Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The mean amount of menstrual blood loss in one cycle is approximately 35 mL but may be as much as 60 mL, with an average loss of 13 mg of iron. Heavy menstrual bleeding occurs in 9% to 14% of healthy women.
Diagnostic tests in women with menorrhagia include measurement of hemoglobin, serum iron, serum ferritin, beta human chorionic gonadotropin, thyroid-stimulating hormone, and prolactin levels; endometrial biopsy and hysteroscopy; sonohysterography; and hysterosalpingography. Magnetic resonance imaging may be helpful in the diagnosis of adenomyosis or surgical planning for leiomyoma but is not part of the initial evaluation.
High doses of oral or intravenous estrogen usually stops acute bleeding episodes in most cases of abnormal bleeding. An alternative regimen is high-dose oral progestogen for a week, with tapering of the dosage thereafter. Tranexamic acid (TXA) stops acute bleeding most rapidly and has particular efficacy for more chronic use in women with abnormal bleeding who ovulate.
Patients who are being treated for abnormal uterine bleeding as a result of endometrial causes (and who are ovulatory) may be given oral contraceptives, nonsteroidal antiinflammatory drugs (antiprostaglandins), TXA or a prolonged course of progestogens, or levonorgestrel released locally from an intrauterine system (LNG-IUS). Those treated with the LNG-IUS have similar outcomes at 1 year to those treated by surgery, and the LNG-IUS is preferred in women with inherited bleeding disorders.
Various endometrial ablation techniques achieve a 22% to 55% amenorrhea success rate at 1 year but an 86% to 99% satisfaction rate with regard to normalizing menstruation. Within 4 years after endometrial ablation, approximately 25% of women treated will have a hysterectomy.
Abnormal uterine bleeding (AUB) can present in many ways, from infrequent episodes, to excessive flow or prolonged duration of menses and intermenstrual bleeding. Alterations in the pattern or volume of blood flow of menses are among the most common health concerns of women. Infrequent uterine bleeding is called oligomenorrhea if the intervals between bleeding episodes vary from 35 days to 6 months, and amenorrhea is defined by no menses for at least 6 months. These are discussed in Chapter 38 . Excessive or prolonged bleeding is discussed in this chapter, and an overview of several therapeutic modalities being used to treat excessive uterine bleeding is also provided.
To define excessive AUB, it is necessary to define normal menstrual flow. The mean interval between menses is 28 days (±7 days). Thus if bleeding occurs at intervals of 21 days or less or 35 days or more, it is abnormal. The mean duration of menstrual flow is 4 days. Few women with normal menses bleed more than 7 days, so bleeding for longer than 7 days is considered to be abnormally prolonged. It is useful to document the duration and frequency of menstrual flow with the use of menstrual diary cards; however, it is difficult to determine the amount of menstrual blood loss (MBL) by subjective means. Several studies have shown that there is poor correlation between subjective judgment and objective measurement of MBL ( ).
Although subjective methods are used in predicting blood loss, and some investigators have used a pictorial bleeding assessment chart, a more accurate system is the alkaline hematic method, which measures hematin. Average MBL is 35 mL. Total volume, however, is twice this amount, being made up of endometrial tissue exudate. In the absence of disease, the amount of MBL increases with parity but not age. An MBL of 80 mL or greater is defined as heavy menstrual bleeding, which occurs in 9% to 14% of women ( ).
Although mortality and serious complications of AUB are uncommon, their effect on health-related quality of life is significant. Direct costs are calculated at more than $1 billion annually in the United States, and indirect costs as a result of lost work, social function, and vitality have been estimated at more than $12 billion annually ( ).
The causes of AUB can be described by a universally accepted systematic nomenclature. This system was reported by the International Federation of Gynecology and Obstetrics (FIGO) in 2011. It subdivides causes of AUB into nine main categories, which are arranged according to the acronym PALM-COEIN : polyp, adenomyosis, leiomyoma, malignancy and hyperplasia, coagulopathy, ovulatory dysfunction, endometrial, iatrogenic, and not yet classified. The causes that constitute the first group ( PALM ) are structural or histologic and are diagnosed through imaging or biopsy. Those that compose the second group ( COEIN ) are nonstructural ( Fig. 26.1 ). The term dysfunctional uterine bleeding (DUB) is no longer favored and should be discarded. In the past this term represented causes of abnormal bleeding when structural causes and other specific defects, such as coagulation defects, had been excluded. Cases that previously would have been described as DUB are now referred to as AUB as a result of ovulatory dysfunction or endometrial causes.
