Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Mycetoma (maduromycosis, Madura foot) is a neglected tropical disease that has its greatest impact on severely impoverished, remote rural communities. It is a noncontagious, localized chronic debilitating infection that affects skin, subcutaneous tissue, fascia, muscle, and bone. The foot is most commonly involved, but any site can be affected. Characteristic clinical features of mycetoma include localized swelling, fistulous tracts, and suppurative drainage that contains dense colonies (called granules, sclerotia) of the offending pathogen. Mycetoma can be divided into two general categories based on the causative agent. Mycetoma caused by one of the filamentous fungi is termed eumycotic mycetoma, and that which is caused by Actinomycetes is termed actinomycotic mycetoma. Striking geographical differences occur in the proportion of mycetoma caused by true fungi and mycetoma caused by Actinomycetes. Agents of eumycotic mycetoma are considered in this chapter, and agents of actinomycotic mycetoma are considered in Chapter 195 , Anaerobic Gram-Positive Nonsporulating Bacilli (Including Actinomycosis).
At least 32 species of filamentous fungi (Eumycetes) are recognized as causes of eumycotic mycetoma ( Table 255.1 ). All are common saprophytes of soil and vegetable matter. Madurella mycetomatis, the most common cause worldwide, accounts for 70% of eumycotic mycetoma infections, and Pseudallescheria boydii (anamorph Scedosporium boydii ) and Falciformispora senegalensis (formerly Leptosphaeria senegalensis ) each account for 10% of infections. Scedosporium apiospermum was formerly considered the anamorph of P. boydii , however, these are now recognized to represent distinct species: P. boydii (anamorph S. boydii ) and Pseudallescheria apiosperma (anamorph S. apiospermum ). P. boydii, Fusarium falciforme, M. mycetomatis, Trematosphaeria grisea (formerly Madurella grisea ) , and Exophilia jeanselmei, in decreasing order of frequency, are the most common causes of eumycotic mycetoma in the US. Within these groups, additional species have been recognized that are separate phylogenetically and have distinct clinical spectra and antifungal susceptibilities. Scedosporium aurantiacum, Pseudallescheria minutispora, and Madurella pseudomycetomatis are among those recognized most recently.
| Order | Species | Geographic Distribution | Color of Grains | Size of Grains (mm) |
|---|---|---|---|---|
| Chaetothyriales | Exophiala jeanselmei | Worldwide | Black | 0.5–1.0 |
| Diaporthales | Phaeoacremonium spp. | South America, India | White or black | 0.5–2.0 |
| Diaporthales | Pleurostomophora ochracea | Sudan | Yellow | 0.5–1.0 |
| Dothideales | Neotestudina rosatii | Central Africa | Whitish | 0.5–1.0 |
| Hypocreales | Fusarium falciforme | Worldwide | Whitish | 0.2–0.5 |
| Microascales | Scedosporium boydii | North and South America | Whitish | 0.2–2.0 |
| Pleosporales | Biatriospora mackinnonii | Central and South America | Black | 0.3–1.0 |
| Pleosporales | Curvularia geniculate | Worldwide | Black | 0.5–1.0 |
| Pleosporales | Curvularia lunata | Worldwide | Black | 0.5–1.0 |
| Pleosporales | Falciformispora senegalensis | West Africa, India | Black | 0.5–2.0 |
| Pleosporales | Medicopsis romeroi | Arid subtropics | Black | 0.3–1.0 |
| Pleosporales | Trematosphaeria grisea | South America, India | Black | 0.3–0.6 |
| Sordariales | Madurella mycetomatis | East Africa, Middle East | Black | Up to 5.0 or more |
The agents of eumycotic mycetoma grow on Sabouraud dextrose agar, with optimal growth occurring between 25°C and 37°C. Isolation is facilitated if the agar contains chloramphenicol to inhibit growth of bacteria and cycloheximide to inhibit growth of extraneous molds, although cycloheximide also can inhibit growth of P. boydii. Incubation may be required for up to 6 weeks for growth. Identification of isolates is based on morphology and pigmentation of colonies, mechanisms of conidia production, and morphology of conidiophores and conidia. Madurella species, Neotestudina rosatii, and Pyrenochaeta species do not sporulate readily, so special sporulation media and physiologic tests for carbohydrate and nitrate utilization may be needed to identify these agents. Detailed information on isolation and identification of the fungi that cause eumycotic mycetoma is available.
