Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In 1993 the first successful adult-to-adult living donor liver transplantation was performed using a left liver graft. Until then, liver transplantations from living donors had been performed only for pediatric recipients. Because of the overwhelming shortage of liver grafts from deceased donors to meet the demand, the number of adult living donor liver transplantation has increased dramatically throughout the world. Recipients’ poor prognosis in adult living donor liver transplantation due to small-for-size graft was soon ameliorated with the use of a right liver graft, which was first transplanted to a 9-year-old child. Right liver graft harvested together with the middle hepatic vein (MHV) was also adopted successfully for an adult recipient for the first time in 1996. Soon right liver graft without MHV became to be preferred for an adult recipient worldwide.
However, the morbidity rate in donor hepatectomy was reported to be significantly higher in right hepatectomies than in left hepatectomies. The 10 early donor deaths reported involved five right liver donations, of which three were from the United States ; this tempered great enthusiasm for living donor liver transplantation in the United States. Although the difference in the incidence rate of complications between right hepatectomy and left hepatectomy has now become small in Japan, right liver donation has resulted in one donor death and one out of the two cases of severe aftereffects during the past 18 years of living donor living transplantation experience.
Donor safety is paramount in living donor liver transplantation. In 2008 at Istanbul the Transplantation Society issued a declaration stating that “the provision of care for living donors before, during, and after surgery—as described in the reports of the international forums organized by the Transplantation Society in Amsterdam and Vancouver—is no less essential than taking care of the transplant recipient,” and that “a positive outcome for a recipient can never justify harm to a live donor.” All countries require “a legal and professional framework to govern organ donation and transplantation activities, as well as a transparent regulatory oversight system that ensures donor and recipient safety and the enforcement of standards.” Furthermore, the declaration addresses the importance of the informed consent process.
One of the most important factors in donor evaluation is to determine whether the donor candidate is medically and psychologically suitable for donation. It is also extremely important to confirm donor understanding of the risks and benefits concomitant to the procedure and that he or she is making an autonomous and noncoerced decision.
Upon confirming the donor’s health suitability and resolve to become a donor, another complex but also extremely important procedure of choosing the appropriate donor resection for the specific recipient awaits. An intricate balance of protecting the donor by performing the smallest resection possible and at the same time providing the recipient with adequate liver mass and best chance for survival has to be sought. The smallest resection that would provide adequate functional mass for the recipient can only be called appropriate .
Volume data of liver segments are usually obtained by hand tracing the computed tomography (CT) images of each slice and calculating the liver volume by integrating those planar dimensions. This technique is gradually being replaced by a region-growing method using three-dimensional CT.
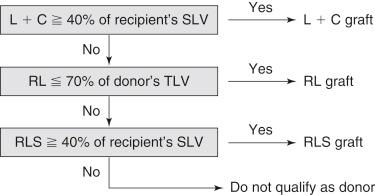
Our graft selection criteria from the viewpoint of graft and remnant liver volume are as follows ( Fig. 51-1 ):
The functional graft size should be at or more than 40% ≥35% for low-risk recipients, ≥30% for normal liver recipients with metabolic diseases ) of the recipient’s standard liver volume.
The resection percentage should be under 70% (under 65% for right hepatectomy with the MHV graft).
When a non–right liver graft meets the first two criteria, a right liver graft should, in general, not be selected.
A right lateral sector graft should be selected when the estimated graft volume is larger than the volume of a left liver with caudate lobe graft and when the right lateral sector graft size satisfies the criterion.
Left liver with caudate lobe grafting is the standard grafting procedure for the left liver.
A left liver graft may be a safer alternative to a right liver graft for donors, and they are now used for properly selected cases in several centers. The caudate lobe was added to the left liver graft in an effort to increase the volume of the left liver graft in 1998. In 2000 a fully functioning caudate lobe was obtained by reconstructing the major drainage vein of the caudate lobe. Although the size of an average caudate lobe is relatively small and the increase in the size of the graft may remain only minimal, particular attention must be paid to the venous drainage of the caudate lobe.
To best ensure donor safety, it is imperative that surgeons have a thorough understanding of common hilar anatomy, as well as the individual anatomy of a specific donor. Accurate recognition of anatomy will also assist in selecting the most appropriate donor in cases where multiple candidates exist. The significance of hepatic anatomical variations and how they surgically affect the left liver living donor transplantation is briefly reviewed.
The extrahepatic portal vein generally has a constant anatomy, with its bifurcation lying to the right and a longer extrahepatic left portal vein. The transverse portion of the left portal vein runs along the right end of the lesser omentum to the ligamentum venosum, whereas the umbilical portion runs up into the umbilical fissure. It is therefore much easier to gain sufficient extrahepatic left portal length than right portal length. The vast majority of donors (more than 90%) have standard anatomy ; however, about 10% of donors have a trifurcation in the portal venous anatomy in which the right lateral portal vein branches off independently from the main portal vein. The right paramedian and left portal veins form a trunk and then diverge. The donor operation is especially technically demanding because the length of the left portal vein is short and is situated deeply behind segment IV. Intrahepatic left portal venous anomalies rarely preclude left lobe donation. The so-called double portal vein is not a contraindication for donation but does require proper identification and extrameticulous dissection in a donor left hepatectomy, and because the extrahepatic portion of the left portal vein is shorter, technically advanced reconstruction is required in the recipient.
