Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
An epithelial neoplasm originating in the long bones of the skeleton, referred to as adamantinoma, which almost exclusively affects the tibia and fibula, and its relationship to a coexisting peculiar fibroosseus lesion, has been intriguing both pathologists and clinicians for more than a century. In 1913, Fischer described a peculiar tumor occurring predominantly in the tibia and occasionally in the fibula that was somewhat similar to a more common odontogenic adamantinoma of the jaw bones. Despite histologic similarity, there has been no proof that these tumors—of the jaw bone and of the long bone—have a similar histogenetic origin. Adamantinoma of long bones is extremely rare; there are approximately 300 well-documented cases published in the world literature. The largest individual series of long bone adamantinoma was reported by a group from the Mayo Clinic. Long bone adamantinomas present with a wide range of morphologic patterns that can mimic various primary or even metastatic bone lesions. The morphologic diversity of long bone adamantinomas also accounts for the continuing controversy regarding their histogenesis. Another interesting phenomenon is the association of these tumors with areas resembling osteofibrous dysplasia (ossifying fibroma of long bones).
The first description of areas of rarefaction associated with long bone adamantinoma interpreted to be osteitis fibrosa was provided in 1942 by Dockerty and Myerding. In a subsequent article by Baker et al., these lesions were interpreted to be fibrous dysplasia. The first comprehensive description of long bone adamantinoma associated with areas resembling fibrous dysplasia was provided in 1962 by Cohen et al. These authors stated that the radiologic pattern of fibrous dysplasia associated with adamantinoma was different from that of classic fibrous dysplasia. Moreover, café au lait spots, which are frequent in polyostotic fibrous dysplasia, were not seen in association with adamantinomas. Therefore these authors were the first to postulate that, despite the histologic similarity to fibrous dysplasia, fibroosseous lesions associated with adamantinoma may represent a distinctive feature related to these tumors. The description of ossifying fibroma of long bone by Kempson in 1966 and the subsequent redesignation of this lesion as osteofibrous dysplasia by Campanacci et al. provided a new basis for studying the relationship between adamantinoma and fibroosseous lesions of the long bones. Further studies of such composite lesions showed that the histologic and radiologic features of the fibroosseous components were more consistent with those of osteofibrous dysplasia. These studies also showed that although osteofibrous dysplasia–like changes are frequently present at the periphery of adamantinomas, in some instances they may dominate the entire lesion. Most current studies have shown that long bone adamantinoma, despite the considerable range of its histologic patterns, appears to be of an epithelial nature.
The correlation of clinical, radiologic, and histologic data of our 25 consultation cases showed that long bone adamantinomas could be divided into two main groups ( Fig. 17-1 ). The first, defined as classic adamantinoma, is characterized by an abundance of tumor cells and destructive growth pattern radiographically. The second group is defined as differentiated adamantinoma and is characterized histologically by a predominance of an osteofibrous dysplasia–like pattern with a small, inconspicuous component of epithelial tumor elements. On radiographs, differentiated adamantinomas are intracortical and often multicentric lesions. The histogenesis of these peculiar neoplasms is still unclear, and various concepts of their origin as derived from eccrine gland, synovial, vascular, or pluripotential mesenchyma have been postulated. The current literature continues to subdivide adamantinomas of long bones into the classic and differentiated osteofibrous dysplasia-like forms. A summary of clinical, radiographic, and histologic features of the two subsets of long bone adamantinoma is provided in Table 17-1 . In the description in this chapter, classic and differentiated adamantinoma are discussed separately.
| Feature | Classic | Differentiated |
|---|---|---|
| Age | Usually after first 20 years | First 20 years |
| Location | Tibia or fibula | Tibia or fibula |
| Radiograph | Expand beyond periosteum or intracortical | Intracortical |
| Histology | Predominance of tumor cells forming basaloid, spindled, tubular, or squamous pattern | Predominance of osteofibrous dysplasia pattern |
| Immunohistochemistry | Predominance of cytokeratin-positive cells | Scattered elements positive for cytokeratin |
Classic adamantinoma is a distinct, rare biphasic neoplasm of long bones that predominantly involves the tibia and occasionally the fibula and is characterized by a variety of histologic patterns. It has an overall histologic similarity to the more common odontogenic adamantinoma of the jaw bones.