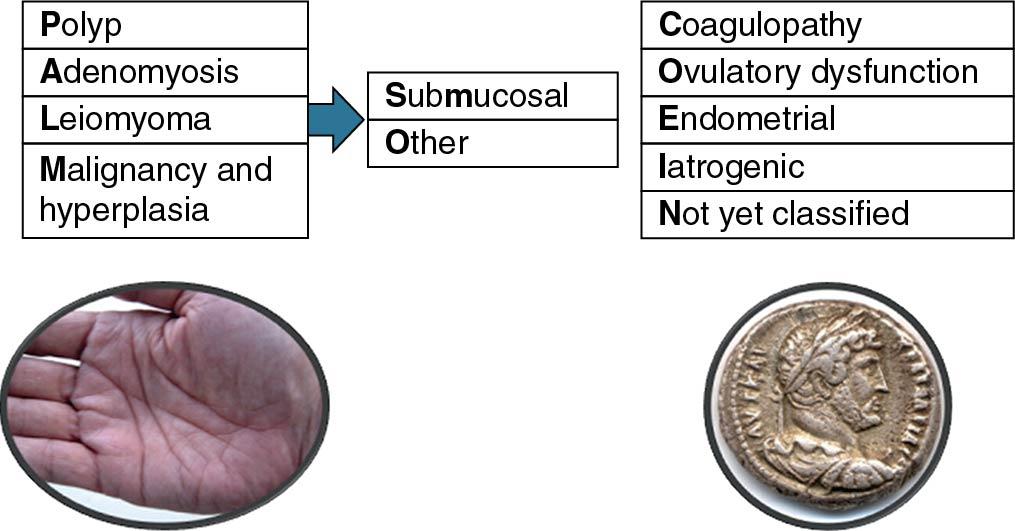
According to FIGO, this classification system should be notated in a consistent and systematic manner. The acronym AUB is followed by the letters PALM-COEIN and a subscript 0 or 1 associated with each letter to indicate the absence or presence, respectively, of the abnormality. For example, a patient with abnormal bleeding caused by a polyp would be described as AUB-P 1 A 0 L 0 M 0 -C 0 O 0 E 0 I 0 N 0 . Because patients may have abnormal bleeding as a result of more than one condition, this notation allows for description of simultaneous factors. For example, a patient with abnormal bleeding that is both irregular and heavy may have endometrial hyperplasia as a result of anovulation. As such, this patient’s bleeding would be described as AUB-P 0 A 0 L 0 M 1 -C 0 O 1 E 0 I 0 N 0 .
What follows is an introduction to each of the pathologic conditions described by the PALM-COEIN system. After this discussion, a diagnostic approach for women with AUB will be outlined. Treatments for acute and chronic bleeding as a result of these conditions conclude this chapter.
Endometrial polyps (AUB-P) are localized overgrowths of endometrial tissue, containing glands, stroma, and blood vessels, covered with epithelium ( ). Endometrial polyps are most commonly found in reproductive-age women, and estrogen stimulation is thought to play a key role in their development. As such, polyps are rarely found before menarche. Molecular mechanisms involving overexpression of endometrial aromatase and gene mutations in HMGIC and HMGI[Y] have also been proposed ( ; ).
The majority of endometrial polyps are benign. A systematic review of the oncogenic potential of endometrial polyps demonstrated that symptomatic vaginal bleeding and postmenopausal status are associated with an increased risk of malignancy. Among symptomatic postmenopausal women with endometrial polyps, 4.5% had a malignant polyp compared with 1.5% in asymptomatic women ( ). A strong correlation exists for both tamoxifen use and obesity and the development of malignancy in endometrial polyps. Diabetes mellitus and hypertension have not been reliably shown to increase the risk for malignancy in an endometrial polyp.
