Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The pediatric duodenum, jejunum, and ileum are subject to a range of acquired pathologies. In this chapter, the etiology, pathophysiology, and clinical manifestations of small bowel obstruction (SBO), infectious and noninfectious inflammatory bowel disease (IBD), and intestinal infiltrative and neoplastic disorders will be discussed.
Acquired SBO may be partial or complete, acute or chronic, and intermittent. It is relatively uncommon in children, particularly in infants under 1 year of age, in whom congenital causes of obstruction are more common (see Chapter 102 ). Congenital anomalies can, however, result in acquired SBO, with an inguinal hernia containing herniated bowel causing obstruction if incarcerated, or adhesions after neonatal gastroschisis repair. Acquired neonatal insults such as necrotizing enterocolitis (NEC) can cause ischemic strictures.
Abnormalities that mimic obstruction include chronic intestinal pseudoobstruction, manifest by variable distension of the gastrointestinal (GI) tract, defined as being “repetitive episodes or continuous symptoms and signs of bowel obstruction, including radiographic documentation of dilated bowel with air-fluid levels, in the absence of a fixed, lumen-occluding lesion.”
As in adults, the majority of acquired SBO in children is secondary to adhesions or hernias. Less common causes include tumors, inflammatory or fibrotic strictures in IBD, volvulus associated with malrotation or Meckel diverticulum, foreign body (FB) ingestion, and small bowel intussusception (see Chapter 107 ).
Adhesions are a common cause of obstruction in patients who have undergone prior abdominal surgery, reportedly affecting 1% to 6% postoperatively, 80% occurring within 3 months. In neonates, adhesions are seen particularly after surgery for repair of abdominal wall defects such as gastroschisis. In older children, adhesive obstruction occurs most often after ileostomy formation and closure (25%) and Ladd's procedure for malrotation (24%), with lower rates for general laparotomy (6.5%). Rates are less than 1% after appendectomy, although higher if perforated.
Hernias are responsible for approximately 10% of bowel obstructions. Inguinal hernias are a relatively common condition in children, with an overall incidence between 0.8% and 4.4%. The vast majority are indirect hernias related to a patent processus vaginalis. The incidence of inguinal hernia is 10 times higher in boys than girls. Inguinal hernias have both a higher incidence (~30%) and a higher risk of incarceration (31%) in premature infants. Internal hernias can be paraduodenal (see Chapter 102 ), occurring around an incompletely fixated falciform ligament or a Meckel diverticulum.
Tumors may cause bowel obstruction from extrinsic compression or as intrinsic lesions triggering intussusception secondary to polyps or to lymphoma, typically Burkitt lymphoma. Tumors are further discussed later in this chapter.
Omphalomesenteric remnants or Meckel diverticula are the residua of the embryologic yolk stalk. Present in 2% to 4% of the population, 4.8% of people will develop a spectrum of GI complications that make diagnosis challenging. When failing to regress, the obliterated lumen can form a fibrous cord, sometimes with cystic remnants, attaching the mid–small intestine to the anterior abdominal wall. Distal SBO in Meckel diverticulum can result from a mechanism similar to adhesions, bowel herniation, or from intussusception. Other manifestations include GI bleeding from ectopic gastric or rarely pancreatic tissue. Gastric ectopia is usually seen without SBO, but ectopic pancreatic tissue is strongly associated with intussusceptions (see Chapter 102 ).
Adynamic ileus can cause bowel distension and may mimic mechanical obstruction. Adynamic ileus occurs after major abdominal operations, and if prolonged it may be difficult to differentiate from an early postoperative or recurrent obstruction. Adynamic ileus may also occur in the setting of burns, trauma, and certain medications. The pathophysiology of postoperative ileus remains incompletely understood, but the disruption of normal autonomic GI motility is multifactorial and related to pharmacologic, inflammatory, hormonal, metabolic, and neuropsychologic influences.
Ingestion of FBs by infants and children, most frequently between 6 months and 6 years, can potentially cause SBO if held up at the terminal ileum. The increasing incidence of multiple magnet ingestion poses unique risks; if self-apposing across different parts of the intestine, they may cause obstruction and/or mucosal erosion and perforation.
SBO in children presents most frequently with crampy abdominal pain, vomiting (variably bilious), anorexia and obstipation, with constant pain, lethargy, and abdominal distention if prolonged. Nausea and bilious vomiting predominate in proximal obstruction, with less distension. Infants and young children may display only irritability and food refusal. Peritonitis, tachycardia, and fever can be indicative of bowel ischemia, with accompanying leukocytosis or lactic acidosis.
Patients with long-standing complete obstruction will demonstrate dilated loops of bowel with differential air-fluid levels on horizontal-beam images and absence of stool and gas in the colon ( Fig. 104.1A and B ). In partial obstruction, gas and stool may be present in the colon; however, a caliber difference between proximal loops of dilated bowel and bowel distal to the obstruction should remain evident.