Classic adamantinoma is extremely rare; while its true incidence is unknown, it clearly accounts for less than 1% of all bone neoplasms. This tumor typically affects patients older than age 20 years ( Fig. 17-2 ), but occasionally it may occur during the first two decades of life. The youngest patient with classic adamantinoma in our series was a 13-year-old boy who subsequently developed lung metastases. More recently, a case of classic adamantinoma has been observed in the tibia of a 3-year-old boy. The involved bones are usually the tibia, fibula, or both. Involvement of other skeletal sites is rare but has been reported. According to our experience, the involvement of other skeletal sites should make the clinician question the diagnosis of adamantinoma and rule out other metastatic neoplasms. Synchronous involvement of the tibia and fibula by two independent foci or by contiguous foci is typical of adamantinoma. The tibia and fibula are synchronously involved in approximately 50% of cases.
Pain, bowing deformity of the tibia, or both of these symptoms are the most frequent clinical findings, and these may be present for decades before diagnosis. A palpable mass may be present.
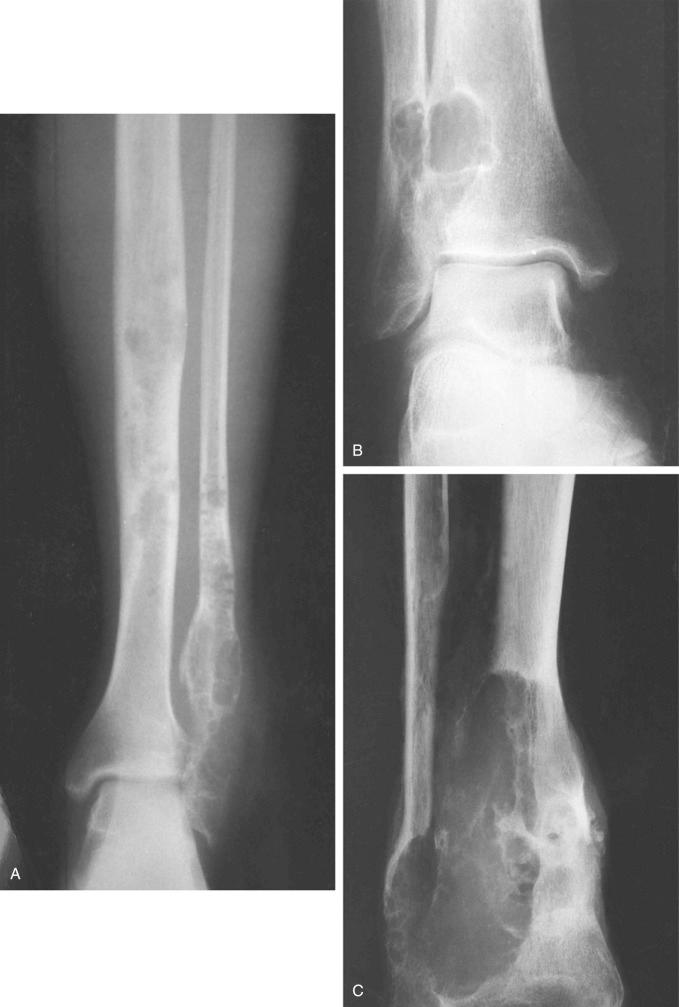
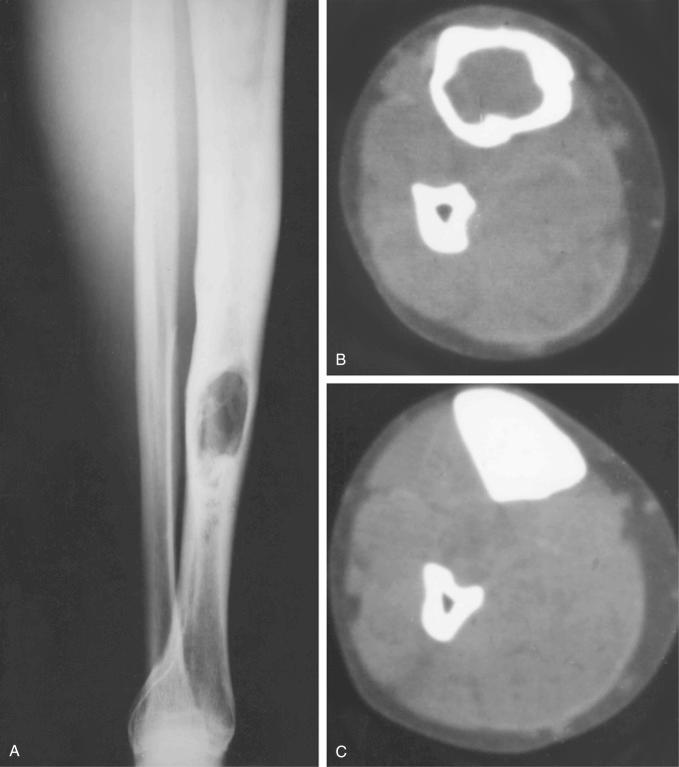
Features of classic adamantinoma on radiographs are distinct and often diagnostic. According to the radiographic appearance, the lesion can be divided into two major groups: intracortical and with radiologic evidence of complete cortical disruption and involvement of the medullary cavity, adjacent soft tissue, or both. Approximately 70% of lesions are located in the midshaft of the tibia. The remaining 30% involve the proximal or distal tibial regions. In the