Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neonatal resuscitation is commonly defined as the assistance given to infants immediately after birth as they transition to newborn life. From a physiologic perspective, this transition involves some of the most complex and profound changes that any human will likely encounter during their life. The airways that are filled with liquid during fetal life must be cleared to allow the entry of air and onset of pulmonary gas exchange, and major vascular shunts must close to separate the pulmonary and systemic circulations. It is truly an amazing feat of nature that the vast majority of infants transition through these changes with such apparent ease. As a result, it is easy to underestimate both the magnitude of the physiologic changes and the complexity and difficulty of rendering assistance to infants struggling to adapt to life after birth.
Very preterm infants commonly require assistance at birth because they are simply too immature to survive unassisted, but there is considerable debate about what assistance is required and how it should be provided. Nevertheless, a fundamental tenet of neonatal resuscitation is to recognize that at birth, newborn infants, particularly very preterm infants, are not “mini adults” but are essentially exteriorized fetuses with liquid-filled airways. As such, the type of assistance given should be tailored to suit the infant’s changing physiology and its specific needs at any moment in time. For instance, what is the logic of applying ventilation strategies that facilitate pulmonary gas exchange when the gas exchange regions of the lung are liquid filled and so no pulmonary gas exchange can occur? Although this is only a transient consideration for most infants, because the airways are rapidly cleared of liquid, it is a lingering consideration in very preterm infants who have problems aerating their lungs ( ).
A key component to a successful neonatal resuscitation is understanding the physiologic changes that occur after birth and having the capacity to monitor the infant as it progresses through these changes so that the right assistance can be provided at the right time. As such, rather than utilizing an algorithm-based approach for describing currently recommended strategies for neonatal resuscitation, we will discuss the physiologic changes that occur at birth and highlight approaches that may best assist different subgroups of infants as their physiology changes. Many well-informed, recent publications have already detailed the currently recommended strategies for undertaking neonatal resuscitation from a practical perspective ( ). We intend to take a different approach and will focus on the physiology. This is because currently recommended strategies for neonatal resuscitation will likely change as our understanding of the physiology improves and better strategies for facilitating the necessary physiologic changes are identified. Indeed, much of the evidence underpinning current neonatal resuscitation guidelines is regarded as weak and/or absent ( ). The reasons for this are unclear, but it could be argued that a lack of scientific clarity regarding the physiology of transition is a major contributing factor. Nevertheless, in the following discussion, it will become evident that some of the emerging science is not consistent with current recommendations. This should not be misinterpreted as a recommendation for changing practice, but as the first important step in designing studies that will provide the required level of evidence needed to better guide practice.
You are called to the delivery room to resuscitate a late preterm infant born at 34 weeks’ gestation by repeat cesarean section. The 1 min Apgar score is 2. You arrive at 90 sec of life. The infant is pale with a heart rate of 30 beats/min. The infant is receiving nCPAP with 100% oxygen, but only gasping intermittently. The Sa o 2 reading on the pulse oximeter is 65%. The anesthesiologist has just begun chest compressions.
What is the next most appropriate next step in this infant’s resuscitation, and what should have been done before you arrived?
Positive pressure ventilation should have been started immediately.
From a teleologic perspective, it is logical that the physiologic changes required for survival after birth are triggered by the one event that cannot occur in utero, lung aeration. Aerating the lung and establishing pulmonary ventilation triggers the physiologic changes that underpin the transition to newborn life ( ). However, it is far too simplistic to assume that the primary benefit of “establishing pulmonary ventilation” is reestablishing oxygen and carbon dioxide exchange lost following umbilical cord clamping. Lung aeration not only triggers the switch to pulmonary gas exchange but also triggers a very large reduction in pulmonary vascular resistance (PVR), which initiates a series of cardiovascular changes that are also essential for survival after birth (see later). Positive pressure ventilation also enhances reabsorption of lung fluid.
With initiation of positive pressure ventilation, the heart rate increases to 120/min and the saturation increases to 85% by 7 min of life. The infant is breathing regularly at 120 breaths/min. Auscultation reveals fine rales and wet sounding rhonchi. You suspect the infant has a “wet lung syndrome.”
