Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter reviews acquired diseases of the thoracic aorta, venae cavae, pulmonary arteries, and pulmonary veins seen in childhood. Acquired pediatric aortic disease is uncommon, but radiologists and imagers play an important role in the care of these patients. Pulmonary embolism (PE) is the most common acquired disease of the pulmonary artery. The most common acquired abnormalities of the pulmonary veins and venae cavae are obstruction or stenosis caused by luminal occlusion or extrinsic compression from mediastinal pathologies.
Acquired pediatric aortic pathology can be categorized into valvar aortic stenosis (see Chapter 73 ), aortic aneurysm, aortic dissection, and vasculitis such as Takayasu disease (see Chapter 81 ). Although aortic diseases may present with one or more of these manifestations, it is the clinical consequences of the abnormalities that determine morbidity and mortality ( Table 82.1 ).
| Manifestation | Causes |
|---|---|
| Aortic aneurysm | Infectious aortitis |
| Inflammatory aortitis | |
| Takayasu syndrome (acute, chronic) | |
| Systemic lupus erythematosus | |
| Sarcoid | |
| Connective tissue disease | |
| Marfan syndrome | |
| Ehlers–Danlos syndrome (vascular type) | |
| Loeys–Dietz syndrome | |
| Arterial tortuosity syndrome | |
| Neurocutaneous disease | |
| Tuberous sclerosis | |
| Trauma or postsurgical (pseudoaneurysm) | |
| Aortic dissection | Connective tissue disease |
| Marfan syndrome | |
| Ehlers–Danlos syndrome (vascular type) | |
| Trauma | |
| Aortic stenosis | Inflammatory aortitis |
| Takayasu syndrome (chronic) | |
| Congenital rubella syndrome | |
| Radiation | |
| Neurocutaneous disease | |
| Neurofibromatosis (type I) | |
| PHACES syndrome | |
| Postsurgical | |
| Coarctation repair | |
| Aortopulmonary shunts |
Normally, the caliber of the aorta gradually decreases in size from the sinotubular junction to the aortic hiatus at the level of the diaphragm. An aortic aneurysm is defined as an abnormal dilation of the aorta, which may undergo progressive expansion. An aortic aneurysm may form if aortic wall stress increases, as in the case of systemic hypertension; if the aortic wall is structurally abnormal, as in connective tissue diseases; or due to infection and resultant wall weakening (see Chapter 81 ). Aortic dissection can occur in children as a complication of trauma or may be related to a connective tissue disease ( Fig. 82.1 ). A dissection is created when blood forces through a tear in the aortic intima and progressively separates the intimal layer from the aortic media, creating a true lumen that originally was connected to the aortic root and a false lumen that was not connected to the aortic root. Dissection can cause end-organ ischemia if the branch arteries supplying the organ are obstructed by the dissection flap. Dissection can weaken the aortic wall sufficiently to cause aortic rupture.
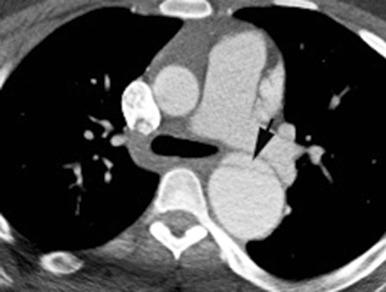
Trauma is a major cause of death in children and results primarily from motor vehicle accidents, pedestrian versus auto accidents, and falls, although penetrating injuries ( Fig. 82.2 ) and child abuse are other important causes of traumatic death. Survival of a child with a traumatic aortic injury until arrival at the emergency department is rare, accounting for one to two cases per year at large metropolitan level I pediatric trauma centers. Operative treatment involves less than 0.14% of all trauma patients, and only 6% of all traumatic ruptures of the aorta occur in patients younger than 16 years. The outcome of traumatic aortic injury in the pediatric population is directly related to timely diagnosis, proper treatment, and hemodynamic status at the time of presentation.
Chest radiographic findings such as pleural capping at the left lung apex, obscuration of the aortic arch, mediastinal widening, pleural effusion, pneumothorax, pulmonary contusion, tracheal and nasogastric tube deviation, and upper rib and clavicle fracture in the setting of blunt trauma should raise clinical suspicion for an aortic injury ( Fig. 82.3 ). Historically, the definitive diagnosis of traumatic aortic injury was made by conventional catheter angiography. Today, computed tomographic angiography (CTA) has supplanted catheter angiography as the diagnostic method of choice. Care should be taken to identify the location of aortic transection, active extravasation of arterial contrast, a dissection flap extending to major aortic branches, pseudoaneurysm, hemothorax and hemopericardium, and other organ and musculoskeletal injuries.
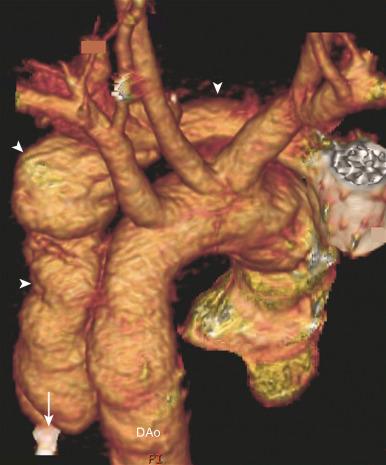
The goals of treatment of pediatric traumatic aortic rupture are identical to those in adults. The mainstay of treatment is operative repair of the aorta. Patients for whom surgery poses a high risk have been treated successfully with endovascular stent grafts. CTA should be performed immediately after stent placement, with a follow-up study in 48 hours to document the stability of the repair. Observational management has also been utilized in patients with comorbidities too severe to allow intervention.