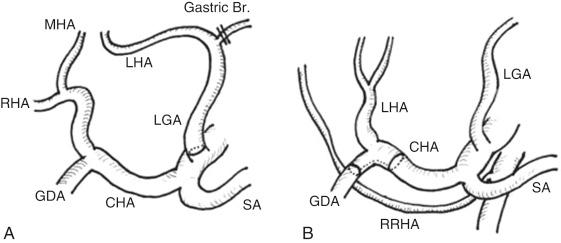
Hepatic arterial anatomy is the most complicated variable anatomical factor. One in four individuals have their left hepatic artery arising from the left gastric artery. In such cases thicker and longer arterial branches can be obtained with ligation and division of the gastric branches from the left gastric artery and arterial dissection at the root of the left gastric artery at graft removal ( Fig. 51-2 , A ). In 7% the left hepatic artery alone branches out from the common hepatic artery because of the replaced right hepatic artery. In such cases a wider orifice can be obtained by removing the left hepatic artery with bifurcation of the common hepatic artery and the gastroduodenal artery (see Fig. 51-2 , B ), which can be used to create a branch patch. In more than half of individuals, the arterial supply to segment IV, often referred to as the middle hepatic artery , originates independently of other arterial branches of the left liver. However, in most cases reconstruction of one of the thickest branches is enough to arterialize the whole left liver graft.
Biliary anatomy is another variable anatomical factor to be kept in mind. Intraoperative cholangiography is invaluable as a means of defining a “road map” of the biliary anatomy and for identifying the exact site of the divisions. Although magnetic resonance imaging now permits highly advanced imaging of the biliary tree for living donors, intraoperative cholangiography remains the gold standard. Just a few millimeters can change the number of bile duct orifices to be identified. Even more important, a millimeter in the wrong direction could have a lifelong crippling consequence for the donor. The right posterior hepatic duct may drain into the left hepatic duct in 12% of individuals, a common variation important to remember especially in left liver donation. Although consequential variations of the bile ducts may be common, they rarely necessitate any special surgical modification. Recognition of a correlation between anatomical variants of the biliary tree and portal vein helps to predict the existence of variation in the bile duct before cholangiography. In patients with a trifurcation pattern portal vein, one out of three are found to have their right posterior hepatic duct draining into the left hepatic duct.
It is important to assess the anatomical relationship of the hepatic veins as they enter the vena cava. This relationship can have important implications when identifying the correct transection point in donors and graft outflow reconstruction in recipients. Most commonly the right hepatic vein (RHV) enters the vena cava separately from the left and the MHVs, which then join together as a common trunk.
Treatment of the MHV is a technical dilemma in adult living donor liver transplantation using left liver graft. A considerably large congested area remains in the graft or donor’s remnant liver after left hepatectomy with MHV. The area drained by the MHV varies among patients, and the MHV was found to be the major draining vein of the right liver in 24% of the cases studied by Couinaud. Lee et al reported necrosis of the right liver graft without the MHV after liver transplantation. Two of the five patients had severe congestion, and one died due to sepsis 20 days after transplantation. However, because the MHV functions also as the drainage vein for segment VI, if the left hemiliver was removed without the trunk of the MHV, graft function was compromised. We therefore, proposed a criterion for MHV reconstruction in left liver grafts. When a thick tributary of the MHV draining the right liver is confirmed in donor operation, left liver grafts should be harvested without the MHV trunk. The hepatic venous congestion in segment IV can be investigated intraoperatively by Doppler ultrasonography after graft reperfusion. If the portal flow in segment IV is hepatofugal and the graft volume with hepatopetal portal flow is calculated to be insufficient for the recipient’s metabolic demand, the MHV branches should be reconstructed.
The caudate vein is another topic for left liver graft with complete revascularization. Under preoperative enhanced CT and intraoperative ultrasonography (IOUS), two thirds of individuals are found to have the caudate vein located on the ventral 60 degrees of the inferior vena cava (IVC).
All donors in elective transplantation are recommended to have autologous blood available preoperatively.
In the days before the surgery, kanamycin and lactulose are administered for chemical bowel preparation, purgative medicine is used for mechanical bowel preparation, and an enema is prescribed on the morning of the operation. Before the induction of general anesthesia, an epidural catheter is placed for perioperative pain control. After the induction of general endotracheal anesthesia, an internal jugular central venous catheter is inserted to measure the central venous pressure. Before laparotomy, prophylactic antibiotics, a first-generation cephem antibiotic, is administered. It is also administered every 6 hours during operation.
Intravenous fluid infusion should be rationed at approximately 4.5 to 5 mL/kg per hour to keep the central venous pressure low.
Inverted-T incision is selected in all cases to obtain best operative field. Midline incision should be placed from just beneath the mammary line to just above two fingers' width from the navel. Transverse incision starts from the left side of the rectus muscle and extends toward the right ninth intercostal space and enters the thoracic cavity. Right thoracotomy is unnecessary in left-side hepatectomy. An alternative incision would be the Chevron incision with Mercedes-Benz modification (with additional upper abdominal midline incision). The ligamentum teres and the falciform ligament are divided at the anterior abdominal wall side for use in properly fixing the graft to the recipient. Dissection around the coronary ligament enables exposure of the root of the common trunk of the LHV and the MHV.
Meticulous inspection and palpation of the liver and IOUS confirm the final indication for the donor hepatectomy. If the liver is impermissibly steatotic, which was not expected preoperatively, or unknown tumorous lesions are found, surgical biopsy or needle biopsy should be carried out for pathological diagnosis. Portal and hepatic venous anatomy is also surveyed under IOUS to check especially for the caudate portal branch that may have originated from the main trunk of the portal vein, tributaries of the MHV, or the principal caudate vein, which also drains the left side of the caudate lobe (spigelian lobe).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here