Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
There were 19,950 new cases of acute myeloid leukemia (AML) and 6590 new cases of acute lymphocytic leukemia (ALL) in the United States in 2016, resulting in 10,430 deaths from AML and 1430 from ALL. Whereas the incidence of AML is relatively low until age 35 years and then rises almost exponentially, ALL peaks in incidence in young children. Risk factors for the development of acute leukemia include genetic predisposition as well as exposure to benzene, ionizing radiation, or previous chemotherapy.
The acute leukemias are clonal disorders, with all leukemic cells in a given patient descending from a common precursor. Recent genome-wide analyses of leukemia have demonstrated considerable complexity with a number of recurrent potential “driver” mutations and a much larger number of random “passenger” mutations in individual cases.
The diagnosis of acute leukemia is generally made by bone marrow examination. In the past, the classification of acute leukemia depended heavily on morphologic examination of the leukemic cells. This is no longer the case, and currently, the most important elements of classification are the immunophenotype (to distinguish AML from ALL), cytogenetics, and mutational analyses. In AML, three primary-risk groups are recognized. Favorable-risk patients are those with CBF translocations and those with normal cytogenetics and either NMP1 or CEBPA mutations without mutations in FLT3-ITD . Adverse-risk patients are those with abnormalities of 3q, 5 or 7, t(6:9), or t(9;22); mutations in RUNX1, ASXL1, TP53, or FLT3 (without mutations in NPM1 ); and those with complex cytogenetics. The intermediate-risk group includes all those not classified as favorable or unfavorable. Acute promyelocytic leukemia, characterized by t(15;17), is a separate entity and requires specific therapy. In ALL, the favorable-risk group includes those with high hyperdiploidy and del9q. The unfavorable-risk group includes t(4;11), low hypodiploidy or near triploidy, and complex cytogenetics. All others comprise the intermediate-risk group. Patients with t(9;22) (Ph+ ALL) and mature B-cell ALL including Burkitt leukemia comprise separate categories of ALL that require specific treatment.
Induction chemotherapy that includes an anthracycline plus cytarabine will result in a complete remission in approximately 70% of patients. Midostaurin may improve outcome in those with FLT3 mutations. Postremission therapy depends on the risk group. Favorable-risk patients are generally treated with 3 or 4 cycles of consolidation chemotherapy, including intermediate-dose cytarabine. With such treatment, approximately 60% of patients younger than age 60 years will be cured. Patients with intermediate-risk disease should undergo allogeneic transplantation while in first remission if they have a matched sibling, a matched unrelated donor, or an acceptable cord blood source. The use of partially matched donors in this setting is less agreed on. Allogeneic transplantation using matched siblings, matched unrelated donors, or partially matched cord blood is recommended for patients with unfavorable-risk disease in first remission.
Supportive care alone, or therapy with low-dose cytarabine, decitabine, or azacitidine are all acceptable alternatives depending on the situation.
Induction therapy for lower risk patients (white blood cell count <10,000/mm 3 ) should include all- trans -retinoic acid plus arsenic trioxide. The addition of a third agent (gemtuzumab ozogamicin, or chemotherapy) may benefit patients with higher risk disease.
Induction therapy in ALL includes a combination of vincristine, prednisone, an anthracycline, and asparaginase, with cyclophosphamide sometimes included. With such regimens, 75% to 90% of patients will achieve a complete remission. Rituximab should be included during induction and postremission therapy for patients with CD20+ B-cell ALL. Postremission therapy generally involves 6 to 8 courses of intensive consolidation therapy, several of which contain high-dose methotrexate, cytarabine, and asparaginase, and several of which include the same drugs used for initial remission induction. Some form of central nervous system prophylaxis is required, as is low-dose maintenance therapy. More intense regimens mimicking those used for pediatric patients result in improved outcomes for patients up to age 40 years. High-risk patients should receive an allogeneic transplant in first remission if at all possible. The role of allogeneic transplantation for patients with standard-risk disease is more controversial.
A tyrosine kinase inhibitor (imatinib, nilotinib, or dasatinib) should be included as part of induction and consolidation therapy. Allogeneic transplantation is recommended in first remission if an appropriate donor is available.
Therapy should include, along with the usual drugs used for remission induction, high doses of fractionated cyclophosphamide, high-dose methotrexate and cytarabine, and rituximab.
