Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
There have been many new developments in the invasive brain–computer interface (BCI) field with respect to computational and processing methods that have deepened our understanding of how the brain works and allowed neural signals to be deciphered and harnessed for a wide array of applications. These applications include thought-controlled computer interfaces for disabled users, and most recently technologies specifically aimed at restoring movement in people living with paralysis. With continued advancements in the areas of implantable microelectrodes, advanced signal processing, and neural decoding methods, the list of applications for BCI continues to expand. In the future we can expect to see increased research and development efforts related to decoding neural patterns and technologies that interface with not only the brain but also nerves throughout the body. These technologies will allow us to tap into and decipher the vast amount of information present to help treat and potentially diagnose a wide variety of conditions. With new brain–computer and neural interfaces and methods to decode neural signals, we will at some point in the future be able to understand the language of the human nervous system, leading to new treatment options for the millions of patients worldwide living with disease and debilitating injuries.
Examples of early research efforts that paved the way for BCIs and advances in neural decoding are studies that recorded electrical activity in the brain to understand better how sensorimotor information is encoded ( ). In subsequent work differing neuronal firing patterns were observed during passive and active movements ( ). Furthermore, neurons were identified that had modulation patterns correlating to force ( ). Important discoveries such as “directional tuning” were made, where neuronal firing rate changes in the motor cortex were associated with different arm trajectories and certain neurons were found to have a “preferred” direction ( ). Later, with the increased availability of more sophisticated data collection and computer systems, more advanced analyses were performed to deepen understanding of the patterns in neuronal populations ( ). Since each neuron in the brain is connected to a large number of other neurons, understanding pattern changes (modulation) of large neuronal populations is critical to further our knowledge of how the brain represents and processes information.
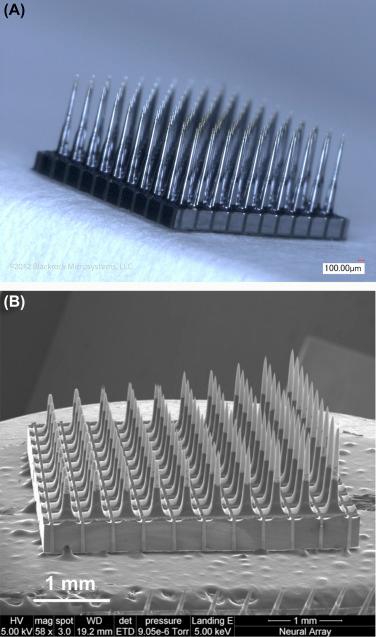
Initially electrodes used to probe and collect data in the brain were made exclusively by hand in the lab (typically limited to a handful of recording sites), making it difficult to study large populations of neurons and network behavior. However, implantable electrode technology evolved as microfabrication techniques were developed to produce more precisely manufactured electrode arrays with more recording sites. One such design, developed at the University of Utah, is fabricated by etching silicon to form “spikes” with recording sites at their tips (see Fig. 29.1 ). Another design, developed at the University of Michigan, is made using a thin-film process to create flexible thin shanks that can be inserted into the brain to record signals. Yet another type of electrode array used in BCIs is an electrocorticography (ECoG) array or grid. ECoG arrays do not penetrate into the cortex and can be placed on top of or below the dura to record neural signals that are useful for neural decoding. Unlike the first two types of electrodes mentioned, the ECoG array cannot record single-unit action potentials and the electrode spacing is typically larger. As discussed later, these differences are important because they affect the specificity of the signal recorded, and need to be considered when developing BCI systems and neural decoding methods for various applications.
In the 1990s an area of research called machine learning, branching from the field of artificial intelligence, began to grow rapidly ( ). Machine-learning algorithms were developed and could be run on personal computers to handle increasingly large datasets, making them well suited for deciphering brain activity data. Machine-learning methods could be used to find and learn patterns in complex datasets for later automatic recognition when these patterns reoccurred. Thoughts in the brain are reflected in the electrical activity patterns generated by neurons “firing.” If this electrical activity is detected and recorded, neural decoding algorithms can be trained to recognize brain activity associated with certain thought patterns, such as thoughts about movement. This fueled research activity in neural decoding, opening the possibility of interpreting brain activity patterns so scientists and engineers could, in a limited way, recognize thought patterns and “read the mind.”
With the advancements of neural interface technology and neural decoding methods, the possibility of linking brain signals to computers and devices was beginning to become a reality. Researchers began to think about the possibility of allowing a disabled person to have thought-control of a computer or assistive device to restore lost functions such as speech, and at some time potentially movement. A new area of invasive BCI technology and new applications had been launched.
Continued research in nonhuman primates demonstrated that it was possible to decode movement-related thoughts and allow the animals to control robotic arms with their thoughts ( ). Researchers began to study the behavior of neuronal populations by using multielectrode arrays and developing more sophisticated neural decoding algorithms to correlate neural activity with movement in three-dimensional space ( ). Neural decoding was later combined with neuromuscular stimulation to allow volitional control of temporarily paralyzed arm muscles in nonhuman primates ( ). These initial research findings provided the foundation for subsequent development of invasive BCI technology and neural decoding methods for human applications.
In the late 1990s researchers began to develop invasive BCI technology and neural decoding methods with clinical applications in mind. One of the first studies involved the use of neural decoding for restoration of speech ( ). Other studies were planned and conducted for participants living with paralysis due to spinal cord injury (SCI), stroke, and amyotrophic lateral sclerosis to allow direct thought-control over cursor movement in personal computers ( ). As the patient imagined or attempted various arm and hand movements, neural decoding algorithms were trained to recognize the neural modulation patterns associated with each movement. Once a decoding algorithm was trained and in place, the patients could use their thoughts to control not only a cursor on a computer but also robotic devices ( ).
