protein. In addition, with age, stratum corneum turnover time lengthens with protracted replacement.
In a recent study of adult female skin, skin surface pH on the forehead, temple, and volar forearm were reported to increase only slightly with age. This information is crucial for the development of medical and cosmetic skin care products.
The most consistent change found in aged skin is flattening of the dermoepidermal junction at sites that were highly corrugated in youth ( Fig. 25-1, A and B ). The flattening creates a thinner looking epidermis primarily because of retraction of the rete ridges. With this reduced interdigitation between layers, there is less resistance to shearing forces. There is also a reduced surface area over which the epidermis communicates with the dermis, accompanied by a reduced supply of nutrients and oxygen. It is likely that much of this effect is influenced from so-called solar elastosis changes in the papillary dermis (see below)—that is, changes in the elastic fiber network, including tropoelastin and fibrillin-1. Even with minimal photoaging, one can appreciate the loss of fibrillin-rich microfibrils in the dermal-epidermal junction, so this can be viewed as an early marker of photoaging. There is general agreement that epidermal cell turnover is 50% lower between the third and seventh decades of life. This is consistent with the observation that wound-healing capacity deteriorates in old age.
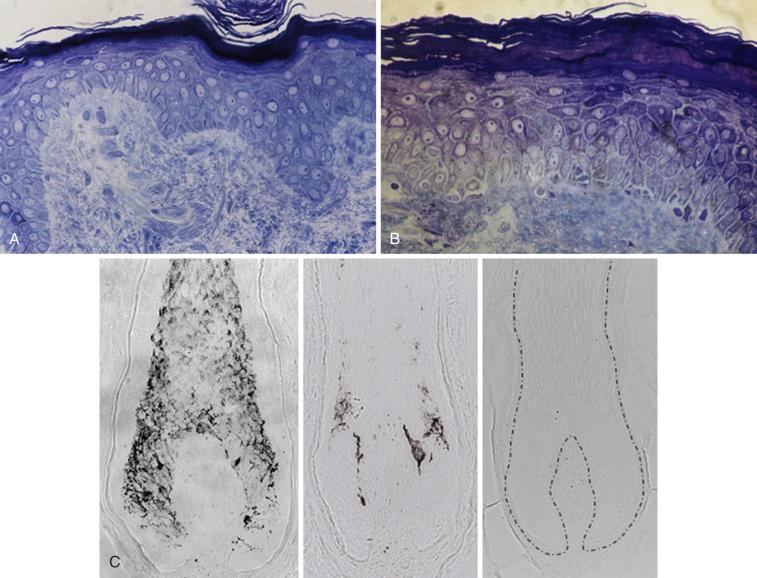
With age, there is increasing atypia of the basal layer keratinocytes. Involucrin, a differentiation marker normally expressed by irreversibly differentiated keratinocytes in the stratum corneum, has been found to have increased expression in sun-damaged skin. This is consistent with the fact that keratinocyte differentiation is impaired by UVR. In addition, in basal epidermal cells, there is downregulation of certain β 1 -integrins, which are markers of keratinocyte differentiation and adhesion to the extracellular matrix, suggesting that proliferation and adhesion of keratinocytes in photodamaged aged skin are abnormal.
With age, there is a reported reduction in the number of functional (tyrosinase-positive and tyrosinase-active) melanocytes in the basal layer of the human epidermis, from 8% to 20% per decade. Paradoxically, there may be an increase in the number of melanocytes in photodamaged skin, although these cells tend to be smaller than normal and often exhibit cellular activation with marked nuclear heterogeneity, large intracytoplasmic vacuoles, and more frequent contact with Langerhans cells. This overall reduction in melanocyte number and/or function in aging skin is also reflected by a reduction in melanocytic nevi in older patients. With reducing melanocyte numbers, there is an associated loss of melanin in the skin, which means less protection against the harmful effects of UV radiation. Consequently, older adults are more susceptible to skin cancers, and sun protection remains very important for this group, despite the fact that most of an individual's harmful sun exposure occurs in the first 2 decades of life.
There are also dramatic changes to pigment cell function in the graying hair follicle that are directly linked to the cyclic activity of the hair growth cycle (see later). One of the most striking changes in aged skin in those of most ethnicities is the dramatic increase in so-called age spots, or solar lentigo lesions. For those of Asian ethnicity, these pigmentary changes contribute more to perceived age than wrinkling. Age spots are usually up to 1 cm in diameter, with major histologic changes to the basal layer of the epidermis, especially the elongation of epidermal rete ridges (in contrast with the epidermal flattening seen with general skin aging). Although it first appears that these areas of hyperpigmentation are due to an increase of melanocytes, this finding has not been confirmed in several reports. In a report by Kadono and associates, the numbers of tyrosinase-positive melanocytes per length of the dermal-epidermal interface appeared to be increased twofold in the solar lentigo versus the unaffected skin. Other studies have reported increased melanocyte size, dendrite elongation, and alterations in melanosomes and their organization, but not increased cell numbers. Endothelin-1 and the stem cell factor appear to be key regulators in the development of hyperpigmentation in solar lentigo lesions, with alterations in the epidermal-dermal melanin axis, including dermal melanin incontinence and factor XIIIa–positive melanophages in senile lentigo and aging skin.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here