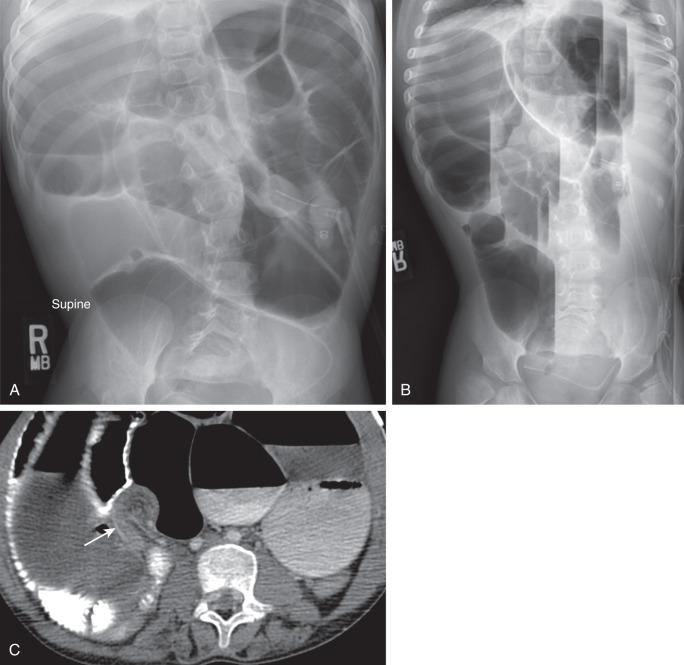
Conventional radiographs may reveal the level of obstruction but in many cases poorly differentiate incomplete obstruction from adynamic ileus. Radioopaque FBs such as magnets or batteries may be evident on x-ray, with serial imaging advocated depending on FB location. The level and, at times, the cause of obstruction may be more readily demonstrated on computed tomography (CT) or ultrasound (US), including FBs that may be radiolucent on radiographs ( Fig. 104.2 ). Small bowel follow-through (SBFT) is rarely done, usually reserved now for young infants.
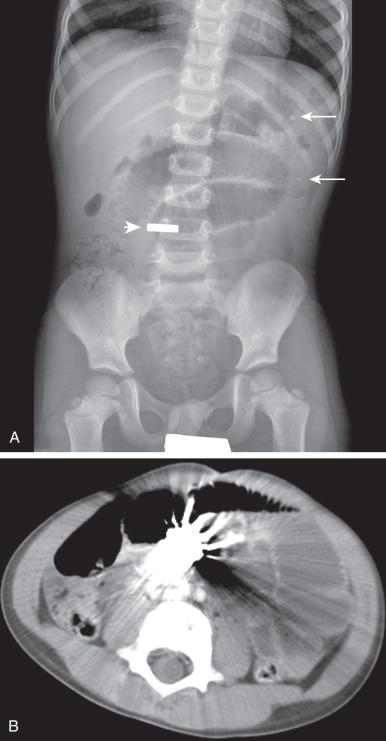
Adynamic ileus can simulate mechanical obstruction on plain radiographs. Supine radiographs of the abdomen show dilated loops of bowel in both conditions; however, mechanical obstruction usually causes proximal dilation with reduced caliber of the distal bowel, whereas adynamic ileus typically causes uniform dilatation of the entire small and large bowel.
In neonates, it is often not possible to differentiate small and large bowel; a prone cross-table lateral view of the rectum can be helpful to assess caliber of distal bowel when obstruction is suspected. In older children, on upright views, mechanical obstruction can show a step-laddered arrangement to bowel, often with pronounced fluid distension accompanying the air-fluid levels. Conversely with adynamic ileus the air-fluid levels are frequently more scattered and with more gaseous distension, both better seen with decubitus views. Decubitus views are also important in evaluating for free intraperitoneal air.
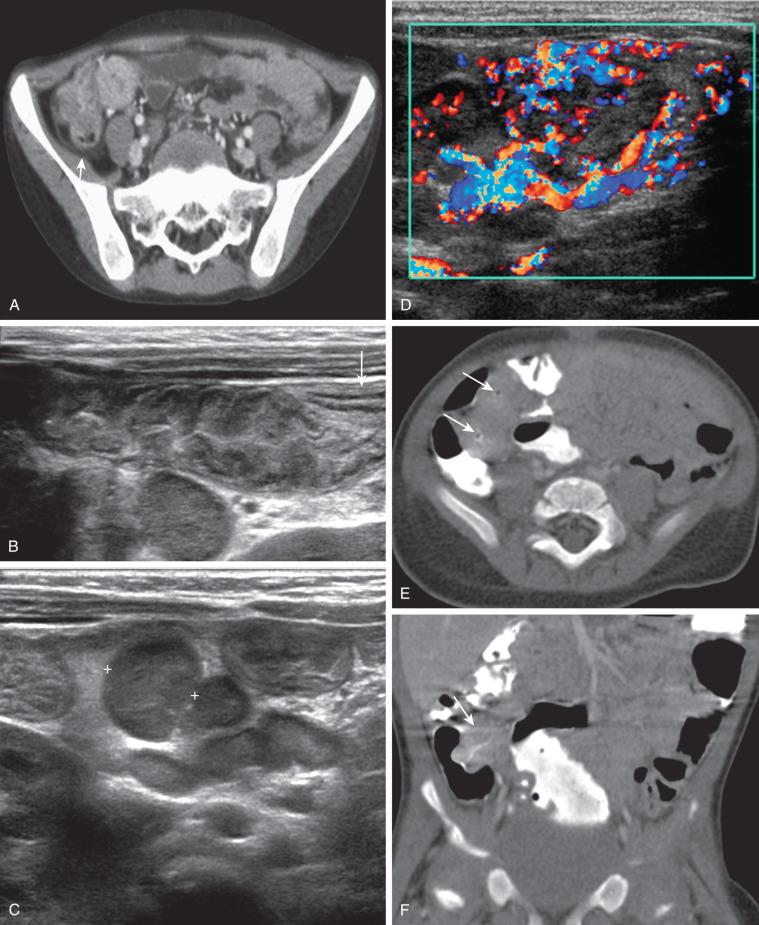
There is a renewed interest in US in diagnosing SBO. Point-of-care US for pediatric SBO is evolving although is not yet validated. In mechanical obstruction, US can show distended fluid-filled proximal loops (>2.5 cm) and collapsed distal loops, hyperperistalsis, and “to-and-fro” motion upstream from the obstruction. In contrast, peristalsis may be diminished in adynamic ileus, with uniformly distended fluid-filled bowel loops. On occasion, intrinsic lesions can be seen, such as strictures in IBD ( ) or intussusceptions (without or with pathologic lead point such as Meckel diverticulum or lymphoma), or extrinsic causes such as a tumor. Infrequently, bowel tethering with or without volvulus or angulation from an adhesion or peritoneal band is evident.