The importance of small and asymptomatic endometrial polyps is less clear. Transvaginal ultrasound detected asymptomatic polyps in up to 12% of women undergoing routine gynecologic examination; small endometrial polyps smaller than 1 cm appear to regress spontaneously ( ). Endometrial polyps were discovered in 32% of 1000 patients on office hysteroscopy about to undergo in vitro fertilization, suggesting a possible association between endometrial polyps and infertility ( ). Women with symptomatic polyps can be treated safely and effectively with operative hysteroscopy.
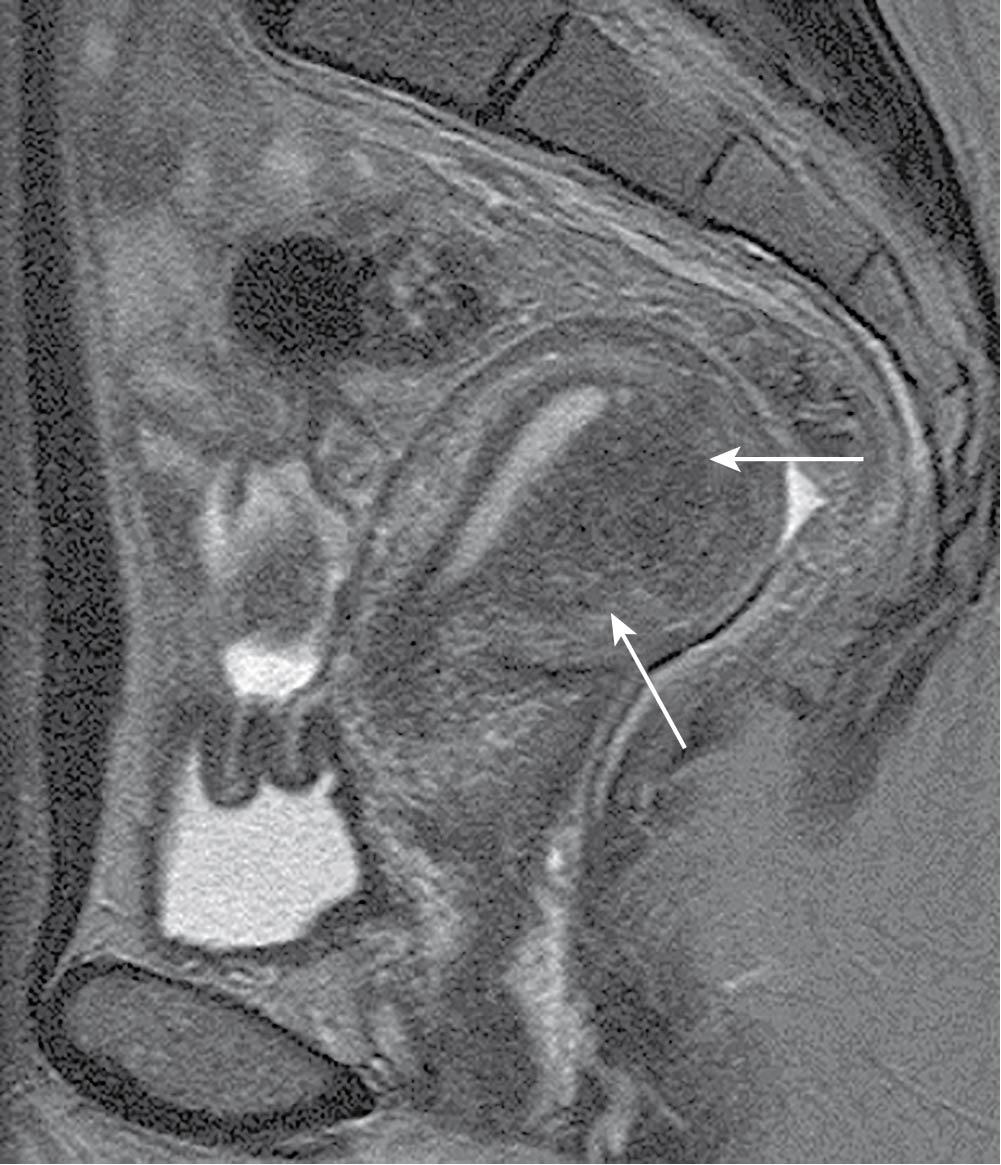
Adenomyosis (AUB-A) is defined by the presence of endometrial glands and stroma in the uterine myometrium. The presence of ectopic endometrial tissue leads to hypertrophy of the surrounding myometrium. Adenomyosis can occur as focal (adenomyoma) or diffuse, with a peak incidence in the fifth decade of life. Multiparity is considered the most significant risk factor for developing adenomyosis, but any process that allows for penetration of endometrial glands and stroma past the basalis layer (e.g., dilation and curettage, cesarean delivery, spontaneous abortion) is thought to contribute. There also appears to be a positive correlation between overexpression of immunoproteins interleukin-6, interleukin-18, and cyclooxygenase-2 and the presence of ectopic endometrial