Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
One of the driving forces in the genesis of the field of interventional radiology was its minimally invasive nature. As catheters and stents replaced the physiologically taxing “open” procedure, patients were able to have an intervention they may not otherwise have survived. Initially, local anesthesia and light sedation were usually sufficient to provide a comfortable experience for the patient with minimal risk, but continued improvements in imaging and device technology have made more complex procedures possible in sicker patients. This has made “local with sedation” a more challenging proposition for the interventionalist. The increasing need for an anesthesiologist seems like a natural progression since the line between the radiology suite and the operating room continues to be blurred. The knowledge and skill of an anesthesiologist play an undeniable role in providing appropriate anesthetic and physiological support for patients undergoing these procedures. The anesthesiologist’s understanding of the procedure and the proper application of pharmacology and other interventions is becoming necessary to decrease morbidity and improve patient care.
It is beyond the scope of this chapter to discuss and detail all of the various procedures performed in the radiology suite. Our goal is to discuss the fundamental issues that exist in the radiology suite, with an emphasis on the more common and more challenging procedures performed there. As the field of interventional radiology continues to expand the number and complexity of procedures performed, the need for presence of an anesthesiologist will continue to expand with it.
Intravenous contrast is commonly used in interventional radiology procedures. The overall incidence of adverse reactions using high-osmolar contrast agents is 5% to 8%, with severe reactions occurring in 1 in 1000 to 2000 examinations. Moderate reactions are those requiring therapeutic measures without hospitalization. Development of nonionic and lower osmolality contrast agents has decreased the incidence and severity of contrast reactions to 1% to 4%. Severe, life-threatening reactions in low-osmolality contrast agents continue to be rare and are reported to be less than 0.2%. Contrast reactions are classified as either anaphylactoid or chemotoxic, with anaphylactoid being more common and chemotoxic typically more severe ( Box 17-1 ).
Minor: Nausea, vomiting (limited), urticarial (limited), pruritus, diaphoresis
Moderate: Faintness, vomiting (severe), urticaria (profound), facial edema, laryngeal edema, bronchospasm (mild)
Severe: Hypotensive shock, pulmonary edema, respiratory arrest, cardiac arrest, seizures
Anaphylactoid reactions can manifest as nausea, vomiting, urticaria, pruritus, bronchospasm, and cardiovascular collapse. The exact mechanism is still unclear but likely involves the direct release of histamine from basophils and mast cells, which directly or indirectly activates the complement, coagulation, fibrinolytic, and kinin systems. It does not appear to be mediated by an antibody-antigen interaction, despite resembling the typical symptoms of an allergic reaction. The magnitude of the reaction is not dependent on the dose or concentration of the contrast agent, and as with true allergic reactions, symptoms are typically seen within 1 hour after administration of contrast. Preexisting activation of these systems in critically ill patients may increase the severity and activation of the contrast reaction. Currently no evidence suggests that shellfish allergy predisposes patients to a contrast reaction, but patients with a history of significant allergies, asthma, or anxiety have an increased risk for anaphylactoid reactions, and premedication should be considered. Patients with previous anaphylactoid reactions may benefit from premedication with corticosteroids and histamine-1 blockers. A commonly used regimen is to give prednisone 50 mg 13 hours, 7 hours, and 1 hour before contrast administration and diphenhydramine 50 mg 1 hour before contrast administration.
Chemotoxic reactions to intravascular contrast are due to the inherent chemical properties of the contrast agent on specific organ systems. Because the contrast itself causes the reaction, the severity is directly proportional to its dose and concentration. Important factors are (hyper) osmolality, calcium binding, and the nature and concentration of its cations (sodium or meglumine). Critically ill patients who are already sensitive to significant volume shifts, arrhythmias, and renal insufficiency are at greater risk for chemotoxic reactions.
Treatment of acute contrast reactions is largely supportive. Once an adverse reaction has manifested, the injection of further contrast material should be immediately stopped. An urticarial rash may be self-limited or the harbinger of a worsening anaphylactoid reaction. The anesthesiologist should be vigilant for the development of bronchospasm, angioedema, hypotension, bradycardia, and sudden cardiovascular collapse. Beta-agonists, intravenous fluids, epinephrine, atropine, and the ability to secure the airway should be readily available in any location where intravenous contrast is administered.