CT can be helpful in revealing the level and occasionally the cause of the obstruction. Administration of oral contrast is not always necessary (see Fig. 104.1C ), and only water-soluble agents should be administered if bowel perforation is suspected. CT is particularly helpful in diagnosing closed-loop obstruction and obstruction related to adhesions, with dilated bowel loops seen proximally and collapsed bowel distal to a transition point of obstruction, sometimes with swirling vessels. The diagnosis of an incarcerated hernia is typically established by history and physical exam, although radiographs ( Fig. 104.3 ) or US may occasionally be used to confirm the diagnosis.
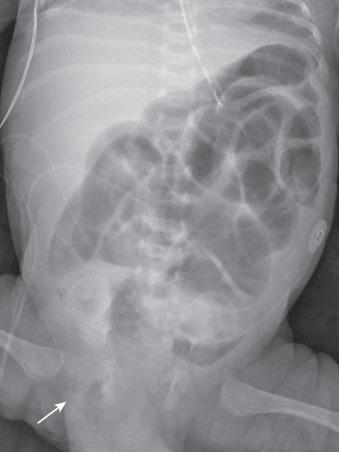
Although a majority of children are managed conservatively, more require surgical intervention than adults to minimize gut ischemia and necrosis, which remains a high risk. This requires careful triage and monitoring for clinical change. Those with symptoms of partial obstruction usually respond well to bowel decompression. Prompt surgical intervention is warranted if fever, leukocytosis, or unremitting pain are present or develop, or conservative treatment does not resolve symptoms of partial SBO within 48 hours.
In the absence of peritoneal signs, the treatment of choice for an incarcerated inguinal hernia is manual reduction, which has a success rate of 80%, followed by repair 24 to 48 hours after reduction. For umbilical hernias persisting beyond 4 years of age, surgery is advocated because of the increasing risk of incarceration with age.
Early postoperative SBO has traditionally consisted of prolonged bowel rest and decompression via nasogastric tube. However small bowel intussusception should be excluded, as this may require laparotomy. In FB ingestion, particularly multiple magnet ingestion in a symptomatic patient, early surgical review is required to consider endoscopic or laparoscopic removal. Some advocate magnet removal even when asymptomatic due to the risk for severe GI injury and death (see Fig. 104.2 ). In chronic intestinal pseudoobstruction, treatment depends on the underlying cause and may include prokinetics; surgery in the form of gastrostomy, jejunostomy, and/or loop enterostomy; and total parenteral nutrition (TPN).
Infectious diarrhea is a major source of childhood morbidity and mortality under 5 years of age, with 1.7 billion episodes reported in 2010, and an estimated 700,000 deaths attributable to it in 2011, 72% under 2 years. Undernutrition is a major risk factor. Viruses, bacteria, and parasites are causative agents of acute gastroenteritis in children, with the leading causes rotavirus and cholera preventable by vaccination.
Viruses are one of the most common causes of acute infectious diarrhea, defined as at least “three loose stools per day for less than 14 days.” Globally, the most common and severe viral gastroenteritis is due to rotavirus, highest in number in Africa but with peak severity and mortality in the Western Pacific (32.6%), and lowest in the Americas (23.4%). Less common severe viral infections are astrovirus, adenovirus, and norovirus (Caliciviridae) .
Bacterial pathogens are estimated to cause between 2% and 10% of infectious diarrhea cases in developed countries ; Vibrio cholerae accounts for 1% globally, with severe diarrhea and mortality rates of up to up to 4.5%. Shigella, Salmonella, Escherichia coli, and Campylobacter are the most common in the United States.