When is lung liquid reabsorbed? How did the mode of delivery influence the resorption of lung liquid?
Resorption of lung liquid begins antenatally and continues during labor and delivery. However, most lung liquid is reabsorbed postnatally when spontaneous or assisted ventilations begin. Infants delivered by cesarean section do not undergo the postural changes of vaginally delivered infants; those changes help to expel liquid from the lungs.
Although there is some evidence to suggest that airway liquid clearance begins late in gestation before labor onset ( ), this is not a consistent finding, and the role of experimental artefacts is unclear with regard to the original observations ( ). Nevertheless, considering the capacity of the lung to clear airway liquid during labor and after birth (see later), whether small amounts of liquid are cleared before labor appear inconsequential. However, it is clear that airway liquid clearance can begin during labor and vaginal delivery ( ). The release of adrenaline in response to the stress of labor activates Na + channels located on the luminal surface of airway epithelial cells, which promotes Na + reabsorption from the airways into lung tissue ( ). This reverses the osmotic gradient for liquid movement across the airway epithelium, leading to liquid reabsorption, rather than secretion as occurs in utero. However, Na + reabsorption requires high levels of adrenaline, is relatively slow, only arises late in gestation, and so is not active in very preterm infants ( ). Similarly, as cesarean section delivery in the absence of labor avoids the stress of labor, this mechanism is unlikely to be activated in infants delivered by cesarean section without labor ( ).
Partial airway liquid clearance can also occur during labor as a result of induced postural changes before and during delivery of the head ( ). The fetus is forced into an exaggerated “fetal position” with the enhanced dorso–ventral flexion causing an increase in abdominal pressure and rostral displacement of the diaphragm ( ). This increases intrathoracic pressures and forces liquid to leave the lungs via the trachea ( ; ). As the fetal respiratory system is highly compliant, only small increases in intrathoracic pressure are needed for large reductions in airway liquid volumes ( ; ). Although this mechanism is applicable to infants born vaginally, as per Na + reabsorption, it is not readily applicable to infants born by cesarean section, particularly in the absence of labor.
Lung aeration has significant implications for respiratory function in the newborn period, and to better understand these consequences, the process of lung aeration can be divided into a series of phases that give rise to separate challenges ( ).
The first phase commences at birth with liquid-filled airways, and so the primary challenge is to clear the airways of liquid, which occurs across the distal airway wall.
Airway liquid is cleared from the airways into the surrounding lung tissue at a much greater rate (over minutes) than it is cleared from the tissue (over hours). As such, airway liquid accumulates within lung tissue for the first few hours after birth, forming “perivascular fluid cuffs,” expanding the chest wall and increasing interstitial tissue pressures, essentially making the lung edematous.
Airway liquid is gradually cleared from lung tissue via the circulation and lymphatics, after which lung function and mechanics stabilize.
What is the importance of spontaneous breathing (or positive pressure ventilation) on promoting the clearance of lung water?
To clear lung liquid from the airways and alveoli, positive pressure ventilation (either spontaneous or assisted) must begin. Ventilation moves the liquid through the airways to the distal respiratory units, where it is absorbed into the lung interstitium and then into lymphatics.
As noted earlier, the majority of liquid remaining in the airway is cleared across the distal airway wall. For this to occur, the liquid must move distally through the airways before leaving the airways and entering the surrounding distal lung tissue ( ). This process has been observed in newborn rabbits using phase contrast x-ray imaging, showing that the air/liquid interface moves distally during each inspiration ( ; ) ( Fig. 1.1 ). As no further distal movement occurred between breaths, lung aeration and the creation of a functional residual capacity (FRC) occurs in a stepwise fashion, increasing with each successive inspiration ( Fig. 1.1 ). This led to the recognition that hydrostatic pressure gradients (between airways and lung tissue) generated by inspiration are largely responsible for airway liquid clearance after birth ( ; ). Importantly, this mechanism provides a rational explanation for why very preterm infants who have little or no capacity to reabsorb Na + are still able to clear their airways of liquid. As the air/liquid interface can also move proximally between breaths, causing a reduction in FRC, it is possible that liquid can reenter the airways between breaths, necessitating its reclearance with the next inspiration ( ; ).
