Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Acute kidney injury (AKI) is common in critically ill neonates. AKI affects survival, hospital expenditures, and long-term outcomes, independent of the severity of illness and comorbidities.
Kidney development continues until 34 weeks’ gestation. Neonatal intensive care unit graduates, especially those with a history of AKI, those born prematurely, and those with intrauterine growth retardation, are at risk for long-term chronic kidney disease.
New technological advancements are making the use of kidney replacement therapy (dialysis) more common, safer, and effective in neonates.
Acute kidney injury (AKI) is characterized by a sudden impairment in kidney function, which may result in dysregulation of fluid balance, acid-base balance, electrolytes, and build-up of nitrogenous waste products. The term injury highlights the spectrum of organ injury and differentiates a damaged organ from an organ that has dysfunction to an organ that has failed. AKI is incrementally staged based on severity by oliguria or rise in serum creatinine (SCr).
The development and utilization of standardized definitions of AKI have created a commonality in defining AKI. Incremental degrees of AKI independently impact survival in critically ill neonates, children, and adults. All three of the above multi-center cohort studies used the Kidney Disease: Improving Global Outcomes (KDIGO) AKI definition. In neonates, a modified version of this definition ( Table 77.1 ) is the consensus, standard definition. This classification system utilizes the lowest SCr as a baseline and subsequent rise in SCr values to stage kidney injury. In April 2013, neonatologists and pediatric nephrologists participating in the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop carefully scrutinized this definition and concluded that, although there are limitations inherent to utilizing SCr, the modified, neonatal KDIGO definition represents the current consensus definition of neonatal AKI for research purposes and clinical care. This group also pointed out that this definition represents the first step in an iterative process to better define AKI, pointing to the need for neonatal-specific evidence to guide future definitions.
| Stage | Serum Creatinine | Urine Output |
|---|---|---|
| 0 | No change in SCr or rise <0.3 mg/dL | ≥0.5 mL/kg/h |
| 1 |
|
<0.5 mL/kg/h for 6–12 hr |
| 2 | SCr rise ≥2.0–2.9 × baseline SCr | <0.5 mL/kg/h for ≥12 hr |
| 3 |
|
|
a Baseline SCr defined as lowest previous SCr value.
b SCr value of 2.5 mg/dL represents glomerular filtration rate of <10 mL/min/1.73 m 2 .