Clostridium difficile can be isolated from the intestine of up to 50% of normal neonates, decreasing to less than 5% by 2 years of age. Illness-associated C. difficile infection is seen with a decrease in other intestinal flora secondary to exposure to broad-spectrum antibiotics, resulting in antimicrobial-associated diarrhea or pseudomembranous colitis.
Rarely, Mycobacterium tuberculosis (TB) can involve the intestine in children.
Giardia lamblia, Entamoeba histolytica , and Cryptosporidium are parasitic causes of infectious diarrhea, all seen globally, and mostly not severe, although occasionally Cryptosporidium can be. The helminthes including nematodes or roundworms, and the platyhelminthes or flatworms, are estimated to infect one-third of those in developing countries, with children and adolescents harboring the largest loads.
Approximately 3.2 million children under 15 years of age are infected with human immunodeficiency virus (HIV) worldwide, often in conjunction with other endemic conditions, such as TB, malaria, cytomegalovirus (CMV), and helminthic infections. Although antiretroviral therapy (ART) has decreased the prevalence of vertical mother-to-child transmission (MTCT) and perinatal HIV in developing countries, approximately 240,000 children are newly infected annually, primarily through postnatal MTCT.
Viral gastroenteritis commonly manifests with vomiting followed by severe watery diarrhea, nausea, abdominal pain, headaches, and low-grade fever, often accompanied by upper respiratory tract symptoms. Others like norovirus are often milder and self-limiting.
Considerable overlap occurs in clinical symptoms of viral and bacterial enteritis. High fever, shaking chills, and blood and mucus in stools is infrequent in viral gastroenteritis. Diagnosis may be established by stool leukocytes and positive stool culture. Intestinal TB can present with fever, anorexia, abdominal pain, distension and diarrhea, and weight loss that may mimic Crohn disease (CD).
Symptoms of Giardia infection vary from asymptomatic to foul-smelling diarrhea with flatulence, abdominal distension, and anorexia. Diagnosis is from stool microscopic smear examination or immunofluorescence antibody testing.
Cryptosporidium infection is usually self-limited in the immune competent child, may be asymptomatic, or can be confused with viral gastroenteritis. In immunocompromised patients, including those with HIV, the course can be protracted with severe, chronic diarrhea and subsequent malnutrition and dehydration, which may result in death.
Helminthic infestations can present with low-grade fever and nonbloody watery diarrhea or gut obstruction as in intestinal Ascaris lumbricoides. Chronic infection can lead to physical and cognitive impairment in children.
Persistent diarrhea is a manifestation of the clinical syndrome of AIDS. HIV can directly affect the gut or coexist with other pathogens, leading to or compounding severe malnutrition and subsequent growth retardation. The child with HIV may also be more susceptible to opportunistic infections (e.g., CMV, Mycobacterium avium-intracellulare, and fungi, especially Candida albicans ).
Imaging is not generally indicated in the evaluation of infectious diarrhea. Abdominal radiographs may demonstrate a nonobstructive pattern of dilated fluid-filled loops (see previous section). Absence of stool throughout the colon is common, with air-fluid levels in the rectum. Pneumatosis intestinalis is rarely seen, more so in immune-compromised patients.
US may show fluid-filled small bowel loops, with or without wall thickening, free fluid, and lymphadenopathy in viral gastroenteritis ( e-Fig. 104.4 ). In intestinal TB, enlarged nodes may be centrally hypoechoic and the ascites finely septated; distortion of the ileocecal region/ascending colon rarely may be seen on barium enema. US may reveal transient small bowel intussusception during periods of hyperperistalsis.
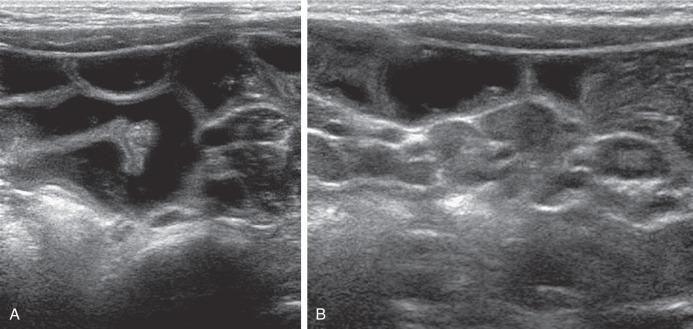
In Ascaris intestinal infestation, the roundworm may be seen at US as four echogenic lines in parallel, best seen with a high-frequency transducer, and US can exclude related complications such as intussusception or biliary ascariasis. Features relating to helminthic infections can also be seen on SBFT, including barium dilution, mild mucosal fold thickening, rapid or delayed transit times, and, on occasion, delineation of the parasitic gut.
The terminal ileum and cecum are often involved in bacterial enteritis, such as with Yersinia , termed infectious ileocecitis. Unlike intestinal TB, infectious ileocecitis may be seen on US, CT, and SBFT and barium enema, as mural thickening with variable involvement of the more distal large bowel ( Fig. 104.5 ). US or CT may also reveal regional lymphadenopathy.
HIV enteropathy may be seen on SBFT as increased nodularity or mucosal effacement, more usually secondary to opportunistic infection with CMV or Cryptosporidium infection.