The agents of eumycotic mycetoma are relatively avirulent, opportunistic pathogens that do not invade intact skin. Infection results from direct fungal inoculation or contamination of wounds. Factors that predispose an exposed person to mycetoma have not been established. Impaired cellular immunity may be important but has not been observed consistently in patients with mycetoma. Fungal agents act as chemoattractants that stimulate the complement system to induce chemotaxis. This leads to the accumulation of neutrophils around granules and in the centers of abscesses and sinus tracts. However, this immune response appears to be an ineffective mechanism for inhibiting fungal replication.
Antibodies to some antigens of the offending agent are produced after established infections and can be measured by counter-immunoelectrophoresis, immunodiffusion, and immunoblot methods. , Specific IgG and IgM antibody directed against the causative organism also can be measured by enzyme immunoassay (EIA). , In endemic regions, antibodies to fungi that cause mycetoma can be detected by EIA in infected patients, as well as in uninfected people, consequently, caution is indicated when undertaking a serologic approach to reaching the diagnosis. , The effect of specific antibody on the course of infection has not been established. Antibody titers decrease in some patients after appropriate treatment. In some endemic regions, resolution of precipitation arcs identified with counter-immunoelectrophoresis techniques are used to guide therapy.
Infection usually begins with a skin wound caused by a thorn contaminated with the causative fungus. Organisms proliferate, with development of localized skin and subcutaneous infection appearing as a papular lesion. Often unrecognized, the lesion enlarges slowly and painlessly over a period of weeks to months. Inflammation also can advance along fascial planes. Hematogenous dissemination does not occur, although lymphatic spread has been described and is estimated to occur in approximately 2% of cases.