A critical component to understanding the shortcomings of the current AKI definitions is to recognize the limitations of SCr as a biomarker. Importantly, SCr is a marker of kidney function and detects damage that has occurred in the preceding 48 to 72 hours. Thus, the current AKI definitions utilizing SCr do not detect kidney damage; instead, they document changes in kidney function. Table 77.2 details the limitations inherent to utilizing SCr.
|
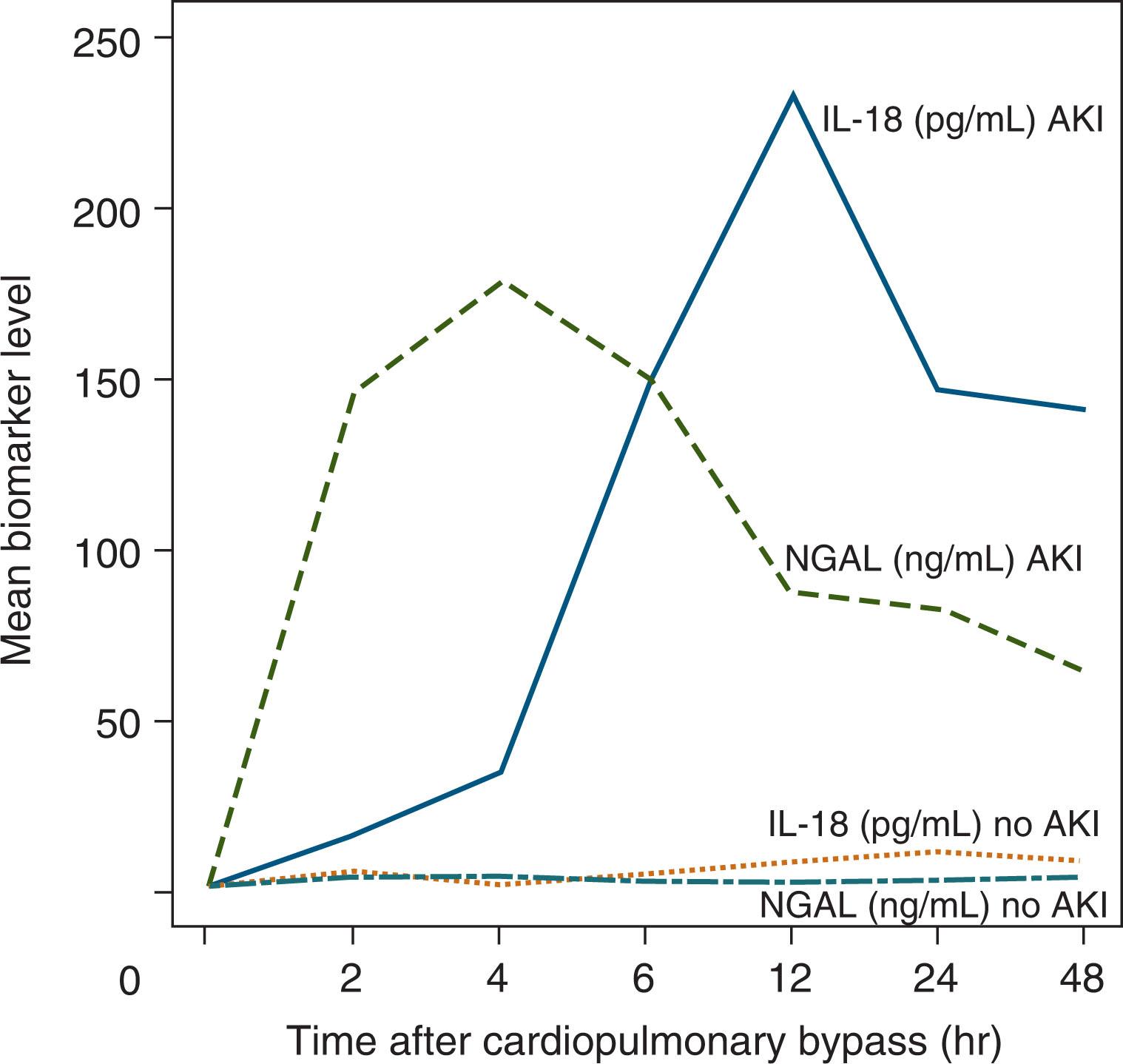
Over the last decade, there has been a significant amount of work to identify urine and serum biomarkers of AKI. Ideally, novel AKI biomarkers will show acute damage hours after an insult, distinguish between different causes and locations of tissue injury, and prognosticate clinical outcomes. Potential biomarkers include cystatin C, urine and serum neutrophil gelatinase-associated lipocalin (NGAL), urine interleukin-18, kidney injury marker-1, copeptin, and liver fatty acid-binding protein among others. Many of these were originally identified and studied in neonates undergoing cardiopulmonary bypass and now are being studied in neonates in the neonatal intensive care unit (NICU) ( Fig. 77.1 ). These serum and urine biomarkers notably vary based on gestational age (GA), day of life, and gender but show promise in their ability to predict AKI in preterm infants, very low birth weight (VLBW, i.e., birth weight <1,500 g) infants, near-term/term neonates, and neonates with perinatal asphyxia. Future work is needed to determine how best these biomarkers can be used at the bedside.
Over the past decade, there has been a significant amount of research utilizing modern staged definitions of AKI to evaluate the incidence and impact of AKI in the NICU. In the largest epidemiologic study to date, the Assessment of Worldwide Acute Kidney Injury Epidemiology in Neonates (AWAKEN) study, more than 2000 infants from 26 NICUs and 4 countries were enrolled allowing investigators to compare AKI incidences and associated outcomes across the GA spectrum in a multicenter, multinational cohort for the first time. Using this general NICU cohort, AWAKEN investigators demonstrated that 30% of neonates admitted to the NICU develop AKI and that the incidence of AKI varied across GA groups. Importantly, they also demonstrated that neonatal AKI was independently associated with both morbidity and mortality.
In the NICU there are a number of high-risk patient sub-populations that warrant separate discussion, including neonates with perinatal asphyxia, those undergoing cardiac surgery or receiving extracorporeal membrane oxygen (ECMO), and VLBW and extremely low birth weight (ELBW, i.e., birth weight <1,000 g) infants ( Table 77.3 ).