Treatment is dependent upon the underlying causative agent. Viral gastroenteritis is typically a self-limited condition but may warrant supportive measures such as rehydration. Bacterial enterocolitis also includes supportive therapy, with antimicrobial therapy not always necessary depending on the severity of illness and immunocompetency of the patient. Agents such as metronidazole and nitazoxanide are active against Giardia and Cryptosporidium , respectively. Treatment of helminthic infections is relatively limited, particularly when considering the global ubiquity of these infections, and it includes diethylcarbamazine and newer agents such as albendazole and praziquantel. Measures to increase vaccination for rotavirus and cholera, improve delivery of ART, and prevent MTCT of HIV will significantly reduce pediatric morbidity and mortality from infectious enterocolitis.
IBD includes CD, ulcerative colitis (UC), and IBD-unclassified (IBD-U). CD, characterized by transmural granulomatous inflammation of the GI tract, can affect anywhere from the mouth to anus, whereas in UC, continuous nongranulomatous mucosal inflammation is limited to large bowel. Inflammation can progress to penetrating and/or fibrostenotic disease. These are chronic, relapsing, and remitting conditions. CD is almost three times more frequent than UC in children, with IBD-U comprising less than 10% of cases. The incidence of IBD is increasing worldwide with wide geographic variability; the highest annual incidence of CD is reported as 20.2 per 100,000 person-years in North America and UC 24.3 per 100,000 person-years in Europe. Pediatric onset occurs in up to 25% of patients, often of greater severity than adult-onset IBD. Increasingly recognized is a small cohort of very young children presenting with IBD less than 6 years, known as very early onset IBD (VEO-IBD).
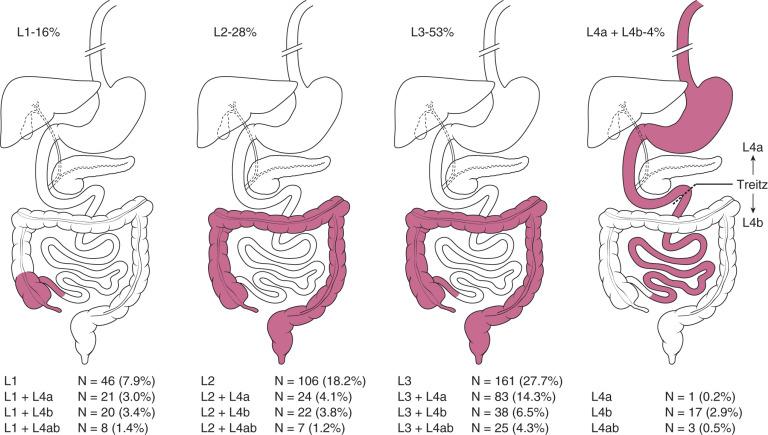
According to consensus guidelines on imaging in IBD, current understanding of causation suggests IBD “results from an inappropriate inflammatory response (immune dysregulation) to intestinal microbes in a genetically susceptible host,” with both genetic and environmental contributors. There are genotypes common to the major phenotypes of CD and UC, some overlapping and some unique, likely accounting for different phenotypic subtypes. This variability is accompanied by different rates of disease progression and severity, more pronounced in children and adolescence, particularly in VEO-IBD. This suggests a phenotype-genotype association, although inconstant, with evidence of genotypic susceptibility linked to single nucleotide polymorphism, with NOD2 having the strongest link to CD. To better understand this phenotype-genotype association, a classification system more accurately capturing pediatric IBD location and extent has been adapted from the adult Montreal Classification, known as the Paris Classification . This incorporates age-based pediatric-specific disease elements such as greater extent/pancolitis in UC, increased upper GI tract disease and coexistent penetrating and stricturing disease in CD, and growth delay. A 5-year review of phenotype in IBD patients from the EUROKIDS Registry with CD identified 16% with ileal/ileocecal disease (L1), 27% with isolated colonic disease (L2), 53% with ileocolonic disease (L3), and isolated upper GI tract disease in 4% (L4 a+b) ( Fig. 104.6 ).
On review of UC patients from this registry, atypical UC features were macroscopic rectal sparing (5%), cecal patches (2%), backwash or reflux ileitis (10%) with pancolitis, and mild upper GI erosions or ulcers (4%). Perianal disease is almost exclusively seen in CD and IBD-U, and phenotypic variability is now being recognized, with increase in rectal and jejunal involvement in perianal CD.
As in adults, children and adolescents with IBD can present with diarrhea, often having mucus and/or bloodstained stool, vague abdominal pain, weight loss, and unexplained anemia. Perianal disease can include fissures, ulcers, abscesses, fistulae, and skin tags, seen in approximately 10% of pediatric CD patients at diagnosis. Distinct from adults is linear growth retardation, as reflected in the Paris Classification, delayed onset of puberty, reduced bone mineral density, and malnutrition. VEO-IBD is more severe with isolated colonic disease, profound impact on growth and development, and can be fatal. Patients may have ongoing subclinical inflammation and be asymptomatic. Approximately 30% of affected individuals have a positive family history, and there is a 50% concordance between monozygotic twins.
Extraintestinal manifestations of IBD include hepatobiliary disease (e.g. primary sclerosing cholangitis in UC) and musculoskeletal disorders such as sacroiliitis, with related symptoms sometimes triggering screening for IBD. They should be kept in mind when imaging for small and large bowel disease.