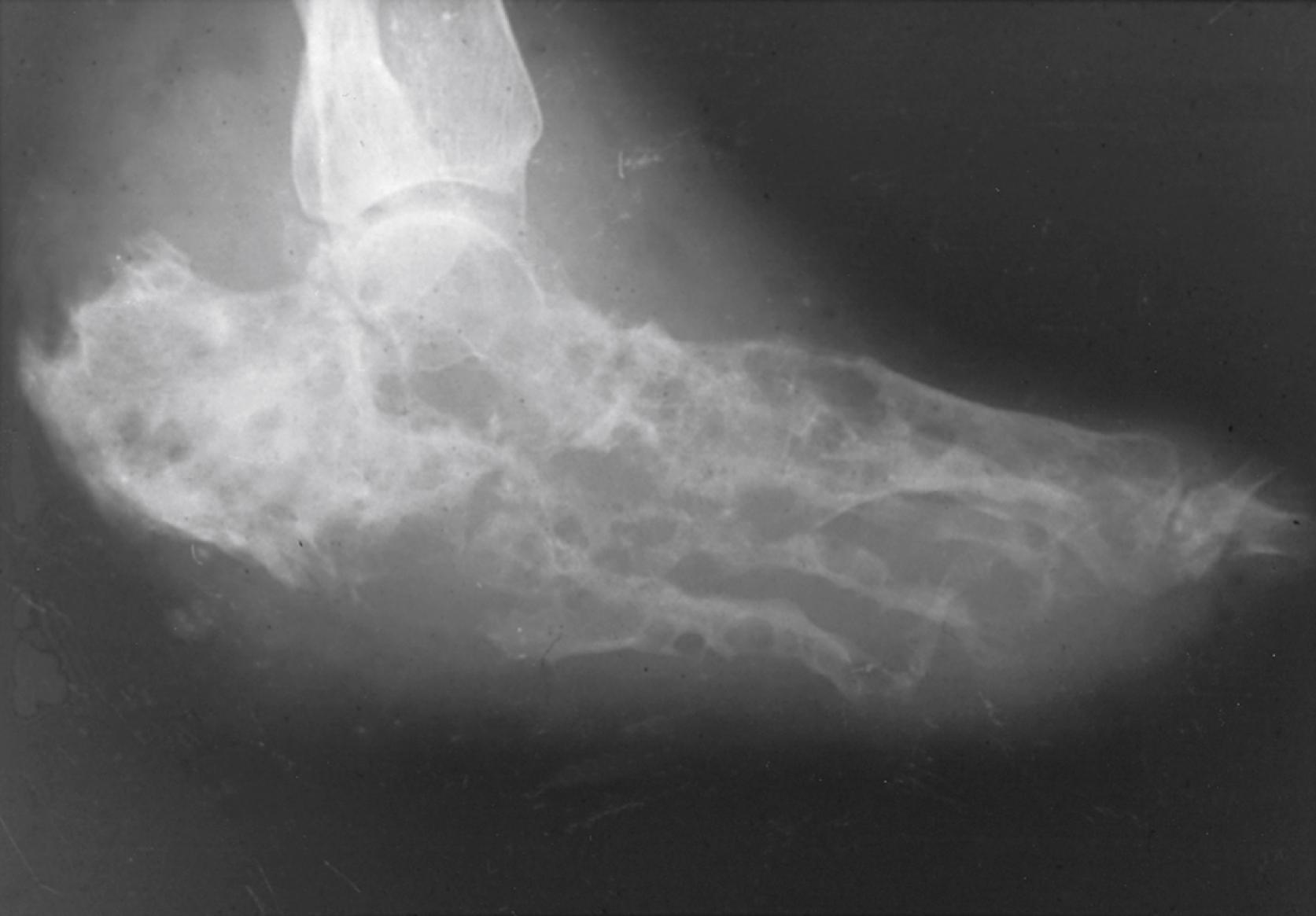
The process slowly destroys contiguous soft tissues and has a predilection for infecting and permanently damaging bone ( Fig. 255.1 ). Multiple sinus tracts are common and exuded drainage contains granules consisting largely of densely packed fungal colonies. Some matrix substances found in granules in vivo are not found in cultures grown in vitro and may represent antigenic material derived from the fungus or the host. The size, shape, and color of fungal filaments and presence or absence of matrix material within granules are features that vary with the offending agent.
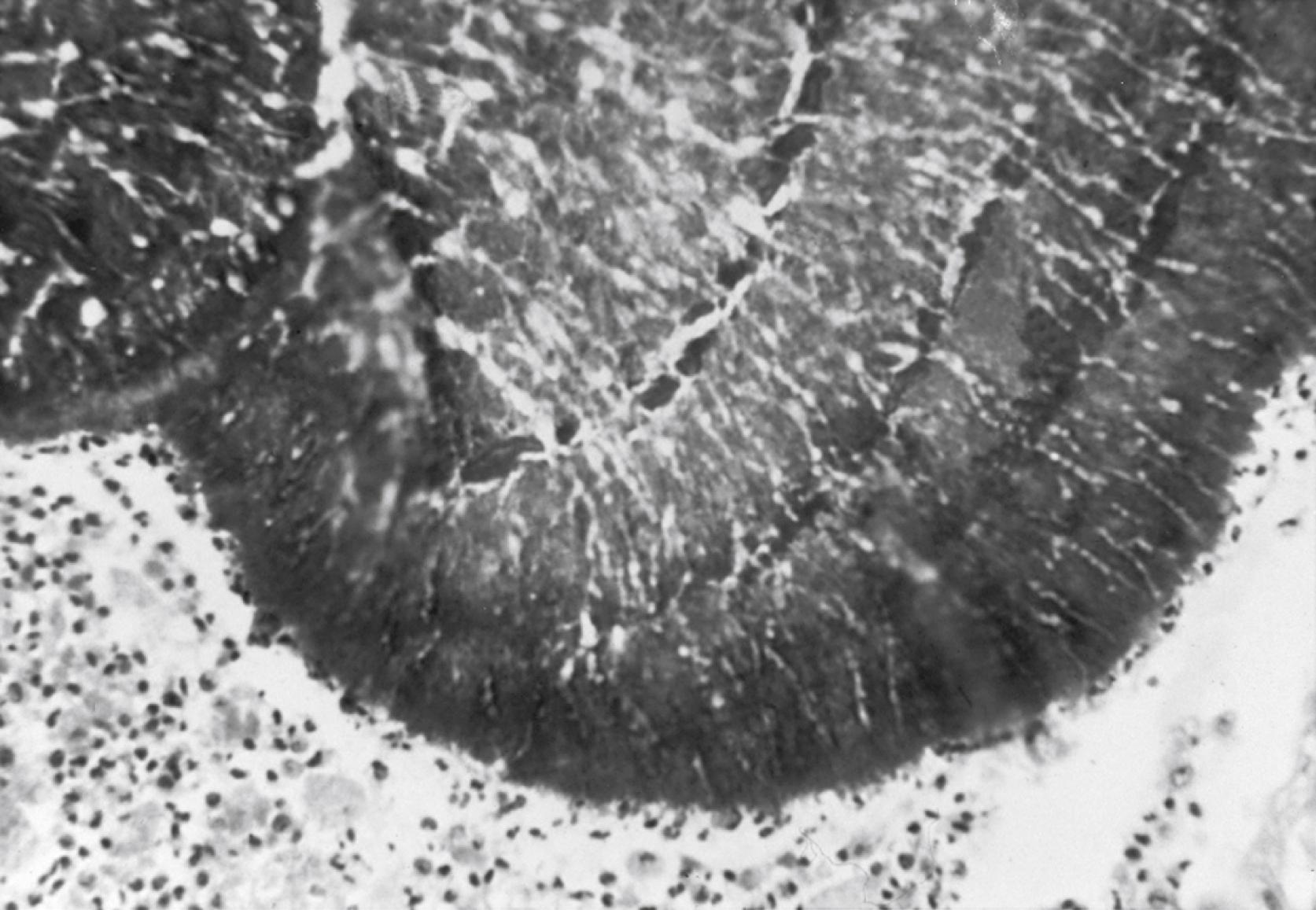
Except for the granules, histopathologic findings in mycetoma are nonspecific. Localized abscesses containing neutrophils and necrotic material surround single or multiple granules. A subacute and chronic inflammatory response consisting of plasma cells, lymphocytes, multinucleated giant cells, and epithelioid cells surrounds the abscess ( Fig. 255.2 ). An eosinophilic deposit (referred to as Splenore–Hoeppli reaction) surrounds the granule and is thought to represent an immunologic response. Fibrosis eventually ensues and contributes to the gross anatomic deformities.
Most mycetoma occurs in tropical or subtropical regions, mainly in areas where rainy seasons alternate with lengthy dry periods. Mexico, India, large regions of Africa, especially Sudan , and South America—most notably Brazil and Argentina—have the highest incidence of reported infections. Eumycotic mycetoma is rare in the most temperate regions of the world and extremely rare in the US. This clinical diagnosis is increasingly seen among individuals who travel from endemic regions. ,