tibia, involvement of the anterolateral cortex and the presence of a moderate to severe bowing deformity are typical. The common appearance is of mixed lytic and sclerotic lesions or multiple lucencies with sclerosis of the intervening bone ( Figs. 17-3 and 17-4 ). The lucent lesions have well-defined margins. Features of a destructive growth pattern with cortical disruption are often seen. On radiographs, some lesions appear as destructive lytic defects involving two adjacent bones, the tibia and fibula. This is typically seen when the lesions are located at the proximal or distal end of the tibia ( Fig. 17-3 ). The synchronous involvement by separate foci in the tibia and fibula is less frequent in this form of adamantinoma (i.e., classic versus differentiated). The eccentricity of the lesions is less evident in the fibula, in which the lesions are more centrally located and represent sharply demarcated lytic foci with sclerotic margins. Expansion of the bone contour without bowing deformity is typical of fibular lesions. A recent study of the role of magnetic resonance imaging (MRI) characteristics found that the method did not add to the specificity of the distinction between histologic subtypes, but was helpful in evaluating soft tissue and intramedullary extension.
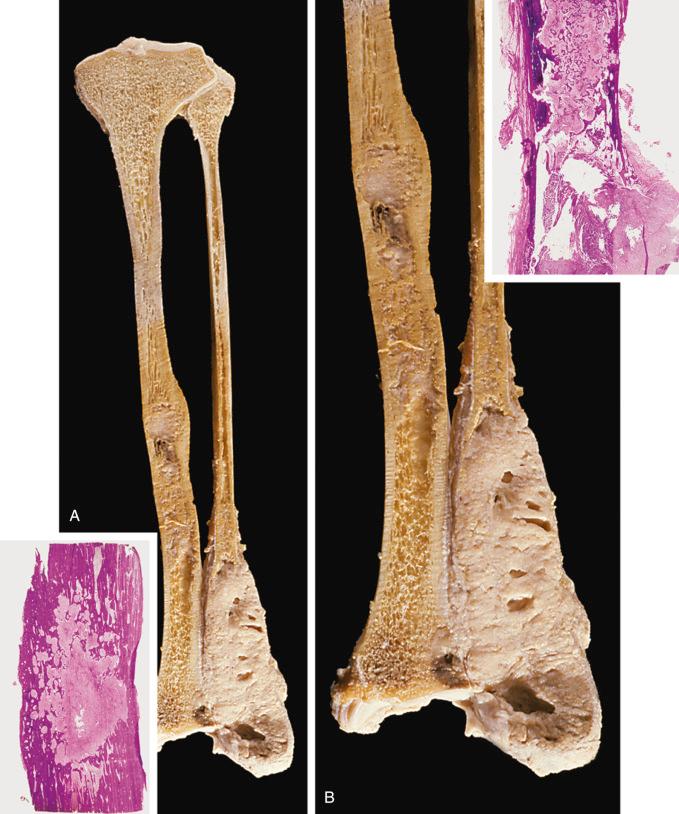
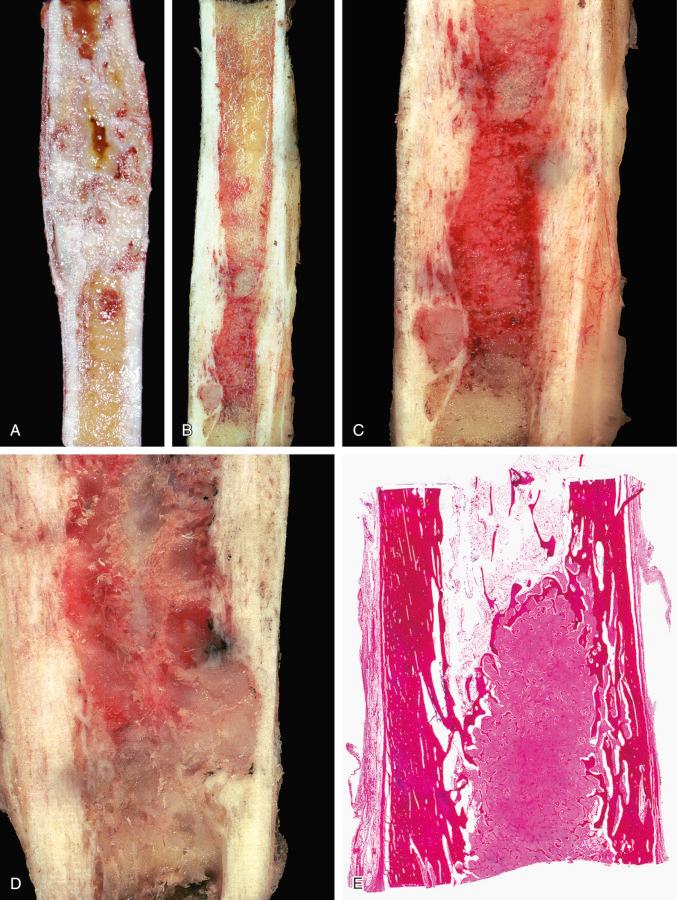
Classic adamantinoma evaluated in a resection specimen presents as a fleshy, soft mass. The lesion can be multifocal or can involve a large segment of the medullary cavity ( Figs. 17-5 and 17-6 ). Gradual transition from soft, fleshy areas to gritty fibrous tissue can be seen. Complete cortical disruption and contiguous involvement of adjacent soft tissue or paired bone may be present.