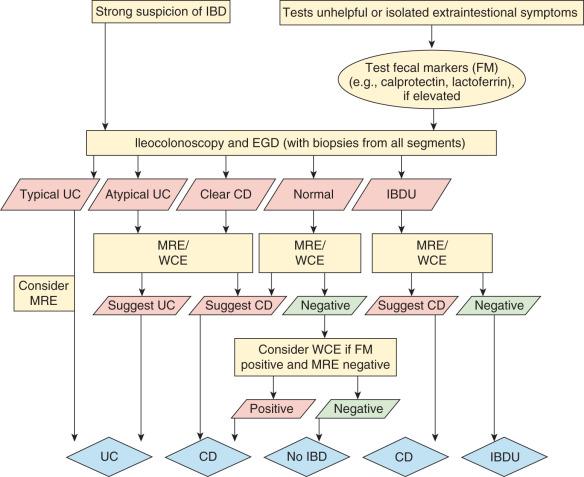
Distinguishing CD and UC can be challenging, with diagnosis dependent upon a complete, standardized diagnostic workup including esophagogastroduodenoscopy (EGD) and ileocolonoscopy, and small bowel imaging with capsule endoscopy or magnetic resonance enterography (MRE) as defined by the revised Porto criteria. This is recommended in all except those with definitive UC, for accuracy and to minimize those designated as IBD-U ( Fig. 104.7 ).
A range of established pediatric clinical activity scores (e.g. PCDAI and PUCAI [Pediatric CD and UC Activity Indices, respectively]) incorporate history, examination, and laboratory data, quantifying baseline and ongoing disease activity; a score less than 10 being remission in PCDAI. As mucosal healing rather than symptom remission is increasingly the goal of treatment, poor correlation of these indices with endoscopic evidence of mucosal inflammation has prompted development of alternate noninvasive image-based scoring systems, MRE in particular, to define activity and guide treatment. Multivariate logistical regression of itemized features of CD activity has been undertaken—bowel wall thickness, edema, ulceration, and relative contrast enhancement for inflammation in the MaRIA Score (validated with endoscopy/biopsy), adding diffusion-weighted imaging in the Clermont score; adding strictures for the Lémann damage score; and for an MRE global score (MEGS) length of disease (validated against fecal calprotectin), with varying sensitivities and specificities. Although validation is ongoing and use is not yet standard in clinical practice, MRE is proving robust in assessing mucosal healing in adults as well in limited studies to date in children, with pediatric-specific MRE-based indices under development.
Imaging algorithms have substantially altered in recent years, in line with changing treatment targets and outcome measures. Detection of IBD and distinction between CD and UC are key at presentation, as well as exclusion of other diseases such as infectious ileitis or appendicitis. At diagnosis and on follow-up, imaging objectives include defining disease location and extent (specifying involved segment length, activity and severity, and where possible characterizing as inflammation or damage), all with treatment implications. In the acute setting, assessment for acute flare, abscess, or obstruction from stricture should ideally occur within 24 hours. Surgical planning is increasingly done with MRE rather than fluoroscopy.
| US Sens/Spec |
CTE Sens/Spec |
MRE Sens/Spec |
|
|---|---|---|---|
| Active inflammation | 75%-94%/67%-100% ^ > o | 67%-100%/62%-100% o * | 55%-88%/79%-89% * > + |
| Fibrosis | N/A | 42%/68% * | 73%/82% * |
| Fistula | 31%-87%/95%-96% o ^ | 70%-78%/87%-97% o | 76%-88%/93%-96% o |
| Abscess | 83%-91%/93% o ^ | 85%-86%/88%-95% o | 86%-100%/93%-100% o |
| Stenosis | 58%-85%/92% ^ o | 85%-92%/100% o | 86%-92%/90%-94% o |
^ Anupindi et al. /Alison et al. —pediatrics;
+ Shenoy-Bhangle et al. —pediatrics;
o Panes et al. —not pediatric specific; CTE/MRE , Computed tomography/magnetic resonance enterography; DWI , diffusion weighted imaging; N/A , not available; Sens , sensitivity; Spec , specificity; US , abdominopelvic ultrasound.
With acute abdominal pain in IBD, radiography can demonstrate perforation or obstruction, with images likely to include an erect chest or upright abdominal image. Although no longer routinely used in IBD assessment, familiarity with the more reliable radiographic signs is still important. These include mucosal irregularity in the colon and small bowel and an abnormal stool pattern with stool absent or limited to a single loop. Extraintestinal manifestations of IBD such as renal or biliary calculi or sacroiliitis may be seen.
Interrogation of bowel is now often used in imaging pediatric IBD because it lacks ionizing radiation, has wide availability and low cost, and is usually well tolerated, even with use of oral contrast. However, its role in initial screening is limited as it has variable accuracy in disease detection (particularly MRE). While used for monitoring disease activity, treatment response, assessment of acute flares, and disease complications, reproducibility has been questioned. The use of nonabsorbable oral contrast and intravenous contrast-enhanced US has been shown to improve sensitivity, specificity, and accuracy for disease detection in CD including strictures. Compared with MRE and capsule endoscopy, conventional or contrast-enhanced US have shown similar sensitivities, but variable specificities. In the small bowel, US is best for terminal and distal ileum but poor for proximal to midsmall bowel. US shear wave elastography is one of a number of promising noninvasive techniques to assess bowel fibrosis.