In endemic areas, the agents of eumycotic mycetoma commonly occur in soil and on plants. Thorn injuries, abrasions, and occupational activities that predispose to skin trauma and soil contamination lead to infection. Consequently, infection occurs most frequently in young adults 15–30 years of age. Children account for approximately 30% of cases, with school age children >11 years of age most likely to develop this disease. , This has severe implications for the future livelihoods and education for those affected and their families, as those affected may be unable to work or attend school. , Mycetoma is more common in men than women with a ratio of 4:1. , Poor nutrition or concomitant infections, often seen in people in resource limited rural areas, are thought to increase the likelihood that exposure will lead to infection; however, a variety of infections have been reported among those who are immunocompetent. ,
Patients with mycetoma typically present at an advanced stage of disease, due to a combination of the initial painless nature of the infection, limitations in socioeconomic status and health education, and poor access to healthcare. , , The initial lesion usually is a small subcutaneous nodule attached to the overlying skin. Fistulous tracts and sinus formation begin early, usually within several months to 1 year following infection. The purulent drainage contains granules. Subcutaneous nodules slowly enlarge, additional nodules appear, and new sinuses form as old sinus tracts heal. The progress of infection is slow, and medical attention frequently is not sought for many months or years. Extension contiguously and along fascial planes can lead to chronic bone infection. Systemic symptoms are absent unless secondary bacterial infection intervenes. Pain is not a characteristic feature and presence of pain often is associated with secondary bacterial infection of bone, or progression of fistulous tracts.