tissue, though these may not be causative ( ; ). Adenomyosis is a histologic diagnosis, but findings of an enlarged, asymmetric uterus on ultrasound and magnetic resonance imaging (MRI) are indicative. Anechoic avascular cysts scattered throughout the myometrium on sonography are considered pathognomonic for adenomyosis on ultrasound. MRI, which is both more sensitive and more specific than ultrasound, will demonstrate thickening of the junctional zone, the area between the endometrium and the myometrium, equal to or greater than 12 mm ( Figs. 26.2 and 26.3 ) ( ). Abnormal bleeding caused by adenomyosis is thought to be a result of altered uterine contractility and is commonly associated with profound dysmenorrhea.
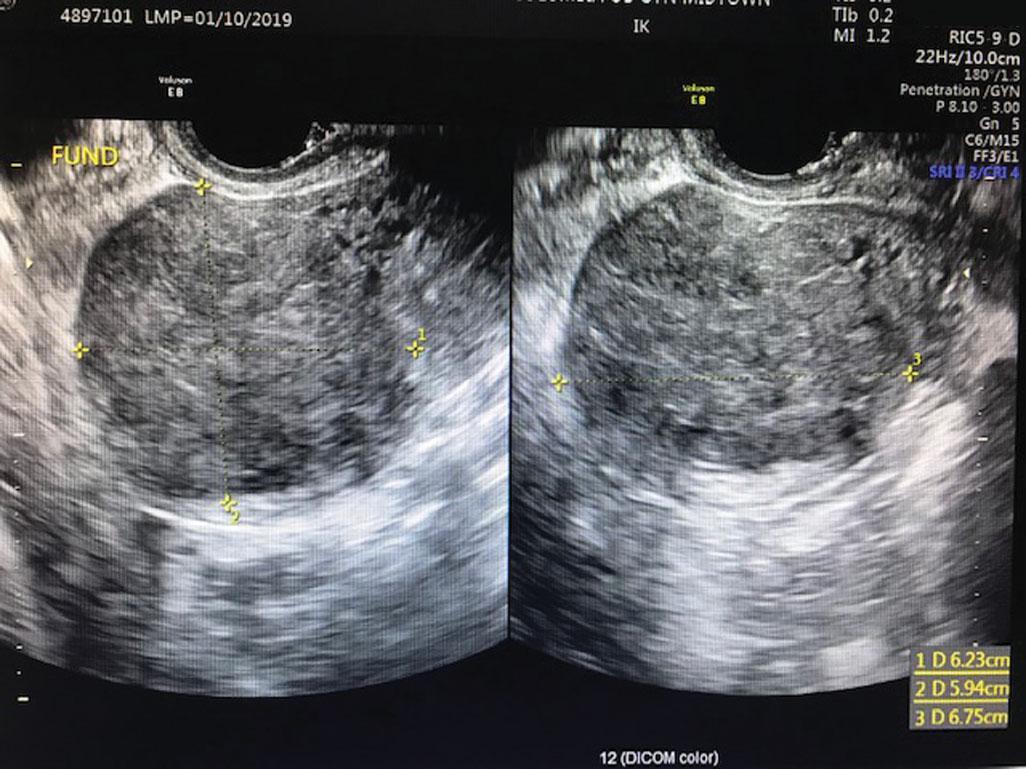
Leiomyoma (AUB-L), or fibroids, are benign tumors of the uterine myometrium with a complex and heterogeneous clinical presentation as varied as their biologic origins. Various genetic mutations are described in leiomyoma, but the pathogenesis is thought to initiate from myometrial injury leading to cellular proliferation, decreased apoptosis, and increased production of extracellular matrix. Critical in this pathway is the overexpression of transforming growth factor beta that leads to fibrosis of these tumors ( ). Transforming growth factor beta also contributes to implantation failure in women with fibroids who are subfertile.