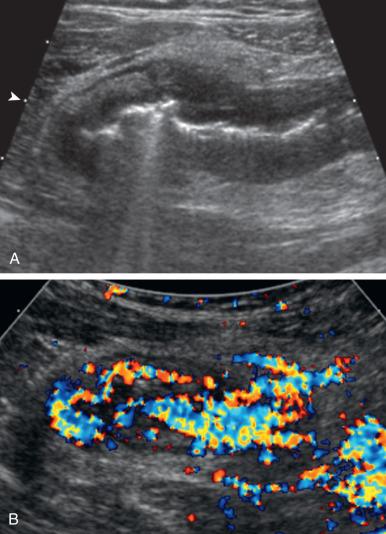
Mural features in IBD include (1) bowel wall thickening greater than 3 mm (normal 1.5–3 mm in the terminal ileum and 2–3 mm in the colon); (2) “stiffness” where thick or distended loop remains noncompressible/unchanging; (3) increased echogenicity/loss of stratification; (4) Doppler hypervascularity ( Fig. 104.8 ); (5) serosal irregularity/speculation; and (6) reduced peristalsis. Vessel density of density greater than 2 vessels/cm 2 and wall thickness greater than 5 mm have shown high specificity (88%) for active disease. Stenoses can be seen as persistent luminal narrowing (≤2 mm), reduced or absent peristalsis, with upstream dilatation and hyperperistalsis. Extramural features include (1) a halo of echogenic, hyperemic mesentery (>4 mm) relating to fibrofatty proliferation, (2) regional lymphadenopathy (short axis ≥8 mm), (3) abscess (sensitivity 83%–91%), and (4) fistula–hypoechoic tracts, occasionally containing echogenic foci if gas, extending to adjacent bowel, genitourinary structures, or skin; they can be associated with tethered or sharply angulated juxtaposed bowel loops (sensitivity 31%–87%). Backwash or reflux ileitis is seen in conjunction with pancolitis in UC with less mural thickening or extramural abnormality. Lymphoid hyperplasia can mimic both entities, again with less diffuse mural thickening, often with rounded hypoechoic lymphoid follicles and normal peristalsis.
SBFT and small bowel enteroclysis have largely been replaced by other modalities, in particular MRE and computed tomographic enterography (CTE) to a lesser extent. Although more sensitive in demonstrating mucosal lesions such as erosions and superficial ulcers, these other modalities better demonstrate mural and transmural features of IBD, with the added advantage of no ionizing radiation with MRE and US. Fluoroscopic studies are now reserved for patients in whom MRE or CTE is contraindicated. Typical features in terminal ileal CD include shallow poorly defined ulcers with surrounding edema; linear, serpiginous ulcers; and a swollen disorganized fold pattern, with loss of pliability on compression. In comparison, lymphoid hyperplasia has small nodular filling defected. Loop separation from wall thickening and/or fibrofatty proliferation, strictures and fistulae can be demonstrated.
CTE is performed after ingestion of sufficient low-density oral contrast to achieve bowel distension and intravenous iodinated contrast, imaging at either 45 seconds (enteric phase) or 70 seconds (portal venous phase). With sensitivities ranging from 60% to 90% and specificities 90% to 100% for detection of CD, it is used in some pediatric centers as baseline imaging to document the presence and extent of IBD (although some prefer MRE to avoid ionizing radiation). However, short acquisition times, less operator dependence than US, wide accessibility, high spatial resolution, and reduced need for sedation or anesthesia make it a viable alternative (see https://acsearch.acr.org/docs/69470/Narrative/ ).
Bowel wall thickening (>3 mm) and hyperenhancement are hallmarks of CD on CTE. Disease activity, with active inflammation evidenced by wall thickening, avid enhancement, submucosal edema, and mesenteric inflammation, correlate well with histologic evidence of inflammation. However, CTE is less predictive of fibrosis than MRE. As with US, stenoses can be evidenced by luminal narrowing and prestenotic dilatation. Additionally, upstream fecalization or the “small bowel feces” sign can be indicative of SBO ( ; Fig. 104.9 and e-Fig. 104.10 ). The peri-intestinal features of comb sign, fibrofatty proliferation, phlegmon, and abscesses are well depicted. Fistulae are variably seen, with abscesses and fistulae often arising just proximal to stenosed bowel. In the acute setting, when larger volumes of contrast may not be tolerated, conventional abdominal CT with no or limited oral contrast may be sufficient for a diagnosis of bowel obstruction, perforation, or abscess (see ). IBD-related pathologies such as renal and biliary calculi, and some musculoskeletal changes can also be depicted on CT.
