Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acute renal dysfunction represents a critical complication of an acute thermal injury and is associated with a significant increase in morbidity and mortality. Currently, the incidence of acute renal failure (ARF) in burn patients varies between 0.5% and 30%, and the risk of mortality associated with renal failure in burn patients has been reported to be as high as 54–100%.
Prior to 1965, there were no reported survivors from ARF following a thermal injury. Clearly the care of patients with renal injuries has improved during the past 50 years. However, while advances have been made in the understanding of burn-associated ARF, very few specific interventions have been clearly shown to change outcomes in these patients. Even renal replacement therapy (RRT), one of the core milestones of modern medical progress, has yet to show a significant improvement in the mortality rate of burn patients suffering from ARF. As such, the optimal treatment for acute renal failure remains prevention.
This chapter will review the definition, etiology, pathophysiology, diagnosis, and treatment of acute renal failure in association with thermal injury ( Fig. 31.1 ).
Intuitively ARF is easily understood as an abrupt decline in renal function. Precisely how to quantify renal function and where to place the threshold for “failure” has long been open to interpretation. Until 2004, with no consensus regarding a definition for ARF, more than 30 different definitions were used within the literature at the time. The need for a common working definition of renal failure prompted an effort to standardize the definition of renal insufficiency, with the International Acute Dialysis Quality Initiative (ADQI) group developing the RIFLE criteria for kidney injuries in 2004. The RIFLE criteria divided renal compromise into five discrete categories: Risk, Injury, Failure, Loss, and End-stage renal disease ( Fig. 31.2 ). In addition to establishing a common definition, the RIFLE criteria provided a means to quantify degrees of acute kidney insufficiency or injury (AKI). In 2007, the Acute Kidney Injury Network (AKIN) introduced an updated definition of acute kidney injury; see Tables 31.1 and 31.2 . The AKIN definition simplified the stratification of renal injury into three stages (I, II, and III), with the RIFLE Failure, Loss, and End-stage renal disease categories folded into grade III. There was also an increased sensitivity (relative to the RIFLE criteria) by virtue of use of an absolute increase in serum creatinine of 0.3 mg/dL or more as sufficient to define a stage I injury. In 2012, the Kidney Disease Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group published a consensus definition of renal injury that essentially merged the two previous frameworks, with slight adjustments.
| SERUM CREATININE CRITERIA FOR THE DEFINITION AND CLASSIFICATION OF ACUTE KIDNEY INJURY | ||||
|---|---|---|---|---|
| RIFLE Criteria—The Acute Dialysis Quality Initiative (ADQI) | AKIN Criteria—Acute Kidney Injury Network | KDIGO Criteria—Kidney Disease Improving Global Outcomes | ||
| Risk | Increase in serum creatinine ≥1.5 times baseline OR Decrease in GFR ≥25% |
Increase to 1.5–1.9 times baseline OR Increase in serum creatinine of ≥3 mg/dL (26.2 µmol/L) from baseline |
Increase to 1.5–1.9 times baseline OR Increase in serum creatinine of ≥0.3 mg/dL (26.2 µmol/L) from baseline |
Stage I |
| Injury | Increase in serum creatinine ≥2.0 times baseline or decrease in GFR ≥50% | Increase in serum creatinine to 2–2.9 times baseline | Increase in serum creatinine to 2–2.9 times baseline | Stage II |
| Failure | Increase in serum creatinine ≥3.0 times baseline OR Decrease in GFR ≥75% OR An absolute serum creatinine ≥354 µmol/L with an acute rise of at least 44 µmol/L |
Increase in serum creatinine to ≥3 times baseline OR Serum creatinine ≥4.0 mg/dL (354 µmol/L) with an acute rise of ≥05 mg/dL (44 µmol/L) OR Initiation of renal replacement therapy |
Increase in serum creatinine to a level ≥4.0 mg/dL (353.6) OR Initiation of renal replacement therapy |
Stage III |
| KDIGO CRITERIA FOR RENAL INJURY | ||
|---|---|---|
| Stage | Serum Creatinine Criteria | UO Criteria |
| I | Increase to 1.5–1.9 times baseline OR Increase in serum creatinine of ≥0.3 mg/dL (26.2 µmol/L) from baseline |
<0.5 mL/kg/h for ≥6 h |
| II | Increase in serum creatinine to 2–2.9 times baseline | <0.5 mL/kg/h for >12 h |
| III | Increase in serum creatinine to a level ≥4.0 mg/dL (353.6) OR Initiation of renal replacement therapy |
<0.3 mL/kg/h ≥24 h or anuria ≥12 h |
While the proliferation of consensus definitions may seem to undermine the basic goal of establishing a universal metric for research and clinical applications, the three systems have proved fairly interchangeable. Multiple studies applying the different scoring systems to the same patient populations have found that they ultimately yield similar results. Specifically in burn populations, Chung et al. found that RIFLE and AKIN identified similar subpopulations when applied to a large cohort of patients.