With previous successes in restoring movement in temporarily paralyzed muscles of a nonhuman primate, other groups began to work toward restoring movement in persons living with paralysis. This involved using an electronic “neural bypass” to reroute signals effectively from the brain to paralyzed muscles in an effort to restore functional movement. In 2016 results from a first in-human study were reported, documenting the use of a cortical implant to restore movement in a paralyzed human ( ). The study participant was a 24-year-old male with stable, nonspastic, C5/C6 quadriplegia from cervical SCI sustained in an accident 4 years earlier. The BCI system decoded intracortically recorded signals and linked them to a custom neuromuscular stimulation system to restore volitional control over hand movement. As shown in Fig. 29.2 , a tiny electrode array (4 mm × 4 mm across) with 96 electrodes (1.5 mm long) was implanted in the motor cortex and a custom neuromuscular electrical stimulation sleeve was used for muscle activation.
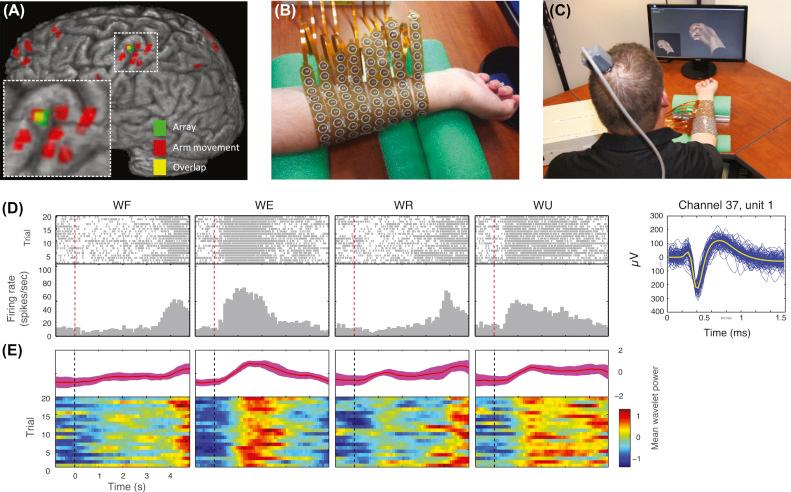
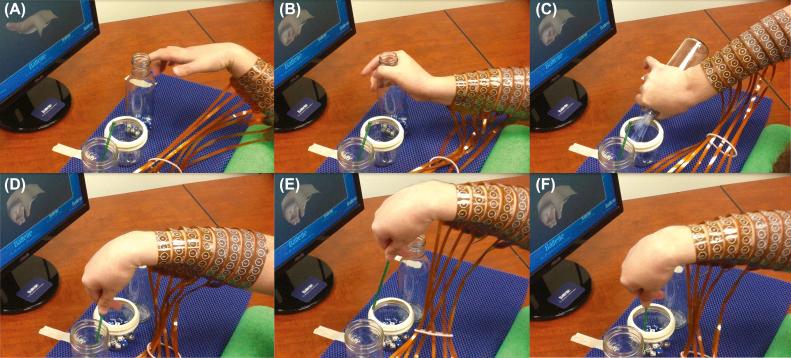
The participant was able to regain continuous volitional wrist and dexterous finger movements through his own thoughts. He was also able to achieve functional movements relevant to daily life, as shown in Fig. 29.3 . This was a large step forward in BCIs and helped pave the way for future medical devices for treating SCI, stroke, traumatic brain injury, severe nerve injuries or degeneration, and other conditions where movement is severely impaired.
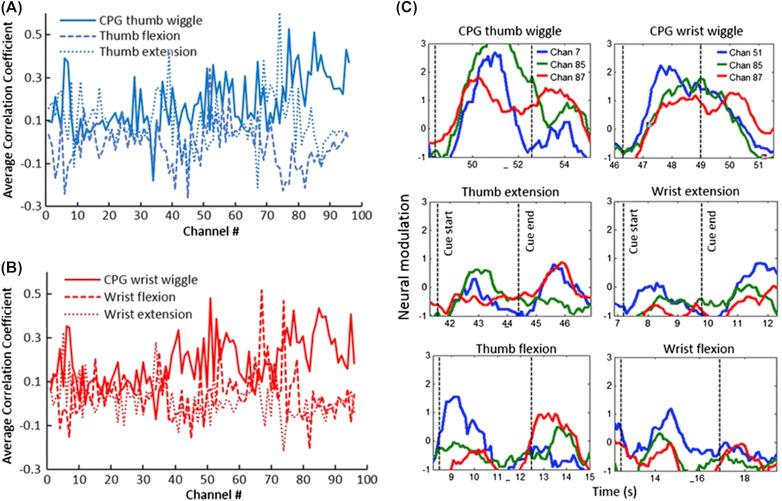
One of the challenges in using an electronic neural bypass to reroute signals around a damaged spinal cord is that the central pattern-generator neural networks used in rhythmic movement are bypassed completely. In recent work artificial central pattern generators were developed in an attempt to replace this lost function. The study participant was able to activate these artificial pattern generators through thoughts ( ), and could think about static and dynamic/rhythmic movements, such as flexing and wiggling the fingers or wrist (as in movements used during teeth brushing or scratching), and switch between the two volitionally, as shown in Fig. 29.4 .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here