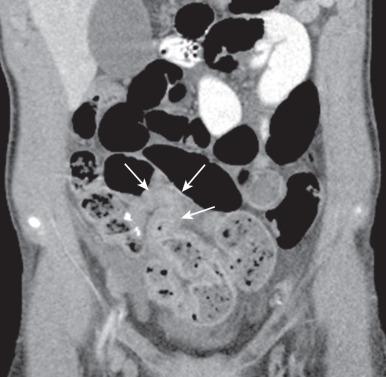
In addition to detecting diseased segments in small bowel CD, MRE helps define extent, activity, and complications. MRE requires ingestion of weight-based doses of hyperosmolar biphasic oral contrast agents, typically up to 1400 mL, intravenous gadolinium-based contrast agents, and antispasmodic agents to optimize small bowel visualization. Conventional rapid and fluid-sensitive sequences provide anatomic and functional information, using dynamic (cine) and static pre- and postcontrast sequences to delineate the bowel wall and mesentery. Advanced imaging techniques include delayed contrast enhancement (DCE) imaging with subtraction, diffusion-weighted imaging (DWI), and magnetization transfer pulses generating ratios (MTR), increasingly used to better define activity and differentiate active inflammation from fibrosis. While validation sufficient to guide therapy is ongoing, enhancement gain greater than 24% between 70 seconds and 7 minutes, with or without ulcers, shows promise in distinguishing minimal and marked fibrosis and inflammation in CD. The diagnostic capabilities plus the lack of ionizing radiation and excellent soft tissue contrast resolution have led to widespread use of MRE in pediatric IBD, particularly for serial imaging, and can be performed satisfactorily at 1.5 and 3 Tesla field strengths.
The majority of CD small bowel findings on CTE apply equally to MRE. Mural features include skip or continuous lesions with bowel wall thickening greater than 3 mm (which can be eccentric), hyperenhancement, mucosal ulceration, pseudosacculation, and strictures with luminal narrowing and prestenotic dilatation (>3 cm). MRE additionally can show mural edema with T2 hyperintensity and mucosal or stratified enhancement in active disease, reduced T2 signal relative to skeletal muscle, and variably transmural enhancement in chronic or fibrotic disease, with diseased segments having abnormally increased or decreased peristalsis on cine imaging ( ). Restricted diffusion is seen in active inflammation with increased mural signal on trace images and reduced signal on the apparent diffusion coefficient (ADC) map ( Fig. 104.11 ). While less reliable on its own, DWI accuracy improves when used in conjunction with conventional MRE sequences, particularly if DWI is viewed first. Extramural features include penetrating lesions: sinuses, fistulae, and abscesses ( Fig. 104.12 ). Abscesses, rim-enhancing fluid collections, can be difficult to distinguish from fluid-filled bowel, and central restriction on DWI improves conspicuity. Sinuses and fistulae have variable signal depending on contents (gas, fluid, feces) and are better demonstrated when DWI supplements T2-weighted or postcontrast T1-weighted sequences. Fistulae occur in 8.2% of pediatric CD within 12 months of diagnosis and 24.5% by 10 years, and additional features include tethering of adjacent bowel loops, and simple or stellate tracts to bowel, skin, or genitourinary structures, often arising just proximal to or at the level of a stricture. Other extraintestinal features are fibrofatty proliferation, vasa recta engorgement (comb sign), regional lymphadenopathy, perimural mesenteric edema and enhancement, intraperitoneal fluid, and phlegmons. Increasingly, qualitative and quantitative MRE analyses are being used as biomarkers of treatment response.

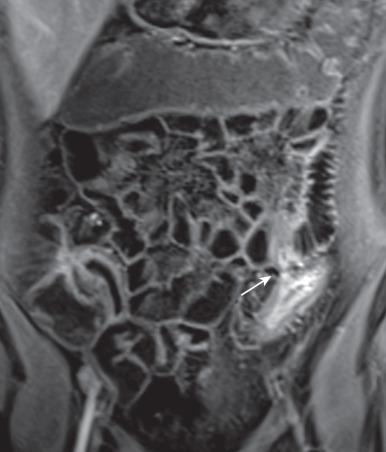
Identified IBD-related extraintestinal findings can include sclerosing cholangitis and biliary calculi, with magnetic resonance cholangiopancreatography (MRCP) usually performed separately (see Chapter 88 ), and enthesitis-related arthropathy and sacroiliitis, with bone marrow edema well seen on DWI.
Radiolabeled white blood cell scintigraphy and 18 F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT or PET/MRI can demonstrate active inflammation in small and large bowel in IBD, documenting extent and assessing treatment response. Decreased 18 F-FDG uptake in areas of fibrosis can aid distinction of fibrotic and inflammatory structures. Radiation burden limits routine use in pediatric imaging.
VEO-IBD rates special mention because of its more serious prognosis and the extra challenges in performing CTE and MRE in children under 6 years of age. While US may be used for initial imaging (with particular focus on colon), increasing utilization of MRE under general anesthesia has had good success, requiring only minor modification in technique.
Fortunately, the risk of IBD-related malignancy is extremely low in children and adolescents, occurring more in CD. Leukemia and lymphoma are most common and usually treatment related. Long-term implications of therapy must thus be balanced against severe IBD outcomes. Disease-related malignancies are less frequent and include small and large bowel adenocarcinoma and cholangiocarcinoma. Diagnostic imaging is neoplasm specific rather than surveillance focused.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here