Normal hematopoiesis involves the tightly regulated proliferation and maturation of pluripotent hematopoietic stem cells to become mature myeloid and lymphoid peripheral blood cells. Acute leukemia is the result of a series of mutational events occurring in an early hematopoietic precursor that prevents the progeny of that precursor from maturing normally but allows them to proliferate in an uncontrolled fashion. The result is the rapid expansion of an immature population of myeloid (in acute myeloid leukemia [AML]) or lymphoid (in acute lymphocytic leukemia [ALL]) cells that replace the normal bone marrow, leading to the loss of production of normal red blood cells (RBCs), white blood cells (WBCs), and platelets. Eventually, the leukemic cells escape from the marrow into the bloodstream and accumulate in lymph nodes, the spleen, and normal organs. If untreated, acute leukemia is usually fatal within a few months. However, with modern therapies, survival after the diagnosis of acute leukemia can be markedly prolonged, and many patients can now be cured.
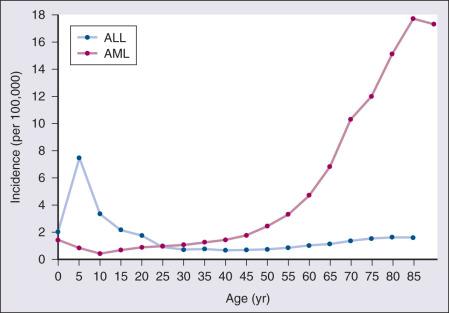
According to the American Cancer Society, there will be 19,950 new cases of AML and 6590 cases of ALL in the United States in 2016, resulting in 10,430 deaths from AML and 1430 from ALL. The incidence of AML has a bimodal pattern with a modest increase among infants, a decline in childhood, and then an exponential rise with advancing age ( Fig. 95.1 ). ALL likewise has a bimodal pattern, with a more striking peak among children aged 1 to 4 years, a decline between ages 20 and 60 years, and a more modest rise in advanced years. The rates for all forms of acute leukemia are higher in males than females and tend to be higher in non-Hispanic whites, with the exception of higher rates of acute promyelocytic leukemia (APL) and B-cell acute lymphoblastic leukemia (B-ALL) among Hispanic whites.
The concordance rate of acute leukemia in identical twins is virtually 100% if one twin develops leukemia during the first year of life but then declines with age. A number of germline mutations are associated with a predisposition to the development of acute leukemia. These can be categorized as syndromic, disorders of hematopoiesis, or cancer or leukemia related. Syndromic germline mutations are those associated with a defined syndrome that have nonhematologic abnormalities plus a predisposition to leukemia and include Fanconi anemia, Diamond-Blackfan anemia, Shwachman-Diamond syndrome, absent radius syndrome, and GATA2 deficiency. Germline mutations associated with disorders of hematopoiesis (usually affecting platelets) and a predisposition to leukemia include abnormalities in RUNX1 , ANKRD26 , and ETV . The final group of germline mutations includes those with a predisposition to the development of leukemia and in some cases other cancers but not with an identifiable syndrome or abnormal hematopoiesis before the development of leukemia. Li-Fraumeni syndrome; constitutional mismatch repair deficiency; and germline mutations in CEBPA , DDX41 , and PAX5 are in this group . With increased availability of high-throughput sequencing technologies, the number of identifiable leukemia predisposition syndromes is likely to grow. Knowledge of such syndromes is of importance in choosing therapies, donor selection in the case of transplantation, and for genetic counseling.
Human T-cell lymphotropic virus type 1 (HTLV1) is an enveloped, single-strand RNA virus that is associated with the development of a distinct form of adult T-cell leukemia found in southwestern Japan, the Caribbean basin, and Africa. The virus can be spread vertically from mother to fetus or horizontally by sexual contact or blood products. Although endemic in the areas noted, the virus only causes leukemia in 2% to 4% of those infected and has a very long latency period, estimated at 30 years or more. Epstein-Barr virus, a DNA herpes family virus, is associated with the endemic African form of Burkitt lymphoma and leukemia.
Ionizing radiation is leukemogenic. An increased incidence of AML, ALL and chronic myelogenous leukemia (CML) was seen in survivors of the atomic bombings of Hiroshima and Nagasaki, which began approximately 1.5 years after exposure, peaked at 7 years, and returned to baseline 25 years later. The risk of leukemia was also increased in individuals treated with radiation for ankylosing spondylitis in the 1940s. Although most of the data from humans showing the leukemogenic potential of ionizing radiation come from individuals exposed to high dose rates, occupational exposure to low dose-rate radiation has more recently been associated with the development of leukemia.