Burn-associated acute kidney injuries can generally be divided into two categories, early and late. AKI presenting within the first 48 hours of the burn injury (i.e., early AKI) typically results from either uncontrolled burn shock, underresuscitation, or protein degradation products. Renal injury manifesting later in the course of acute burn care (i.e., late-onset AKI) typically represents either a medication toxicity or a complication of sepsis.
Within the first 24–28 hours of a massive burn injury, AKI results from the pathologic response to thermal injury. Early burn-associated AKI is multifactorial, with hypovolemia, inflammatory mediators, cytokines, extensive tissue destruction and release of denatured proteins, iatrogenic causes (nephrotoxic agents), and cardiac dysfunction all contributing to the renal insult. In the setting of delayed resuscitation, hypovolemia is the most immediate threat to renal function. And yet AKI can still develop in the thermally-injured patient despite aggressive fluid resuscitation and a normal urine output. In such cases, renal injury might reflect a fluid-refractory, inflammatory shock in response to the burned tissue, cardiac dysfunction, or injury from nephrotoxins, which can be either endogenous (denatured proteins) or exogenous (medications) in nature.
In large surface area burns, decreased renal blood flow results from massive fluid shifts and losses. Local and systemic cytokine release leads to “capillary leak,” shifting fluids from the intravascular to interstitial space. Burn-induced compromise of the water-tight dermal barriers results in rapid evaporative losses from the extravascular compartment, which facilitates further extravasation of the intravascular compartment in a vicious cycle. Given the sheer speed and volume fluid shift seen in burn shock, profound intravascular hypovolemia can result. Renal blood flow is restricted in a compensatory response to hypovolemia resulting in renal ischemia. The ischemic insult is known to produce oxygen free radicals that cause direct tubular damage as well as disruption of tight junctions, resulting in obstructing casts that further reduce effective glomerular filtration rate (GFR).
Hypovolemia can develop within the course of minutes in the absence of appropriate fluid replacement. As such, hypovolemia represents by far the most likely source of AKI in any burn patient showing signs of renal insult within 24 hours of their burn.
Unfortunately, overadministration of fluids can be just as harmful as underresuscitation. Studies have shown that AKI can develop in burn patients despite fluid resuscitation volumes in excess of that recommended by the Parkland formula and despite normal average urine output (0.5–1.0 mL/kg per hour). Furthermore the risks of overresuscitation have been well-documented and include pneumonia, acute respiratory distress syndrome (ARDS), compartment syndromes, and an overall increase in mortality.
Despite the physician's greatest effort to monitor endpoints of resuscitation, obligatory intercompartmental fluid shifts will occur during resuscitation. These intercompartmental fluid shifts can be particularly hazardous if they occur into fascial bound compartments, such as the peritoneal cavity. Numerous studies from trauma literature have described the adverse physiologic effects of increasing intraabdominal pressure on visceral perfusion. Intraabdominal hypertension (IAH) is a known pathological process that may occur during initial burn resuscitation as defined by intraabdominal pressures (IAPs) of greater than 12 mm Hg. Abdominal compartment syndrome (ACS) is defined as an IAP of greater than 20 mm Hg with at least one concomitant organ failure. The exact level of abdominal hypertension required to compromise visceral perfusion varies depending on a variety of patient factors and is difficult to predict. O'Mara et al. demonstrated that the volume and type of fluid resuscitation affects the development of ACS in the burn patient and suggested that fluid resuscitation with crystalloid of greater than 0.475 L/kg should alert the clinician to possible IAH/ACS and to monitor for decreased cardiac output, decreased lung compliance, or decreased renal perfusion. In a mixed population of critically ill patients, a multicenter prospective trial has demonstrated that the occurrence of IAH during the ICU stay was an independent outcome predictor.
Rhabdomyolysis frequently presents within 24 hours of thermal injury and represents a well-documented risk for AKI and subsequent renal failure. Rhabdomyolysis can arise secondary to direct thermal damage or compartment syndrome and is commonly seen following a severe electrical injury. The release of myoglobin into the systemic circulation results in blockage of renal tubules, constriction of afferent arterioles, and the generation of oxygen free radicals. Myoglobinuria occurs when serum myoglobin exceeds 1500–3000 ng/mL. Myoglobinuria does not always result in renal injury, but certain risk factors have been identified. The risk of renal injury is directly related to the amount of iron-containing molecules released, the state of hydration, and the degree of associated acidosis. Elevated creatinine at baseline and creatinine kinase levels greater than 5000 U/L have been associated with the development of AKI and the need for RRT, both in general trauma populations and in studies of burn patients.