Although the prevalence of fibroids among women is approximately 70%, as many as 50% of these will be symptomatic ( ). Mechanisms by which fibroids cause abnormal bleeding are varied and depend on size, location, and number. Subclassification of leiomyomas describes their location throughout the myometrium ( Fig. 26.4 ). Intracavitary fibroids (type 0) and submucosal fibroids, where more than 50% are intracavitary (type 1) or less than 50% are intracavitary (type 2), as well as intramural fibroids, which are large, may increase the overall surface area of the endometrial cavity or alter uterine contractility. These effects in turn lead to abnormal and excessive uterine bleeding. Whereas hysterectomy for fibroids remains among the leading indications for the procedure in the United States, treatments are diverse and include hormonal or surgical ablation of the endometrium, uterine artery embolization, radiofrequency ablation, and myomectomy through a variety of surgical approaches.
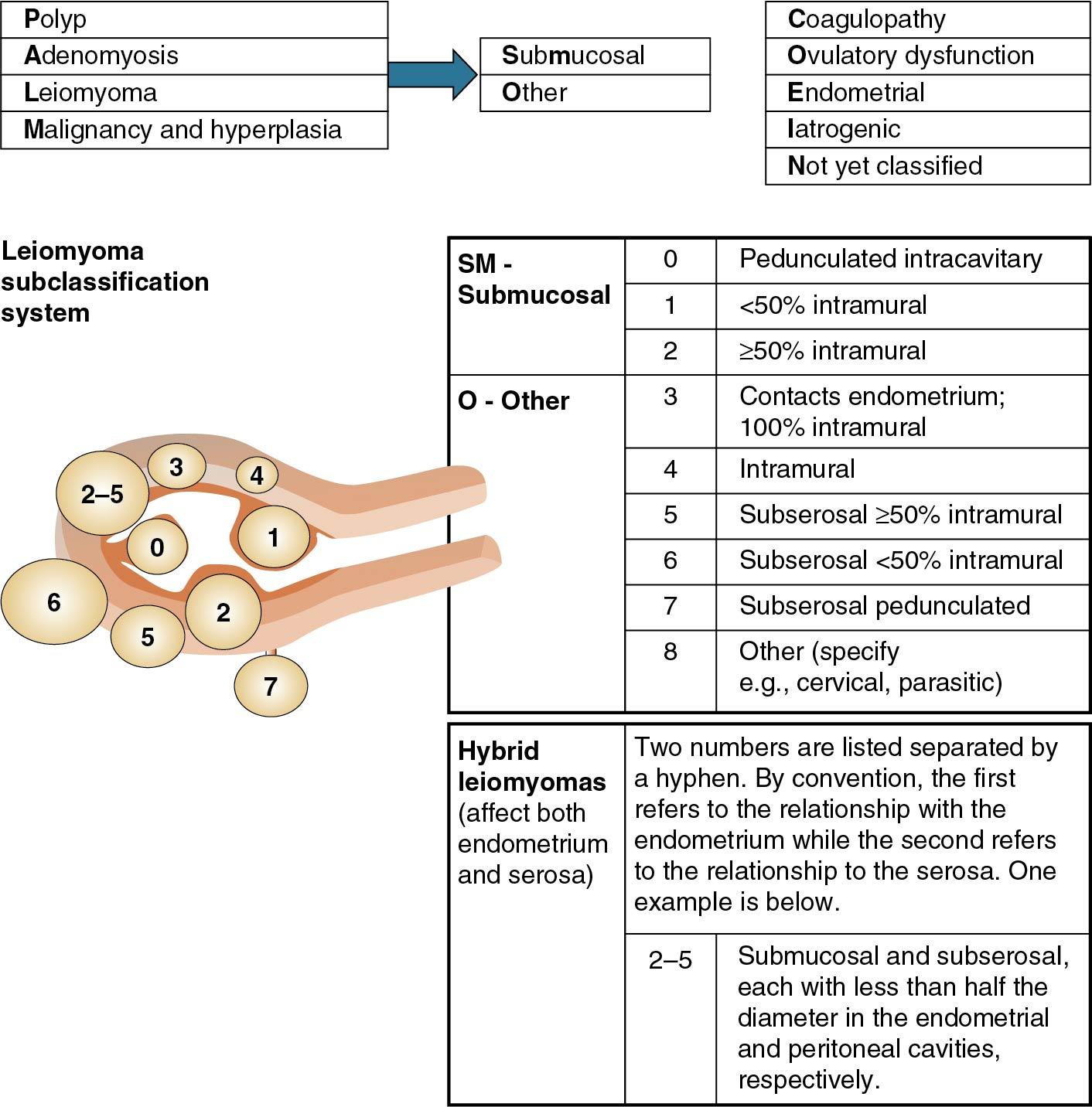
According to the FIGO system, leiomyomas can be notated in the PALM-COEIN system with a subscript 0 in their absence or by the number 1 when present. Additionally, the letters SM can be inserted to indicate a fibroid’s location as submucosal.