| Population | Study | Definition | Incidence of AKI | Findings |
|---|---|---|---|---|
| General NICU Population |
|
Neonatal modified KDIGO criteria | 29.9% | AKI varies by gestational age
AKI is associated with higher mortality and longer length of hospital stay. |
| Perinatal Asphyxia/HIE |
|
Neonatal modified KDIGO criteria | 38% | AKI predicted prolonged mechanical ventilation, length of stay, and abnormal brain MRI findings at 7–10 days of life. |
| Cardiopulmonary Bypass Surgery |
|
AKIN criteria | 62% | Severe AKI (stage III) was associated with increased mortality and length of stay after adjusting for severity of illness. |
| ECMO |
|
KDIGO criteria | 51% |
|
|
RIFLE criteria | 64% | Increased risk of mortality at highest level of AKI (failure). | |
| VLBW Infants |
|
Neonatal modified KDIGO criteria | 26.2% | AKI is associated with nephrotoxic medication exposure. |
|
Neonatal modified KDIGO criteria | 18% | Adjusting for severity of illness AKI was associated with increased mortality. | |
|
AKIN criteria | Matched case-control study | AKI is associated with increased mortality after adjustment for confounders. |
Infants with perinatal asphyxia represent a population at high risk for the development of AKI. Selewski et al. (2013) evaluated 96 newborns undergoing therapeutic hypothermia for perinatal asphyxia and found that 38% developed AKI. In this cohort, AKI was associated with adverse outcomes, including prolonged mechanical ventilation by a mean of 4 days ( P < .01) and prolonged hospitalization by 3.4 days ( P < .03). In the same cohort, those with AKI were more likely to have abnormal brain magnetic resonance imaging (MRI) findings at 7 to 10 days of life, implicating AKI as a potential marker and/or mediator of poor neurologic outcomes. Recently, in a randomized controlled trial (RCT) of 120 term neonates with perinatal asphyxia, those randomized to therapeutic hypothermia had lower rates of AKI (32% vs. 60%, P < .05), suggesting therapeutic hypothermia may protect against the development of AKI.
When examining infants greater than 34 weeks of gestation with perinatal asphyxia within the AWAKEN cohort, Kirkley et al. (2019) found that 42% (47/113) developed AKI and that those with perinatal asphyxia and AKI remained in the hospital with an average of 8.5 days longer than those with asphyxia but no AKI. More recently, Cavallin et al. (2020) demonstrated that AKI was associated with an increased likelihood of unfavorable neurodevelopmental outcomes at 24 months (100% vs. 59% in neonates without AKI; P = .01).
Several factors contribute to the risk of postoperative AKI in neonates undergoing cardiac pulmonary bypass (CPB) surgery, including prematurity, CPB characteristics and duration, surgical complexity, perioperative morbidities, hypotension, deep hypothermic circulatory arrest, and hypoxia. Reported rates of post-CPB AKI in neonates range from 45% to 64%. In this population, AKI is consistently found to be independently associated with morbidity, including prolonged length of intubation, postoperative ventilation days, ICU stay, and hospitalization, as well as with the increased risk of mortality.
Neonates on ECMO are predisposed to AKI for a number of reasons, including those inherent to their underlying critical illness (sepsis, ischemia, respiratory failure, cardiac failure, hypotension, nephrotoxic medications) and elements associated with ECMO (hemodynamic fluctuations, hemolysis, systemic inflammation). Several early studies of infants and children who received ECMO suggest AKI is associated with mortality. In a retrospective cohort study of 7941 neonates in the Extracorporeal Life Support Organization (ELSO) registry, where AKI was defined as infants who had an SCr greater than 1.5 mg/dL or an ICD-9 code for acute renal failure, neonatal mortality was 2175/7941 (27.4%). Nonsurvivors experienced more AKI than survivors (413/2175 [19.0%] vs. 225/5766 [3.9%]; P < .01), and more received renal replacement therapy (RRT, also known as kidney support therapy [KST]) (863/2175 [39.7%] vs. 923/5766 [16.0%]; P < .01). After adjusting for confounding variables, the adjusted odds ratio (OR) for mortality was 3.2 ( P < .01) following AKI. Zwiers et al. (2013) evaluated AKI in 242 neonates on ECMO, reporting an AKI incidence of 64% and a mortality of 65% when AKI progressed to the highest stage.