The histologic patterns of adamantinoma are extremely variable within each case and among cases. However, five basic patterns can be recognized: basaloid, spindle, tubular, squamous, and osteofibrous dysplasia–like.
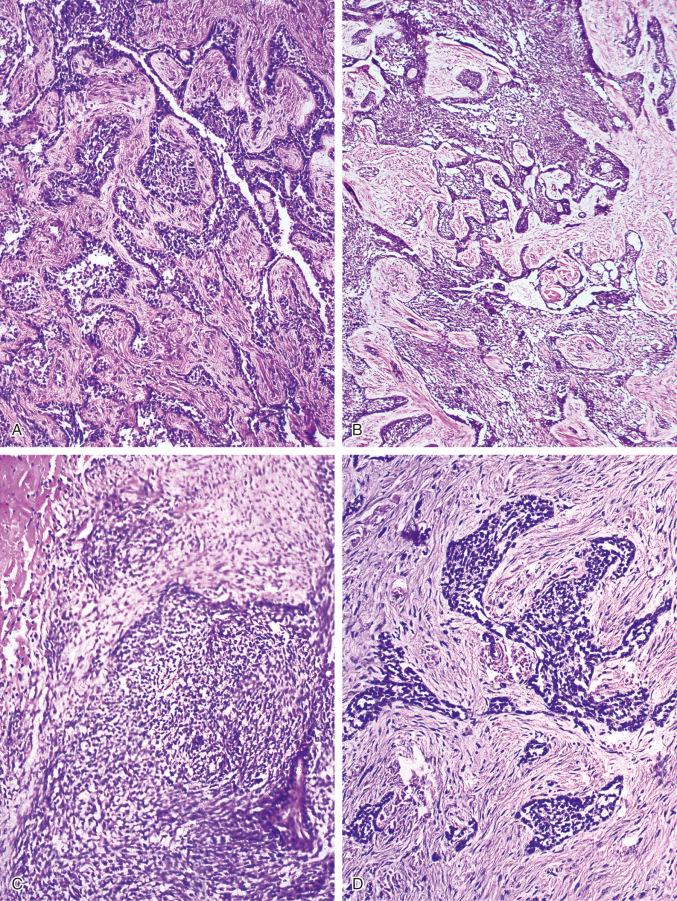
The basaloid pattern consists of nests or large masses of epithelioid tumor cells that have a peripheral layer of cells oriented roughly at right angles to the inner masses of cells ( Figs. 17-7 and 17-8 ). Peripheral cuboid or columnar cells with elongated nuclei occasionally produce sharply demarcated palisading structures. Cystlike spaces of various sizes within the masses of cells usually separate the outer layer of cells from the inner masses. The solid areas are composed of cells forming sheets with a roughly parallel arrangement of their oval nuclei.