Although infection can begin at any superficial anatomic site, mycetoma usually occurs on extremities. Approximately 70%–80% of mycetoma lesions affect feet and 12%–15% involve hands; other sites on extremities and on the head and neck account for most of the remaining cases. , Chronic changes and destruction eventually affect most underlying structures ( Table 255.2 ). The eventual result is a disfigured, scarred, swollen site with draining sinuses and functional impairment.
| Tissue | Abnormality |
|---|---|
| Skin and subcutaneous fatty tissue | Fistulas, granulomas, change in color, zones of fibrosis |
| Muscle | Degenerative myositis |
| Tendon | Few alterations because of resistance |
| Nerve sheath | Hypertrophy |
| Lymphatics | Inflammatory reaction; embolization of granules possible |
| Lymphatic ganglia | Hypertrophy; dissemination rare |
| Bone | Periostitis, osteolysis, osteofibrosis |
| Peripheral nerve | Altered by sclerosis |
In the presence of a lesion with tumefaction (indurated swelling), fistulous sinus tracts, and drainage that contains granules, the clinical diagnosis of mycetoma is straightforward. However, because treatment and prognosis differ for mycetoma caused by Actinomyces species or filamentous fungi, a specific diagnosis is necessary, and biopsy is required. A combination of diagnostic tools is indicated for definitive diagnosis. Histopathologic findings in mycetoma are nonspecific and consist of acute and chronic inflammation, granulomatous changes, and fibrosis. On biopsy specimen, “grains” may or may not be observed because they are scattered along sinus tracts and vulnerable to sampling error. When present, they are large and surrounded by neutrophils. Careful examination of granules in tissue ( Fig. 255.2 ) or purulent material suggests the causative agent. Accurate confirmation requires fungal culture. Granules range in size from 0.2 to 5.0 μm and often are visible to the unaided eye. The color, texture, size, and shape of the granules provide important clues to the etiologic agent. Eumycotic mycetoma has granules that are black or white and soft or hard, with a cement-like matrix. Gomori methenamine silver nitrate staining of eumycotic granules shows wide, interwoven septate hyphae 2–5 μm in diameter; large swollen cells up to 15 μm in diameter also may be present at the periphery of granules. Actinomycotic infections have gram-positive, interlaced, thin filaments 0.5–1.0 μm in diameter, with coccoid and bacillary forms.
To facilitate recovery of fungus, granules obtained from purulent material or tissue should be washed in normal saline that contains antimicrobial agents added to inhibit growth of contaminating bacteria. Sabouraud dextrose agar is inoculated and incubated at 25°C and 37°C and examined for growth at 2-day intervals for 6 weeks. A secure etiologic diagnosis is established when a single agent is isolated from several granules.
Diagnostic modalities such as polymerase chain reaction (PCR) and mass spectrometry, such as matrix-assisted laser desorption/ionization—time of flight (MALDI-TOF), are becoming more readily available to assist in reaching a definitive conclusion once a pathogen has been isolated. Most recently, real-time PCR has been used to rapidly identify Madurella species in an endemic context. , Less invasive modalities, including radiographic findings, have been used to aid in diagnosis. Radiographic abnormalities can be seen in soft tissue and bone and include areas of increased soft-tissue density (caused by granulomas) adjacent to normal soft tissue. Osseous lesions have a variable appearance ( Fig. 255.1 ); most are lytic cavities in bone that may contain large numbers of granules. Cortical bone also can be eroded by extrinsic irritation from contiguous soft-tissue inflammation. Periosteal new bone formation can be seen as spicules oriented perpendicular to the long axes of long bones. MRI has been helpful in suggesting the diagnosis of mycetoma when a “dot-in-circle” is appreciated. , Ultrasound evaluation has the capacity to distinguish between the eumycotic mycetoma and actinomycetoma by appearance of their macrogranular characteristics. Osteopenia results from disuse or impairment of perfusion. These diagnostic modalities are useful for earlier diagnosis, which may allow for earlier therapy and less amputation. Some radiographic improvement can occur in response to successful treatment. Although serologic tests have been investigated for use in diagnosis and to monitor therapy, standardized reagents are not available commercially.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here