More recently, the multidisciplinary, international Kidney Intervention during Extracorporeal Membrane Oxygenation (KIDMO) study group has produced several manuscripts investigating the epidemiology and impact of AKI, fluid overload, and RRT utilization in neonates and pediatric patients receiving ECMO. Utilizing the KDIGO criteria, KIDMO investigators found that 66% of neonates receiving ECMO develop AKI. In their cohort, AKI occurred by 48 hours of ECMO support in 93% of cases and was independently associated with increased duration of ECMO and increased odds of in-hospital mortality. When examining only neonatal patients within the cohort, they found that neonates receiving ECMO for cardiac diagnoses experienced higher rates of AKI than those cannulated for respiratory indications or congenital diaphragmatic hernia (CDH; cardiac 68% vs. respiratory 33% vs. CDH 38%; P < .01), and an interaction suggested that risk of mortality differed by diagnosis in the presence or absence of AKI in neonates receiving ECMO; in the absence of AKI, CDH independently predicted mortality while fluid overload and RRT receipt both independently predicted mortality regardless of the underlying diagnosis. These findings suggest that physiologically distinct ECMO diagnoses warrant individualized treatment strategies.
There are now multiple single-center studies describing the epidemiology of AKI in VLBW and extremely low birth weight (ELBW) neonates with particular attention being paid to premature neonates. Rates of AKI in this population range from 12.5% to 56% and AKI is independently associated with increased morbidity and mortality. In a recent 2020 study of an overlapping population, extremely low gestational age neonates (ELGANs), Askenazi et al. reported AKI occurred at least once in 38% of their cohort, and severe AKI, defined as KDIGO stage 2 or higher AKI, occurred at least once in 18.2%.
Several studies examining the association between common morbidities of prematurity, including bronchopulmonary dysplasia (BPD) and necrotizing enterocolitis (NEC), and AKI have recently been published. One of the most common morbidities of prematurity is BPD, affecting 10% and 40% of surviving VLBW and ELBW infants, respectively. In 2015, Askenazi et al. showed an association between AKI and BPD in premature infants; neonatal AKI was independently associated with a higher risk of oxygen requirement/death at 28 days old (RR 1.45, 95% CI 1.07 to 1.97; P < .02). In the AWAKEN cohort, neonates born between 29 and 32 weeks of gestation who developed AKI had a higher likelihood of moderate or severe BPD/death than those without AKI after controlling for multiple factors (adjusted OR 4.21, 95% CI 2.07 to 8.61, P < .01).
An association between AKI and NEC has also been reported. NEC is an inflammatory disease of the intestines that typically affects VLBW and ELBW infants and significantly increases the risk of mortality. Bakhoum et al. (2018) studied a population of premature infants with NEC and found that AKI occurred in 42.9% of infants (Stage 1 NEC: 18.2%, Stage 2 NEC: 13.0%, Stage 3 NEC: 11.7%) and was independently associated with mortality (adjusted hazard ratio (HR) 20.3, 95% CI 2.5 to 162.8; P < .01). Criss et al. (2018) found AKI in 54% (98/181) of neonates with NEC and reported that those with AKI experienced higher mortality (44% vs. 25.6%, P < .01) and a higher chance of death (HR 2.4, CI 1.2 to 4.8, P < .01). When examining risk factors for and outcomes of severe AKI (i.e., stage 2 and 3 AKI), Garg et al. (2021) found severe AKI in 33% (66/202) neonates after NEC and in 58.7% (61/104) neonates after surgical NEC. They also reported severe AKI was associated with significantly longer hospitalization (124 days [IQR 88 to 187] vs. 82 days [IQR 42 to 125]; P < .01).
Prerenal azotemia occurs in response to decreased kidney blood flow (RBF). Causes of prerenal azotemia in neonates include loss of effective circulating blood volume, dehydration, capillary leak, increased abdominal pressures, and decreased cardiac output. Nonsteroidal anti-inflammatory drugs (NSAIDs) such as indomethacin and angiotensin-converting enzyme inhibitors (ACE-Is) can also decrease RBF ( Table 77.4 ).
| Prenatal Azotemia | Intrinsic Acute Kidney Injury | Postrenal (Obstructive) Renal Failure |
|---|---|---|
|
|
|
When low RBF occurs, kidney autoregulation preserves glomerular filtration rate (GFR) by increasing kidney sympathetic tone, activating the renin-angiotensin–aldosterone system, and increasing activation of hormones such as vasopressin and endothelin. An increase in filtration fraction (GFR/RBF × 100) increases peritubular oncotic pressure, resulting in enhanced proximal tubular sodium and water reabsorption. These hemodynamic changes lead to a decrease in water and sodium losses which helps to maintain systemic volume expansion. In some newborns, anticipated oliguria does not develop because of poor vasopressin secretion and/or weak kidney responsiveness to vasopressin, immature/poor tubular function, or prolonged/severe hypoperfusion. In prolonged states of kidney hypoperfusion, parenchymal damage occurs, and a pre-renal state may transition to kidney tubular cell damage (acute tubular necrosis, ATN).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here