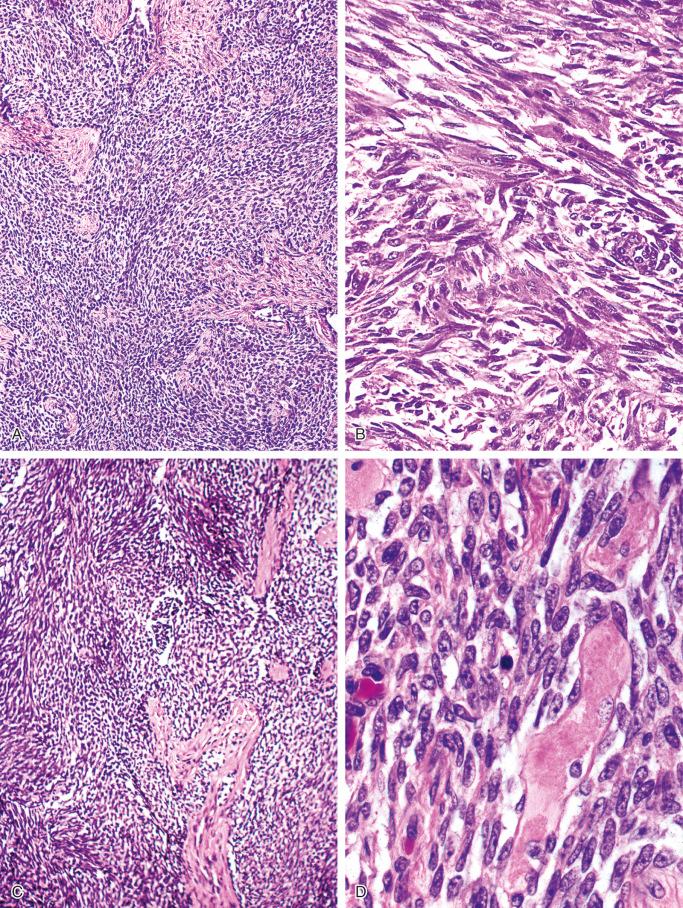
The spindle pattern is characterized by the presence of spindle-shaped cells with elongated nuclei ( Fig. 17-9 ). This pattern also lacks the peripheral palisading orientation of the cells that is characteristic of the basaloid pattern. The areas of pure spindle-cell pattern resemble mesenchymal spindle-cell tumors such as fibrosarcoma, even exhibiting a herring-bone pattern typical of that tumor.
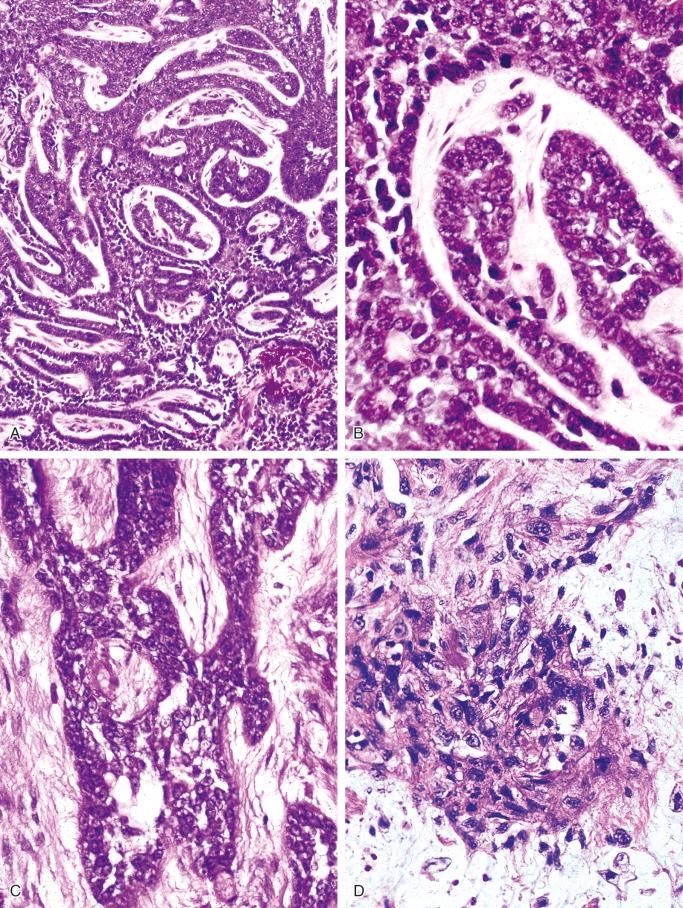
The tubular pattern consists of small, flattened or cuboid cells that line spaces of varying sizes ( Fig. 17-10 ). In some areas the tubular pattern is produced by small glandular spaces separating spindle cells. Sometimes, tubular structures lined by one or several layers of cuboidal cells or flattened cells are embedded in fibrous stroma. Tubular structures give the impression of vascular channels of various sizes or resemble glandular structures. Small tubular spaces occasionally resemble primitive capillary channels. Careful examination, however, sometimes reveals the presence within these structures of cells with features of epithelial differentiation or even keratinization.