Contrast-induced nephrotoxicity (CIN) is thought to result from the chemotoxic effects of the contrast on the renal tubules or by direct vasoconstriction. It is described as a sudden deterioration in renal function after the intravascular administration of iodinated contrast material that cannot be explained by another nephrotoxic event. It is rare in patients without preexisting renal dysfunction or critical illness, but studies suggest an increased risk is also seen in patients with diabetes mellitus, heart failure, older age, hypertension, and anemia. A dose-dependent correlation exists between the severity of injury and the dose of contrast, so the risks for worsening renal injury must be weighed against the benefits of the study or intervention planned. Strategies to help prevent CIN in patients with renal dysfunction are hydration with crystalloid and the administration of N-acetylcysteine and sodium bicarbonate. N-acetylcysteine 600 mg given 24 hours and 12 hours before and 12 hours and 24 hours after administration of contrast coupled with prehydration before administration of nonionic, low-osmolality contrast decreased the incidence of CIN in patients with preexisting renal disease as assessed by serum creatinine. Sodium bicarbonate also decreased the incidence of CIN in patients with preexisting renal dysfunction with the administration of 154 mEq/L of sodium bicarbonate as a bolus of 3 mL/kg/hr for 1 hour before contrast administration, followed by an infusion of 1 mL/kg/hr for 6 hours after the procedure.
Patients with diabetes and preexisting renal dysfunction who take metformin have developed severe lactic acidosis after an iodinated contrast study. If possible, metformin should be discontinued at the time of or before the procedure, withheld for 48 hours subsequent to the procedure, and reinstituted only after renal function has been reevaluated and found to be normal.
The primary safety concern for the anesthesiologist in the interventional radiology suite is ionizing radiation. Computed tomography and simple x-rays carry little risk, and fluoroscopy poses the greatest. The three primary sources are scatter radiation from the patient, direct radiation from the beam, and leakage from the radiation source, with scatter radiation from the patient providing the greatest risk.
A typical fluoroscope comprises a radiation source and an image intensifier. The orientation of the fluoroscopy beam in the anterior–posterior plane places the radiation source below the patient and the image intensifier above the patient. This minimizes scatter radiation by deflecting it down to the floor, away from medical personnel. Biplane machines employ an additional laterally facing fluoroscope to improve visualization, decrease contrast dose, and shorten procedure time. The lateral scope’s radiation source is classically positioned on the opposite side of the interventionalist to decrease his radiation exposure. Anesthesia personnel are frequently located on the same side as the radiation source and can be exposed to four times greater radiation levels than the interventionalist. The anesthesia team should communicate to the interventionalist when they need to access the patient, during which fluoroscopy should not be used, to decrease unnecessary radiation exposure. Anesthesia providers should avoid placing their hands into the x-ray beam unless absolutely necessary for patient safety ( Figure 17-1 ).
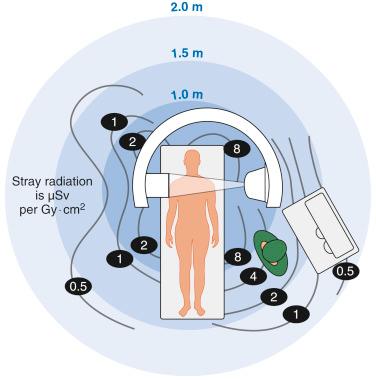
The two primary methods to decrease overall radiation exposure are distancing oneself from the source of radiation and shielding. Standard lead aprons with thyroid collars decrease the radiation from fluoroscopy by a factor of 10 and should be worn by all anesthesia personnel while in the radiology suite. Providers wearing aprons that do not provide adequate shielding in the back should avoid turning away from the beam while it is in use. When possible, transparent leaded acrylic shielding also should be placed between anesthesia providers and the patient to further decrease x-ray exposure. Anastasian et al showed that anesthesiologists’ eyes are exposed to a significant amount of radiation that could lead to cataracts and recommend that providers who spend considerable time in interventional radiology should consider using the lightweight leaded glasses that are commonly worn by interventionalists.
In addition to lead shielding, anesthesia providers should distance themselves as far as safely possible from the patient. The dose of radiation to which a provider is exposed is inversely proportional to the square of the distance from the source of radiation. Interventions such as administering a bolus of medication, adjusting ventilator settings, or checking neuromuscular blockade all increase radiation exposure. Even small distances can decrease radiation exposure significantly; therefore “remote” access using intravenous extension tubing or ventilator extension tubing may allow the anesthesia provider to be positioned farther away.