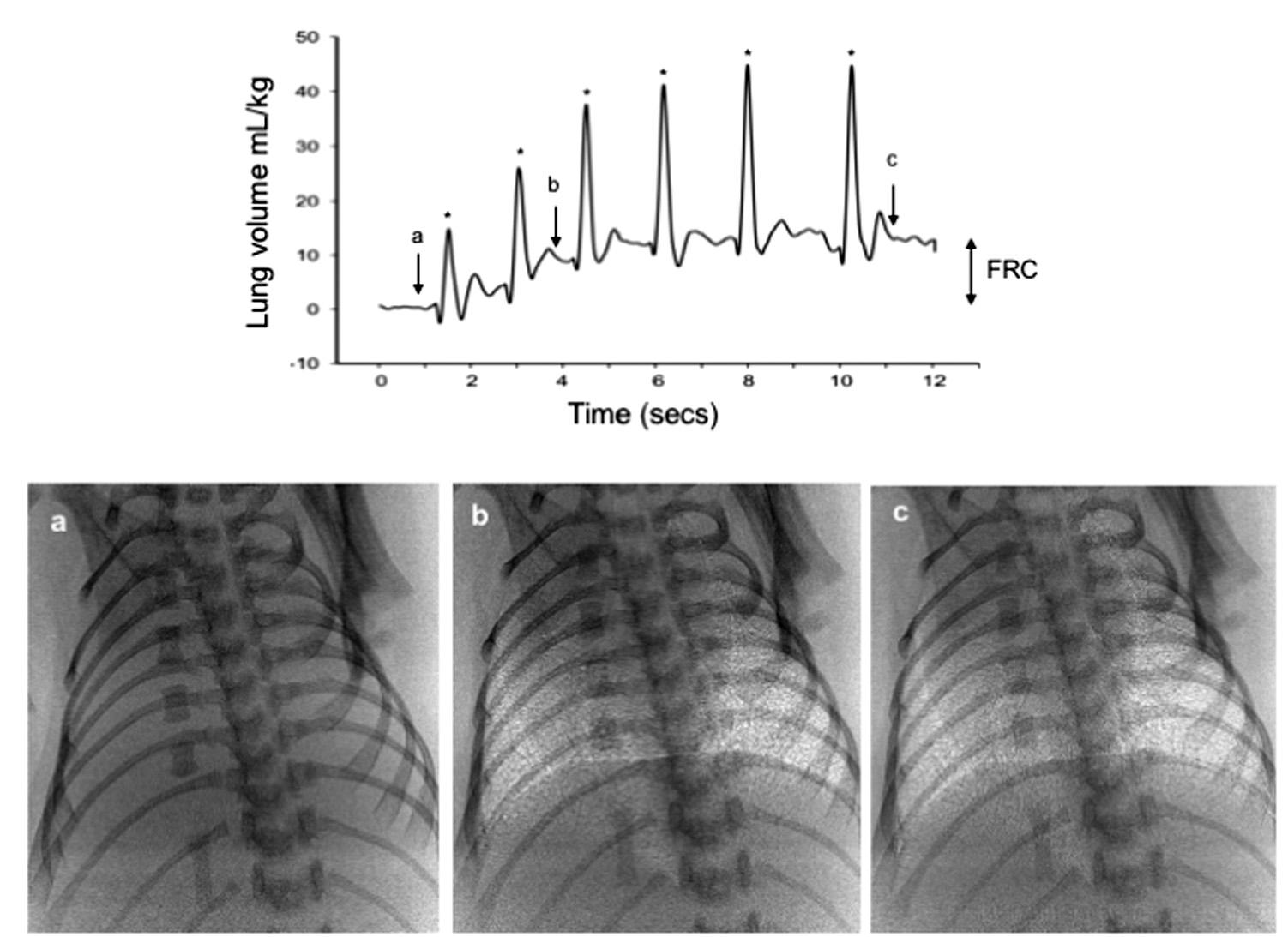
During the transition to postnatal life, what are the factors that govern whether airway liquid clearance is fast or slow?