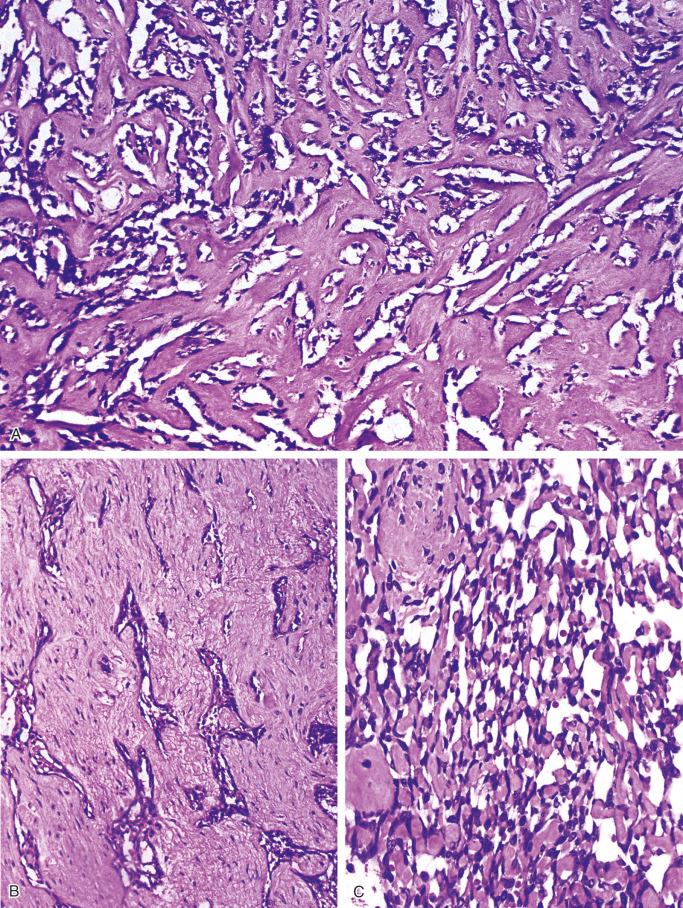
The squamous pattern is characterized by areas that show evidence of squamous differentiation, including eosinophilic cytoplasm and the presence of round, oval, or polygonal cytoplasm with occasional pyknosis of the nucleus ( Fig. 17-11 ). Cells with these features form areas of various sizes embedded within solid masses of nonsquamous cells. Features of squamous differentiation also are commonly seen in the peripheral parts of the solid tumor nests, with individual squamous cells embedded in surrounding stromal tissue.
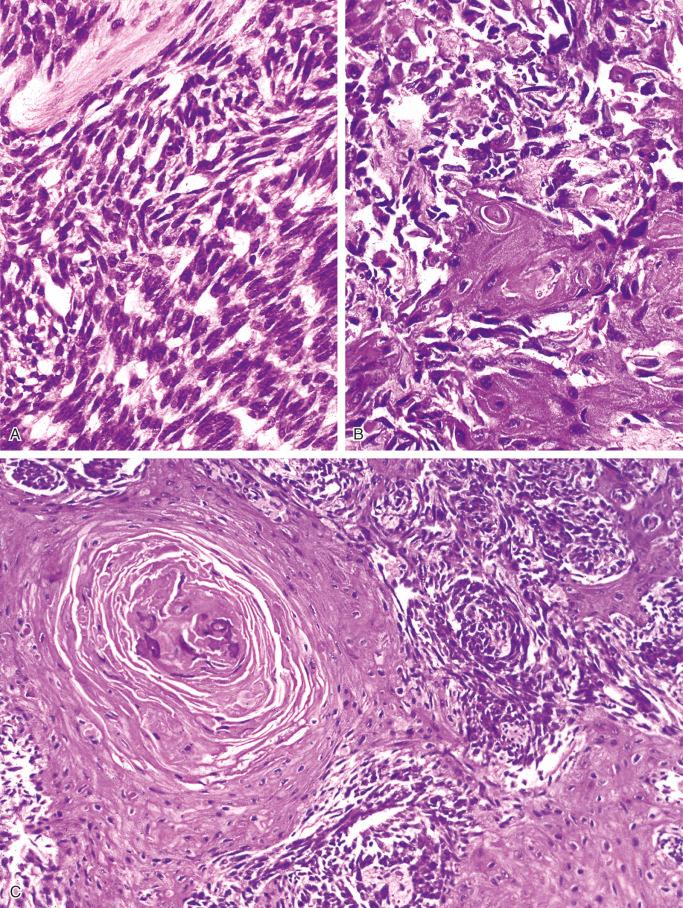
The osteofibrous dysplasia–like pattern is seen at least focally in all cases of adamantinoma with adequate sampling of the tumor and its periphery. It represents areas of loose fascicles of elongated fibroblast-like cells that often have a storiform pattern. Trabeculae of bone surrounded by rims of osteoblasts are present within this tissue. The zonal architecture of the fibrous dysplasia–like changes can be seen in adequately sampled lesions, which show progressive maturation and increasing numbers of bone trabeculae at the periphery of the lesion. When examined with the aid of polarized light, the increasing maturation from woven bone trabeculae in the central parts to predominantly lamellar bone at the periphery of the lesion is seen. The main feature differentiating this pattern from typical osteofibrous dysplasia is the presence of small nests of epithelial cells in the fibroblastic stroma that show occasional squamous differentiation. In addition to the solid epithelial nests, small tubular structures lined by cuboidal or flattened cells scattered in the fibrous stroma are commonly found.