Digital subtraction angiography (DSA) and road-mapping are two techniques that aid the interventionalist during complex vascular procedures. These image acquisition techniques are highly sensitive to patient movement and breathing, and image quality is degraded easily. Patients are required to remain motionless and apneic for periods of up to 15 seconds to acquire adequate images. Because of the high levels of radiation, the anesthesiologist may choose to leave the immediate area, which is still within the standards for basic anesthetic monitoring endorsed by the American Society of Anesthesiologists (ASA) if the patient can be monitored remotely.
The diversity and complexity of interventional radiology procedures have dramatically expanded over the past two decades. Although the vast majority of procedures are performed under light or moderate sedation by a nurse and supervised by the interventionalist, an anesthesiologist is indicated if the patient has significant comorbidities or other issues that will make moderate sedation challenging or the procedure requires a general anesthetic. Procedures performed in the United States are typically performed with more sedation than those performed in Europe, but general anesthesia is employed more often in Europe. The reason for this is unclear, but patient expectations and patient anxiety may play a role. A discussion with the interventionalist before every procedure should include the expected procedure length, procedural stimulation, positioning, and need for patient cooperation. Biliary and percutaneous urological procedures that are particularly stimulating have been performed successfully under neuraxial blockade, obviating the need for general anesthesia or deep sedation.
Just as in the operating room, procedures performed in the radiology suite may last from 10 minutes to several hours. It is imperative for patients undergoing long procedures under moderate or deep sedation to be positioned as comfortably as possible. Foam padding, lumbar support, and a comfortable pillow all help ensure patient comfort during the procedure and may decrease the amount of sedation necessary. During femoral cannulation the patient must be completely supine, but a pillow may be placed under the knees for added support once the femoral sheath is in place. Some patients may not be able to lie supine for extended periods because of comorbid conditions, such as arthritis, chronic obstructive pulmonary disease, obstructive sleep apnea, pleural effusions, or congestive heart failure, and these should be discussed with the radiologist. Some procedures may allow for or require patients to be in the semirecumbent position. Extra care should be taken in patients undergoing placement of venous-access devices, because the patient position will increase the risk for venous air embolism during cannulation, especially in the spontaneously breathing patient. Some procedures (e.g., placement of percutaneous nephrostomy tubes and kyphoplasty) require the patient to be placed in the prone position, and specialized equipment and materials should be employed—just as they are in the surgical suite.
Depending on the type of procedure, access to the patient may be severely limited. The traditional arrangement with the anesthesiologist at the head of the bed is altered by the presence of fluoroscopy equipment and the radiologist. Certain procedures that focus around the head and neck, such as interventional neuroradiology procedures and transjugular intrahepatic portosystemic shunting (TIPS), make it especially difficult to access and secure the airway in an emergency situation. The patient’s arms are typically tucked and secured to allow for free movement of the fluoroscopy machine, which will require intravenous access and monitoring equipment, such as arterial lines, to be placed before the procedure starts ( Figure 17-2 ).
Maintaining normothermia during a procedure can be challenging in interventional radiology suites because the temperature is typically at or below 68° F because of the sensitivity of the radiology equipment. Strategies that actively warm the patient, such as forced-air warming blankets and fluid warmers, and those that minimize heat loss, such as humidifiers on the ventilator circuit and low-flow anesthesia, should be employed for long procedures. Applying the usual forced-air warming blankets can be difficult because of the need for access by the radiologist.
Because anesthesia is required for only a small number of cases, the anesthesiologist typically uses space “borrowed” from the interventionalist. Important considerations include positioning the anesthesia machine a sufficient distance from the patient to allow the fluoroscopy machine adequate travel for imaging. Extensions on intravenous tubing, arterial pressure tubing, and ventilator tubing are usually required to allow adequate distance from the patient. Neurointerventional radiology typically employs a biplane fluoroscopy machine to help minimize radiation. The rotation of this device requires that all tubing, monitors, and ventilator circuits travel inferior to the patient. The ASA standards should be followed for equipment in all off-site cases and include adequate oxygen supply and backup, suction, a waste scavenging system, electrical outlets with backup power supply, emergency equipment for cardiopulmonary resuscitation, trained support staff and two-way communication for assistance, and a rehearsed plan. Nitrous oxide should not be used with anesthesia machines that rely on activated charcoal filters (F/AIR filter canisters, Harvard Apparatus, Holliston, Mass.) because it cannot be effectively removed by these systems.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here