Malignancies (AUB-M) associated with the female reproductive tract include vulvar, vaginal, cervical, endometrial, uterine, and adnexal (ovarian or fallopian tube) cancers. Although vaginal cancers can cause abnormal bleeding, there are only approximately 3000 new cases reported annually in the United States. Bleeding from cervical malignancy classically presents as coital bleeding or intermenstrual bleeding; thus a thorough cervical evaluation is an important part of the workup of any woman with these symptoms. In a series of 73 women with coital bleeding referred for evaluation, squamous cell carcinoma of the cervix was present in 1.4% of patients, and 15% had cervical intraepithelial neoplasia.
AUB is the most common presenting symptom of endometrial cancer. Although endometrial cancer presents most often in the seventh decade, 15% of cases are diagnosed in premenopausal women, and 3% to 5% present in women younger than age 40 ( ). Conditions that lead to increased circulating levels of estrogen are risk factors, for example, obesity is associated with increased estrone levels as a result of peripheral conversion by aromatase in adipose tissue, but the primary source of estrogen in premenopausal women remains the ovary. Impaired ovulation and the absence of progesterone withdrawal can result in sustained exposure of the endometrium to estrogen. This hyperestrogenic state can lead to the pathologic progression from normal endometrium to hyperplasia and ultimately to adenocarcinoma.
Lynch syndrome, or hereditary nonpolyposis colorectal cancer, is an autosomal dominant disease caused by a disruption in the mismatch repair (MMR) genes. Lynch syndrome also carries a 40% to 50% lifetime risk of endometrial cancer, with a significant proportion of endometrial cancers occurring before age 45. In addition, estrogen-producing ovarian tumors may manifest in AUB. Granulosa theca cell tumors are the most common tumors to have this presentation, although many ovarian tumors can produce estrogen.
Systemic diseases, particularly disorders of blood coagulation (AUB-C) such as von Willebrand disease and prothrombin deficiency, may initially present as AUB ( ). Routine screening for coagulation defects is mainly indicated for adolescents with prolonged heavy menses beginning at menarche. In adults, screening for these disorders is of little value unless otherwise indicated by clinical signs such as bleeding gums, epistaxis, or ecchymosis. Research has indicated that 20% of adolescent girls who require hospitalization for AUB have coagulation disorders ( ). Coagulation defects are present in approximately 25% of those whose hemoglobin levels fall to less than 10 g/100 mL, in one-third of those who require transfusions, and in 50% of those whose severe menorrhagia occurred at the time of the first menstrual period. Others report that a coagulation disorder is found in only 5% of adolescents hospitalized for heavy bleeding ( ).
Both studies indicated that the likelihood of a blood disorder in adolescents with heavy menses is sufficiently high that all adolescents should be evaluated to determine whether a coagulopathy is present.
Disorders of platelets are most often quantitative, but defects in platelet membrane or storage granules can result in normal circulating levels with altered function. Hemophilias A and B are X-linked recessive deficiencies of factor VIII and factor IX, respectively. Women who are carriers for these disorders can have reduced levels of factors VIII and IX, some less than 30% of normal and enough to be considered to have mild hemophilia. Rare inherited coagulopathies of the other clotting factors (V, VII, X, XI, XIII) include menorrhagia as a potential symptom. Other disorders that produce platelet deficiency, such as leukemia, severe sepsis, idiopathic thrombocytopenic purpura, and hypersplenism, can also cause excessive bleeding.