The classic adamantinomas are characterized microscopically by the abundance of tumor cells and a frequent mixture of several histologic patterns. The osteofibrous dysplasia–like pattern is seen focally only and never dominates the histologic picture.
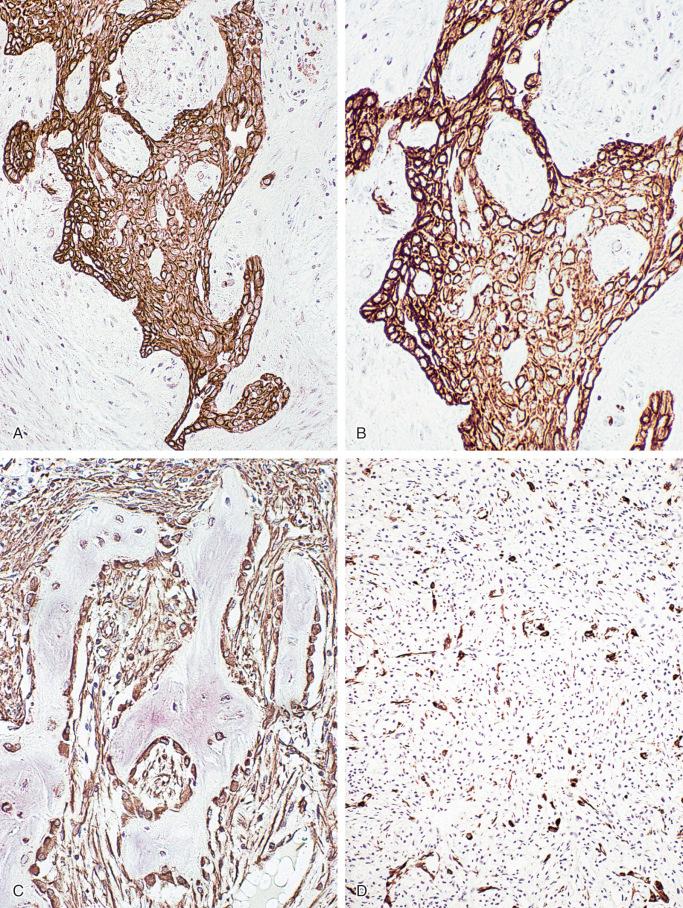
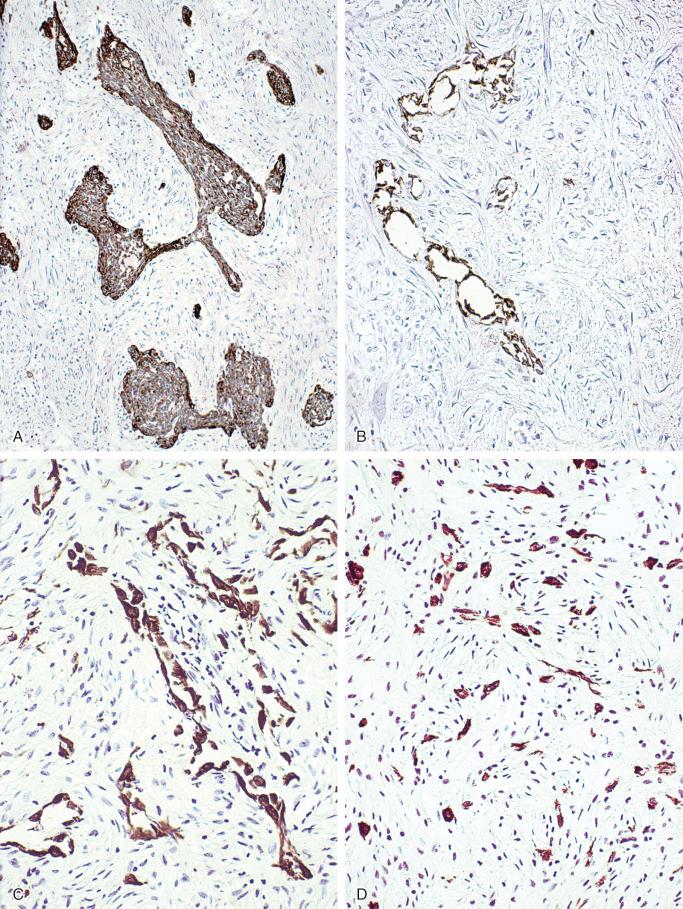
Ultrastructurally, adamantinoma is epithelial in nature with prominent desmosomes, tubular structures, and features of keratinization seen focally. Tumor cells of basaloid, spindled, tubular, and squamous patterns are consistently strongly positive for keratin ( Figs. 17-12 and 17-13 ). The fibrous stroma and bone trabeculae of the osteofibrous dysplasia–like pattern are negative for keratin; however, small nests of epithelial cells and tubular structures scattered in the stromal tissue are strongly positive for keratin. More detailed studies reveal that adamantinomas of long bone express classes of keratins similar to those found in the basal layer of the epidermis. Typically the epithelial component shows expression of keratins 5, 14, 9, 1, 13, and 17. Keratins 8 and 18 are not expressed. Interestingly, epithelial components additionally coexpress epithelial membrane antigen, p63, vimentin, and podoplanin indicative of a mixed epithelial/mesenchymal phenotype. Staining for vimentin is uniformly strongly positive in tumor cells of all patterns and in fibroblast-like stroma, including blood vessels and osteoblasts. Staining for factor VIII, Ulex europaeus, CD31, and CD34 is negative in all patterns of tumor cells, fibrous stroma, and osteoblasts; however, it is clearly positive in endothelial cells lining the blood vessels. All tumor cells and various components of the osteofibrous dysplasia–like pattern are uniformly negative for S-100 protein. Staining for actin is negative in all types of tumor cells. In contrast, fibroblast-like stromal cells are focally weakly positive for this marker. Classic adamantinomas express E-, P-, and N-cadherins, which are typically not found in osteofibrous dysplasia–like adamantinomas.
Cytogenetic studies revealed numerical chromosomal aberrations with extra copies of chromosomes 7, 8, 12, 19, and 21 that could be detected in both classic and osteofibrous dysplasia–like adamantinomas. The similarities of those aberrations in two subtypes of adamantinomas further support the relationship between classic and osteofibrous dysplasia–like adamantinomas. Structural chromosomal aberrations with translocations and marker chromosomes resulting in complex karyotypes are typically seen in classic adamantinoma and are not observed in osteofibrous dysplasia–like adamantinomas. Taken together