Extensive exposure to benzene or benzene-containing solvents can lead to marrow damage culminating in the development of aplastic anemia, myelodysplasia, or acute leukemia. Most studies examining the issue have found a small but consistent increase in AML among cigarette smokers.
Although the exact percentage is uncertain, as much as 7% of AMLs and a smaller percentage of ALLs are thought to be the consequence of prior exposure to chemotherapy or radiation for a primary malignancy or autoimmune disorder. Treatment-related leukemias can be grouped into several syndromes. AML developing after alkylating agent therapy has a latency of 5 to 7 years, often first appears as a myelodysplastic syndrome (MDS), and is frequently associated with abnormalities involving the long arms of chromosomes 5 or 7. Treatment-related AML is also seen after exposure to topoisomerase II inhibitors, such as etoposide and teniposide, or anthracyclines. These leukemias develop relatively rapidly, often within 2 years of exposure, are not generally preceded by a myelodysplastic phase, and frequently have rearrangements involving 11q23, the locus for MLL (the mixed-lineage leukemia gene) or 21q22. A number of other drugs have also been implicated in the subsequent development of acute leukemia, including bimolane, an agent used to treat psoriasis, and recently, lenalidomide, an agent used in the treatment of myeloma or myelodysplasia. Patients with lymphoma who receive autologous hematopoietic cell transplants are at increased risk for the development of leukemia, which may, in part, be predicted by the therapy received before the transplant. Secondary leukemias with inv(16), t(9;22), and abnormalities involving 3q21 have been reported. Not all secondary leukemias fall into these patterns, and because patients are usually treated with multiple agents, identifying a single offender is often impossible. Although there remains some debate, in general, patients with treatment-related leukemias do not fare as well as patients with de novo leukemia, even after adjusting for known risk factors.
In a large population-based study, 20% of cases of AML were found to have evolved from a prior hematologic malignancy, either myelodysplasia (MDS) in 12% of cases, or a myeloproliferative syndrome (MPS) (polycythemia vera, idiopathic myelofibrosis, or essential thrombocythemia) in 8%. The average duration from diagnosis of MDS until evolution to AML is roughly 18 months, and the time for evolution from MPS is 43 months. AML evolving from MDS or MPS responds less well to chemotherapy than does de novo AML and generally is considered adverse. The term “secondary AML” is sometimes used to describe both therapy-related AMLs and those arising from an antecedent hematologic malignancy. Although there are common features, including trilineage dysplasia and a high incidence of unfavorable cytogenetic changes, the current World Health Organization (WHO) classification schema distinguishes between the two, with separate definitions for therapy-related myeloid neoplasms and AML with myelodysplasia-related changes.
The acute leukemias are clonal disorders, with all leukemic cells in a given patient descending from a common precursor. The initial proof of clonality in leukemia came from studies of the disease in females who were heterozygotic for the X-linked glucose-6-phosphate dehydrogenase (G6PD) isoenzyme. In normal heterozygotic women, because of random X-linked inactivation, any single blood cell will express one or the other isoenzyme, and hematopoietic cells overall will be a 50–50 mix. Leukemia cells in G6PD heterozygotic females, however, were found in every case to be all of one isoenzyme or the other, indicating their origin from a single precursor.
Within the clonal populations of cells in patients with acute leukemia, there must be a subpopulation of cells capable both of self-renewal and proliferation. Such cells have been termed “leukemia stem cells.” Although there is no gold standard for their identification, the ability of human leukemic cells to engraft long term in immunodeficient mice is generally accepted as a marker for a leukemia stem cell. Normal hematopoietic stem cells capable of long-term engraftment in NOD/SCID/IL2R mice are confined to the CD34++CD38- subpopulation. In contrast, leukemia stem cells capable of long-term engraftment in such mice can be found in both CD34+ and CD34- fractions, as well as in CD38+ and CD38- populations. Recently, a 17-gene expression score has been developed that describes leukemia stem cells based on their ability to engraft in immunodeficient mice and predicts for relative resistance to chemotherapy.
The persistent growth of the leukemic clone leads to the failure of the marrow to produce normal RBCs, WBCs, and platelets, but the mechanisms by which this happens are not well understood. Although marrow failure may result at least in part by leukemia cells physically crowding out normal progenitors, frequently, peripheral blood counts start to fall weeks or months before the appearance of leukemic blasts in the marrow, and cases of hypoplastic acute leukemia are common.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here