Variables that regulate the rate of resorption of liquid include:
The surface area of the lung
Airway resistance (liquid has a higher resistance than air)
Resistance to moving the liquid across the walls of the distal airways
Tightness of the epithelial barrier
The initial resistance to air entering the lungs at birth is governed by both the resistance to moving liquid through the airways and by the resistance to moving this liquid across the distal airway wall. As water has a much higher viscosity than air, the resistance to moving air into the lungs is much greater when the airways are liquid filled compared with a few moments later when they are air filled ( , ). Consequently, airway resistance decreases markedly during the initial phase of lung aeration, as progressively more of the airways aerate and the reduction follows an exponential function that is difficult to predict ( , ). On the other hand, little is known about the contribution that the resistance to liquid movement across the distal airway wall makes to the overall resistance to airway liquid clearance. Based on the volume of liquid that can be cleared during one inspiration (up to 3 mL/kg), and knowing the duration of inspiration (100–200 mSec), the liquid flux across the pulmonary epithelium can be as high as 15 to 30 mL/kg/sec or 0.9 to 1.8 L/kg/min. Although transient, this is surprisingly high for liquid movement across the relatively tight pulmonary epithelium ( ). A large surface area is one obvious factor that allows the lung to clear liquid at this rate, but the “tightness” of the epithelial barrier likely resists water transfer.
The immature lungs of preterm infants have airways that are smaller in diameter and have few if any alveoli. As reducing the radius of a tube increases its resistance by the 4th power and as the absence of alveoli markedly reduces the lung’s surface area, the resistance to airway liquid clearance is higher in preterm infants than in term infants ( ). As a result, either the process of lung aeration will be much slower, or preterm infants will require larger inspiratory efforts or higher inflation pressures to overcome this higher resistance. This concept is at odds with current resuscitation guidelines that suggest using lower inflation pressures during the initiation of lung aeration in very preterm infants compared with term infants ( ). This recommendation is based on an extrapolation from studies in aerated lungs suggesting that higher pressures cause lung injury. However, considering it is the volume change and not the pressure per se that causes lung injury ( ) and that a liquid-filled lung is orders of magnitude less compliant than an air-filled lung ( ), this recommendation may be flawed and requires further investigation.
Previous studies have demonstrated that the fetal pulmonary epithelium is relatively tight, which restricts the entry of even relatively small molecules into lung liquid during development ( ). However, at birth these pore sizes markedly increase, which likely reduces the resistance to liquid movement across the epithelium in term newborns ( ). However, it is unknown whether this occurs in preterm infants or whether it occurs to a greater degree due to the immaturity of the epithelium. Although this could reduce the resistance to liquid clearance, it may also contribute to the entry of plasma proteins into the lumen, which will interfere with surfactant function.
In the delivery room, how will I know when this infant’s lungs are optimally aerated?
Heart rate, oxygen saturation and expired CO 2
Heart rate and peripheral oxygen saturation levels, measured using a pulse oximeter and/or ECG leads, are commonly used to assess when neonatal resuscitation has been “successful” ( , ). The idea that an increasing heart rate is a sign of lung aeration is based on the concept that a low heart rate indicates a vagal-induced bradycardia in response to perinatal asphyxia ( ). As such, an increasing heart rate is assumed to reflect improved oxygenation following the onset of pulmonary gas exchange. However, it is now clear that an increase in heart rate can also occur after birth in the absence of an increase in oxygenation ( ). In this instance, the increase in heart rate is secondary to an increase in PBF (in response to lung aeration), which increases venous return and left ventricular preload ( ). Nevertheless, whether the increase in heart rate results from increased oxygenation or an increase in PBF, both only occur as a result of lung aeration.