Chronic anticoagulation as a result of heparin, low-molecular-weight heparin, direct thrombin inhibitors, and direct factor Xa inhibitors is necessary for prevention of thrombosis in women with inherited thrombophilias, those with mechanical heart valves, and those with rare anatomic disorders such as May-Thurner syndrome. In the absence of other gynecologic pathologic conditions, these patients present most often with heavy menstrual bleeding. Although it may seem that these patients could be considered to have iatrogenic abnormal bleeding as a result of prescribed medications, the heavy bleeding actually is a result of a derangement in the coagulation cascade and is thus categorized here.
The predominant cause of ovulatory dysfunction (AUB-O) in postmenarchal and premenopausal women is secondary to alterations in neuroendocrine function. In women with AUB-O, there is continuous estradiol production without corpus luteum formation and progesterone production. The steady state of estrogen stimulation leads to a continuously proliferating endometrium, which may outgrow its blood supply or lose nutrients with varying degrees of necrosis. In contrast to normal menstruation, uniform slough to the basalis layer does not occur, which produces excessive uterine bleeding.
Anovulatory bleeding occurs most commonly during the extremes of reproductive life—in the first few years after menarche and during perimenopause. In adolescents the cause of anovulation is an immaturity of the hypothalamic-pituitary-ovarian (HPO) axis and failure of positive feedback of estradiol to cause a luteinizing hormone (LH) surge. In perimenopausal women a lack of synchronization between the components of the HPO axis occurs as the woman approaches ovarian decline at menopause.
The pattern of anovulatory bleeding may be oligomenorrhea, intermenstrual bleeding, or heavy menstrual bleeding. Why different patterns of bleeding occur within a distinct entity of anovulatory bleeding is unclear but is probably related to variations in the integrity of the endometrium and its support structure. Up to 20% of women reporting normal menses may also be anovulatory.
What are the causes of anovulation? Apart from the extremes of reproductive life, as noted, women in their reproductive years often have a cause for anovulatory bleeding. This is most commonly because of polycystic ovary syndrome (PCOS), which may be suggested by other symptoms and signs, such as acne, hirsutism, and increased body weight (see Chapter 41 ). If not PCOS, anovulation can result from hypothalamic dysfunction, which could have no known cause or be related to weight loss, severe exercise, stress, or drug use. In addition, abnormalities of other nonreproductive hormones can lead to anovulation. The most common hormones involved are thyroid hormone, prolactin (PRL), and cortisol.
Hypothyroidism, evidenced by an elevated thyroid-stimulating hormone (TSH) level, can lead to anovulatory bleeding. Unexplained causes of endometrial problems in the face of normal ovulation (discussed later) may also be explained by subtle hypothyroidism. Hyperprolactinemia (PRL level >20 ng/mL) can also lead to anovulatory bleeding, as can hypercortisolism; however, Cushing syndrome is rare and may be considered only if other signs are present (e.g., obesity, moon facies, buffalo hump, striae, weakness). Accordingly, TSH and PRL assays should be part of the normal workup of anovulatory women.
Iatrogenic bleeding (AUB-I) is abnormal bleeding resulting from medications. The most common of these are hormonal preparations, including selective estrogen receptor modulators, and gonadotropin-releasing hormone (GnRH) agonists and antagonists. All hormonal long-acting reversible contraceptives result in some degree of anovulation and irregular or intermenstrual bleeding; however, with time, most patients become amenorrheic. The prevalence of amenorrhea with depomedroxyprogesterone acetate users at 90, 180, 270, and 360 days are 12%, 25%, 37%, and 46%, respectively, as determined by a systematic review ( ). In addition, chronic progestogen therapy of various types can lead to irregular spotting and bleeding. Similarly, irregular bleeding is an expected consequence of levonorgestrel intrauterine devices initially, but 20% of users are amenorrheic by 1 year. Implantable progestin devices have similar amenorrhea rates, but more than 40% of patients have irregular or prolonged bleeding. This pattern of bleeding is the most common reason for discontinuation of the subdermal implants within the first year ( ).