these observations support the progressive nature of chromosomal aberrations when disease evolves from osteofibrous dysplasia–like adamantinoma to classic adamantinoma. Occasional translocations involving the 13q14 region of the tumor and germline in the same patient have also been reported. In general, on total DNA measurements, the epithelial component of adamantinoma is highly aneuploid and osteofibrous dysplasia is euploid.
Adamantinoma of long bones can be confused with a variety of primary and metastatic epithelial and soft tissue neoplasms.
In limited biopsy specimens, epithelial elements, especially basaloid/tubular or squamous patterns, can be misdiagnosed as metastatic carcinoma . Identification of a peculiar mixture of various patterns present in the majority of adamantinomas, as well as the location of the lesion in the tibia, the fibula, or both areas, should help avoid this error.
Dominant spindle-cell or small tubular patterns can be misdiagnosed as fibrosarcoma or a vascular neoplasm . Identification of the epithelial nature of cells by appropriate immunohistochemical stains and correlation with radiographic data are helpful in identifying the lesion as adamantinoma.
More difficult problems exist in biopsy specimens containing predominantly fibroosseous areas that can be confused with fibrous dysplasia or ossifying fibroma (osteofibrous dysplasia). The distinction from fibrous dysplasia can be easily made if radiographic features of the lesion are considered. Fibrous dysplasia typically presents as an intramedullary lesion. Scrupulous search and immunohistochemical stains can disclose inconspicuous epithelial elements in a dominant osteofibrous dysplasia–like pattern. In such instances, the distinction between classic and differentiated adamantinoma should be made ( Table 17-1 ).
In rare instances, morphologic overlap has been observed between adamantinoma and Ewing's sarcoma . These perplexing cases have been referred to as both Ewing's-like adamantinoma and as adamantinoma-like Ewing's because they may not only share phenotypic epithelial features (cytokeratin immunoreactivity and well-formed desmosomes ultrastructurally), but also O-13 antigen, S-100, and NSE immunoreactivity, dense core granule presence ultrastructurally, as well as 11;22 translocation [t(11;22) (q24;q12)], resulting in the fusion of EWSR1 and FLI1 genes. (See also discussion in Chapter 11 .) Although such exceptional cases definitely enter into the differential diagnosis of adamantinoma, they are properly regarded as variants of Ewing's sarcoma and not the other way around, in our opinion.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here