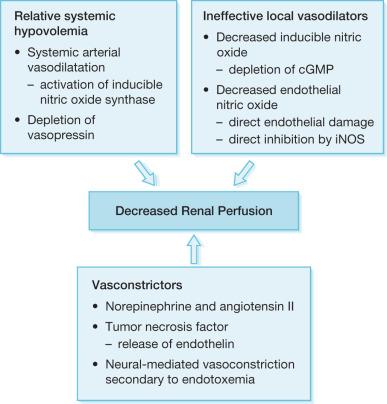
Patients suffering burns greater than 50% total body surface area (TBSA) are subject to decreased cardiac output, increased myocardial workload, and myocardial ischemia. Several authors have suggested theories to explain the decreased cardiac output associated with thermal injury: (1) increased sympathetic activity with impaired adrenal response, (2) hypovolemia resulting in myocardial ischemia, and (3) direct myocardial suppression. Of the potential theories, direct myocardial suppression by tumor necrosis factor (TNF; i.e., myocardial depressant factor) has gained substantial interest. TNF is known to be released by myocytes stimulated by endotoxin or direct thermal injury. The effects of TNF on cardiac function include reversible biventricular dilatation, decreased ejection fraction, and decreased stimulation to catecholamines ( Fig. 31.2 ). This is typically a transient phenomenon, and, with adequate support, it usually resolves within 24–72 hours. Without appropriate support, though, frank heart failure can develop, bringing a myriad of complications with long-lasting or even permanent sequelae. Although most early cardiac dysfunction caused by TNF can be reversed by inotropic support, the key is early diagnosis to prevent ineffective renal perfusion and thus prevent the morbidity and mortality associated with renal insufficiency.
Cardiac dysfunction is known to result in reduced renal blood flow and hence to contribute to AKI. Although diminished cardiac output following thermal injury has been attributed to decreased preload or hypovolemia, there is also evidence of direct myocardial suppression, The impact of this suppression can range from clinically undetectable to frank cardiac shock. Often, this presents as a lack of appropriate cardiac compensation for distributive burn shock. Myocardial dysfunction after thermal injury is commonly overlooked by physicians due to the concentrated effort to correct the overwhelming state of hypovolemic shock and electrolyte abnormalities. In a patient presenting with burn shock (i.e., hypoperfusion) and found to have a cardiac index in “normal” range (rather than the expected supra-normal values), serious consideration should be given to inotropic support, particularly when accompanied by the finding of a low systemic vascular resistance index.
In any case of suspected cardiac dysfunction, a typical workup should be performed to rule out any coronary or mechanical etiology. Absent any such finding, an effective burn surgeon must rapidly reestablish adequate renal blood flow by correcting the diminished preload state while keeping in mind the impact of burn injury on the entire cardiovascular system.
AKI presenting later in the course of treatment for a massive burn typically occurs as a component of multiorgan failure, most commonly from sepsis. Given the high risk for infection and the difficulty of diagnosing such infections in this patient population, underlying infection and sepsis must be seriously considered in any patient found to have new or worsening renal failure more than 48 hours out from their initial injury.
Iatrogenic renal injury is also an important consideration in late burn-associated AKI, particularly given these patients' frequent exposure to multiple antibiotics and diuretics.
Early aggressive resuscitation and excision have significantly influenced the course of ARF immediately associated with thermal injury. Acute renal dysfunction associated with the septic syndrome nonetheless continues to cause significant mortality.
Sepsis and septic shock are the most common cause of death in the ICU and are seen in up to 87% of cases of acute renal dysfunction in the burn ICU. Several authors have found the degree of sepsis to be directly related to the incidence of acute renal dysfunction ( Table 31.3 ). The pathophysiology of AKI associated with sepsis is multifactorial in nature but begins clinically with a generalized arterial vasodilatation secondary to a decreased systemic vascular resistance ( Fig. 31.2 ). Initially bacteria or their products activate sepsis-associated mediators (cytokines) locally at the site of direct invasion. The homeostatic balance between production and inactivation of these mediators is altered, allowing for systemic release and causing direct damage to the endothelium and vasoparesis, as well as a procoagulant state. It has been theorized that acute renal insufficiency associated with sepsis is the result of each of these pathological processes.
a Sepsis associated with lactic acidosis or altered mental status.
The vasoparesis seen in sepsis results in a profound state of hypotension, which activates the neurohumoral axis. In an effort to maintain systemic arterial circulation, the sympathetic nervous system and the renin–angiotensin–aldosterone axis respond by increasing cardiac output and by direct renal arteriolar vasoconstriction. Furthermore, the systemic inflammatory response results in the release of additional vasoconstricting cytokines (i.e., TNF, endothelin), locally secreted vasodilators (endothelial and inducible nitric oxide) to counterbalance these sepsis-associated vasoconstrictors. Ultimately, this compensatory response comes at the cost of renal perfusion by further exaggerating the prerenal state.
Finally, as mentioned previously, sepsis induces a procoagulant state by affecting the expression of complement and the fibrinolytic cascade. This alteration in the homeostasis of coagulation may result in a state of disseminated intravascular coagulation (DIC) with direct injury to the kidney by glomeruli microthrombi. The net result is a lack of perfusion to the kidneys during sepsis that will ultimately culminate in ischemic acute tubular necrosis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here