Hyperprolactinemia can result from central nervous system dopamine antagonism of certain antipsychotic drugs. The prevalence of hyperprolactinemia among women taking risperidone was 88%, and among women taking conventional antipsychotics it was 47% in one study. As previously described, elevations in prolactin are disruptive to the HPO axis and can contribute to anovulation, with 48% of those women on risperidone experiencing AUB ( ).
It is well known that common combined and progestin-only oral contraceptives (OCs) may result in breakthrough bleeding (BTB). BTB most likely reflects alterations in the structural integrity, vascular density, and vascular morphology of the endometrial vasculature as a result of alterations in the expression of steroid receptors and the integrity of the endometrial epithelial layer ( ). Compliance issues and interactions between OCs and other medications, such as antibiotics and anticonvulsants, may alter circulating levels of steroids, allowing follicular recruitment and increased endogenous levels of estrogen. These variations are a common cause of irregular bleeding in contraceptive users.
Women who present with heavy menstrual bleeding in the absence of other abnormalities are thought to have underlying disorders of the endometrium (AUB-E) or are otherwise unclassified. In the past this category was called “ovulatory dysfunctional uterine bleeding.”
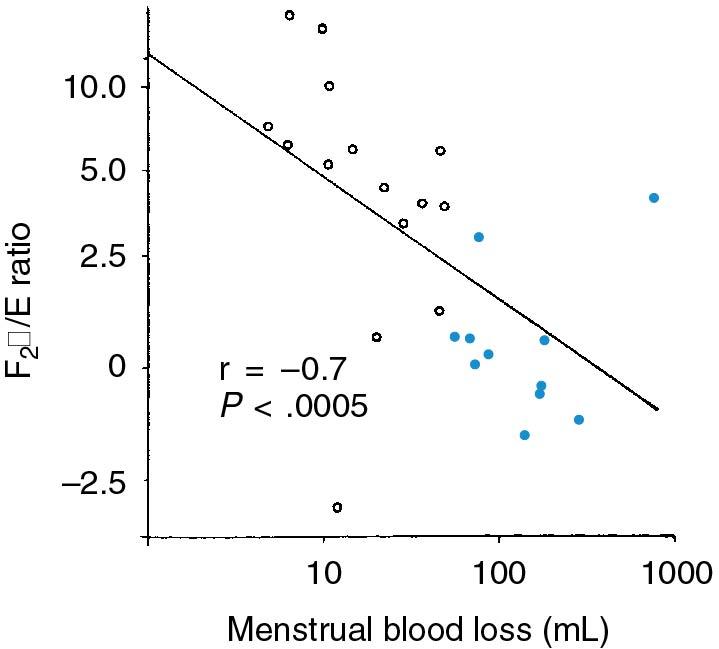
The primary line of defense for excessive bleeding during normal menses is the formation of the platelet plug. This is followed by uterine contractility, largely mediated by prostaglandin F2 alpha (PGF2α). Thus prolonged and heavy bleeding can occur with abnormalities of the platelet plug or inadequate uterine levels of PGF2α. It has been shown that in some women with heavy menstrual bleeding, there is excessive uterine production of prostacyclin, a vasodilatory prostaglandin that opposes platelet adhesion and may also interfere with uterine contractility. Deficiency of uterine PGF2α or excessive production of prostaglandin E (PGE; another vasodilatory prostaglandin) may also explain ovulatory DUB ( ). The ratio of PGF2α/PGE correlates inversely with MBL ( Fig. 26.5 ). In addition to these, other uterine factors affecting blood flow, such as the endothelins and vascular endothelial growth factor, which controls blood vessel formation, may be abnormal in some women with heavy menstrual bleeding. Unfortunately, no commercially available assays exist, and endometrial causes of AUB remain a diagnosis of exclusion in most cases.
Chronic inflammatory changes of the endometrium as evidenced by plasma cell infiltration indicate endometritis; however, the causal relationship between inflammatory changes and abnormal bleeding is unclear, resulting from a variety of factors including infection, vascular endothelial damage, or alterations in vasculogenesis. Subclinical infection with Chlamydia trachomatis has also been associated with AUB.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here