PE is an uncommon but potentially fatal disease in children. In pediatric patients with deep venous thrombosis and PE, the mortality rate from all causes has been reported to be as high as 16%, whereas the mortality rate directly attributable to deep venous thrombosis or PE is 2.2%. The most common risk factor for PE in children is catheter thrombosis, which develops in as many as 50% of patients with central venous catheters. Other risk factors include peripartum asphyxia, dehydration, septicemia, trauma and burns, surgery, hemolysis, malignancy, immobility, and renal disease such as nephrotic syndrome. Two or more risk factors are often seen in children with PE. Abnormal coagulation factors associated with PE in adults that also have been reported in children are antiphospholipid antibodies, factor V Leiden mutation, and deficiencies in protein S, protein C, and antithrombin III.
The clinical diagnosis of PE is often difficult because, in many cases of venous thrombosis, younger children present with subtle symptoms related to their good cardiopulmonary reserve. Older children most commonly present with pleuritic chest pain. Symptoms of PE may be masked by intrinsic lung disease or other underlying illness. Unlike in adults, a negative d -dimer assay in pediatric patients has not been proven useful to exclude PE. Also, children may have other concomitant illnesses that can raise d -dimer levels, so the specificity of this assay in children is low. A high level of clinical suspicion for PE in the presence of risk factors is imperative.
Chest radiographs may show cardiac enlargement, pleural effusions, or peripheral lung opacities, but these findings are not specific for PE. Traditionally, catheter pulmonary angiography was considered the diagnostic gold standard. In current clinical practice, catheter pulmonary angiography has been replaced by noninvasive CT pulmonary angiography (CTPA). The accuracy of the detection of PE by CTPA in an adult population has been studied in the Prospective Investigation of Pulmonary Embolism Diagnosis II trial. With use of CT technology available before 2003, the sensitivity and specificity in this trial were reported as 83% and 96%, respectively.
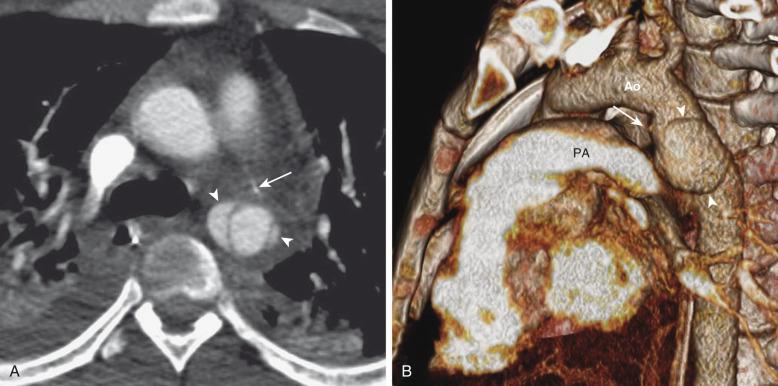
CTPA is the primary modality used to diagnose PE in children ( Fig. 82.4 ), although its accuracy in children has not been studied by rigorous clinical trials. CTPA in children can be technically challenging because of the size of the pulmonary arteries in small children, their inability to cooperate with breath-holding, and concerns about radiation exposure. The principal strategy for reducing radiation dose is to lower exposure factors such as x-ray tube voltage and tube current. Both maneuvers increase image noise and can confound visual detection of PE. As a result, the CTPA protocol for children requires meticulous attention to optimize spatial resolution, scan speed, and exposure factors.
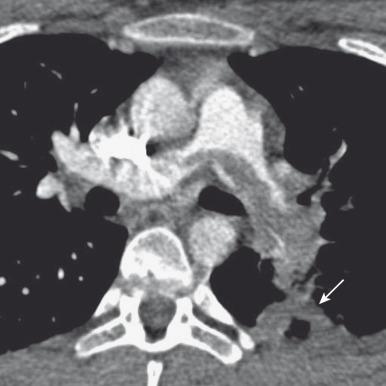
Dual-energy CT has been recently introduced for evaluation of lung perfusion. This technique involves acquisition of CT data with two different kilovolt potentials and allows for post-acquisition analysis of lung tissue components. For imaging of pulmonary embolism, lung perfusion can be quantified by mapping the injected iodine contrast medium distribution throughout the lungs to assess for perfusion defects. This technique is complimentary to standard CTPA as peripheral perfusion defects can be seen with dual-energy CT that locate smaller peripheral thrombi that may not be seen on standard CTPA. Dual-energy image data can also be selectively analyzed for central pulmonary arteries tissue characterization for the presence of thrombus. MRA has been performed for assessment of PE, but has lower sensitivity (88% relative to CT in adults) for its detection. Scintigraphic ventilation-perfusion scanning was historically used for PE detection, but its use has been supplanted by CTPA.
Anticoagulation is the mainstay of medical therapy for PE. Thrombolytic therapy is reserved for patients with hemodynamic instability. Surgical or interventional pulmonary thrombectomy has been successful for treating central or saddle emboli.
PE can also be caused by materials other than bland thrombus. Septic emboli can be the result of endocarditis and of thrombophlebitis. Lemierre syndrome describes jugular vein thrombosis associated with anaerobic infection of the head and neck (classically by Fusobacterium necrophorum ), and more than 50% of these cases are complicated by septic emboli to the lungs. Tumor emboli may be seen in patients with Wilms tumor, neuroblastoma, osteosarcoma, and hepatocellular carcinoma, because occasionally these tumors invade the inferior vena cava (IVC) or embolize to the lungs. Rarely, tumor emboli originate from primary cardiac tumors, such as atrial myxomas. Foreign bodies that embolize to the lungs include broken catheter tips and guide wires, misplaced embolization coils, and other endovascular devices. Finally, fat emboli may occur after major orthopedic trauma or surgery.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here