Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This chapter will:
Review in depth and criticize some of the foundations for current acid-base discourse.
Describe a model based on the strong requirement for charge balance in any fluid. To facilitate this, the notion of strong ion difference, which is the difference in concentrations between totally dissociated bases minus totally dissociated acids, is employed. It will be demonstrated that the strong ion difference is integral to classic understanding of acid-base transport.
Discuss physiologic and clinical applications and theoretical objectives.
Provide a short practical section that shows how diagnoses can be refined and explicitly analyzed on the basis of the discourse.
Repeatedly, areas of uncertainty and in need for fresh investigation are pointed out. Supplementary notes are available with code to reproduce some of the findings from https://figshare.com/articles/Critical_care_nephrology_chapter_65_Acid–Base_physiology/4231364/1
Traditional acid-base modeling has developed in two main directions. One proposes integrated whole-body acid-base balance based on indirect accounting of acid fluxes and separating endogenous acid production, renal acid excretion, and intestinal alkali absorption. The other current tradition has focus on individual transporters and is particularly well developed in the renal domain. Although frequently presented side to side, these discourses have not been integrated, and quantitative understanding is fragmented.
In this chapter acid-base physiology is developed with a focus on explaining why pH in any fluid has exactly the observed value. In the current discourse, pH is a function of the balance of movements of protons in and out of the fluid. An older tradition, which we will revive, emphasized the requirement for charge balance in any fluid and on that basis formulated algorithms for finding H + and pH.
It frequently is stated that multiple regulatory systems attempt to keep pH at control value. However, it appears that the control systems respond to physical effects of pH or H + rather than to pH or H + as such. This is more than a semantic argument, as can be seen, for instance, in the discussion of the effect of temperature on acid-base status. One position states that hypothermic patients should be alkaline just because of the effect of temperature on dissociations of buffers and water without invoking any actual transport of protons, and another discourse holds that only changes in transporter settings can alter pH. As will be seen, formulating acid-base physiology in terms of charge balance will lean heavily toward the former standpoint. Likewise we shall see examples of the fact that frequently it is more the circumstances and mechanisms of, say, acidosis that explains the morbidity rather than it is the actual pH.
A problem related to the question of what is regulated obviously is what is sensed by cells and organs. A recent important trend is that one of the universal “acid” sensors, the soluble adenylyl cyclase, is sensitive directly to HCO 3 − in a completely pH-independent way. This arrangement has several advantages related to the faster movement of HCO 3 − than of H + in the cytosol and direct link to metabolism in Krebs cycle and mitochondria.
The concept of buffering is paramount for conventional acid-base modeling, and it is revealing that the classical results can be obtained directly from the charge-balance model, which also, however, exposes crucial dependencies on strong ions, ignored in the classical discourse.
Also, by explaining buffering, we examine the connections between the proton-focused discourse and the charge-balance paradigm to show that some of the disagreements are mainly apparent because strong ions are implicated in both discourses.
The standard textbook paradigm of acid-base physiology attempts quantification by observing fluxes of substances perceived to generate or neutralize protons. These are endogenous acid production, renal net acid excretion (NAE), and net gastrointestinal alkali absorption. Renal NAE is classically calculated as the sum of ammonium (NH 4 + ) and titratable acidity (TA) less excreted bicarbonate (HCO 3 − ):
It can be shown that the model is misspecified, because it leads to the absurd prediction that urine will carry a negative net charge under the development of acidosis from a whole-body perspective. Therefore NAE is also in doubt because it has never been quantitatively validated but rather has been discredited in experimental studies, demonstrating that NAE as calculated conventionally is unrelated to acid-base status. In practice it is not a large problem because the textbook model has never been used in clinical practice.
In contrast, the model for acid-base physiology based on charge balance proposes instead that pH in any compartment is a function of strong ions, weak acids, and pCO 2 . When looking to the kidneys it is clear that they are instrumental in regulating primarily the homeostasis of electrolytes. It always has been problematic for physiology how the kidneys could integrate these requirements with the necessity of regulating also acid-base. From the viewpoint of charge-balance this conflict is apparent only because the kidneys regulate acid-base by way of regulating electrolytes, because titratable acidity and ammonium excretion are expressed fluently in terms of strong ions.
The charge-balance concept is based on the strong requirement that positive and negative charges are equal, because otherwise very big potentials develop. Apart from strong ions, which have pH independent charges, chemistry defines how pH determines all other charges. Thereby pH can be obtained based on total concentrations of fluid constituents.
It is important to acknowledge that this balance pertains strictly to macroscopic fluid in balance, whereas microscopically, across membranes, or close to electrogenic transporters, voltages certainly develop. However, the concept is equally useful because these forces on the microscopic level are instrumental in energizing the transports that eventually make for macroscopic balance, and even on the microscopic level, the observed voltages require only very small charge imbalances (e.g., to generate a potential of 60 mV across an axon with diameter 10 µm requires separation of less than 1/40,000 of cytoplasm K + as demonstrated by Wright).
To develop the model, a large pool of H + is contributed from water dissociation. It has been stated that water dissociation should be irrelevant to acid-base, because a proton and hydroxyl ion always are contributed together. Elaborating further isn’t necessary, the author suggests assembling a consistent model including water dissociation and examining its utility.
There is considerable discussion of the actual form in which H + is present in water with the hydronium (or Eigen) ion H 3 O + being the common favorite, although recent studies support H 13 O 6 + . Nonetheless exchange of single protons occurs, and the extra charge is unavoidable irrespective of its associations, so the model provided here will be invariant regardless.
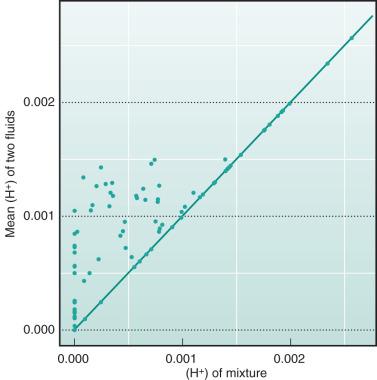
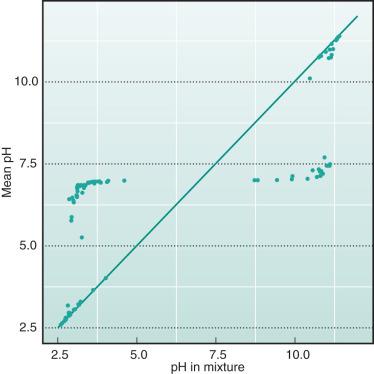
An initial theoretical experiment is performed to demonstrate the crucial fact that H + in biologic fluids is not as easily identified as Na + , for example. The example is employed in Eq. 2 with two fluids randomly sampled with SID between −3 mM and 3 mM, which were mixed 1 : 1. The mean values of H + and pH were compared with the corresponding values in the mixture. As is seen from Figs. 65.1 and 65.2 , H + and pH in the most simple mixture are not predictable from the H + and pH in constituent fluids.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here