An alternate indicator for lung aeration is the use of expired CO 2 , which is closely related to the degree of lung aeration ( ). Indeed, it is such a sensitive indicator that it can detect breath-by-breath changes in lung aeration in parallel with the changing tidal volumes and increases much more quickly in response to lung aeration than an increase in both heart rate and oxygenation in infants ( ; ; ). The close relationship between end-tidal expired CO 2 levels and tidal volumes is because CO 2 exchange is surface-area limited during lung aeration. As CO 2 has a high solubility, its diffusion across the pulmonary epithelium is very efficient and is not normally surface-area limited. As such, end-tidal CO 2 levels are commonly used to estimate pulmonary arterial blood P co 2 levels and can be used to calculate cardiac output ( ). However, when the lung is not fully aerated, the surface area available for gas exchange at end inspiration is dependent on the size of the tidal volume ( ). When tidal volumes are larger, the surface area for gas exchange increases, which increases the efficiency of CO 2 exchange.
Although CO 2 monitoring in the delivery room is currently not routine, in combination with tidal-volume monitoring, it provides a reliable method for assessing the effectiveness of pulmonary ventilation immediately after birth. Indeed, considering that the dead space of the lower airways is 2 to 3mL/kg and that the pharynx is expandable ( ), it is possible to achieve significant tidal volumes (3–4 mL/kg) without gas entering the gas exchange regions of the lung. As such, the baby would appear to be ventilated, but oxygenation levels and heart rate would likely remain low. However, the absence of any expired CO 2 would indicate that the gas exchange regions are not being ventilated. Some of the new respiratory function monitors include the ability to measure expired CO 2 levels, although increasing dead-space volume is an issue, and are most effectively used in combination with tidal-volume monitoring. Alternatively, a colorimetric CO 2 indicator, which changes color in response to expired CO 2 , can be used to indicate when gas exchange has commenced ( ).
During resuscitation, what alternate resuscitation strategies might be used to improve uniform lung aeration and better ventilation in this infant?
Increase in positive end expiratory pressure (PEEP) or sustained lung inflation
Recognition that airway liquid clearance after birth results from the generation of hydrostatic pressure gradients (between airways and lung tissue) has provided opportunities for developing strategies that facilitate this process in very preterm infants. Indeed, in a simplistic sense, all that is required is to apply a gas pressure to the airways to overcome the high resistance of moving liquid through the airways and across the distal airway wall. This rationale is consistent with the current recommendations for using either intermittent positive pressure ventilation (iPPV) or continuous positive airway pressure (CPAP) in combination with the infant’s spontaneous breathing to assist preterm infants initiating pulmonary gas exchange after birth ( ; ).
However, the big question is how much pressure should be applied? Indeed, if the applied pressure is too low, it will be insufficient to overcome the resistance required to move the liquid distally through the airways. If it is too high, then there is a risk of causing overinflation and lung injury in lung regions that have already aerated ( ). To add to the complexity, as the airway resistance dramatically decreases (by ∼100 fold) with airway liquid clearance, the pressures required to move the liquid at any given flow rate will also greatly reduce ( ). Considering the huge variability expected between individual infants at birth, particularly with the amount of liquid present in the airways and the level of inspiratory effort each will apply, stipulating a single set inflation pressure or CPAP level to assist preterm infants to aerate their lungs at birth ignores this complexity. Clearly the pressure required will be different in different infants and will change as the lung aerates. Although we now have a grasp of the complexities involved in facilitating lung aeration in very preterm infants, the challenge is to apply this knowledge in a useful and practical manner ( ).
During lung aeration, ideally the respiratory support applied should change in accordance with the change in resistance caused by airway liquid clearance. High airway pressures could be applied initially when the resistance is high, which decrease as the airway resistance decreases to avoid overinflation and lung injury. However, to decrease the applied pressure in synchrony with the decreasing resistance requires complex feedback information regarding the changing airway resistance. Although modern ventilators can measure airway resistance on a breath-by-breath basis, they are rarely used in the delivery room even if the infant is intubated. Instead, low-technology devices such as resuscitation bags or t-piece devices are more commonly used, mostly in combination with noninvasive ventilation ( ; ). These provide little or no opportunity to measure airway resistance and provide little information on how to modify the required ventilation parameters as lung mechanics change unless it is combined with a respiratory function monitor. It seems counterintuitive that sophisticated ventilators and monitoring equipment are commonly used in the NICU once the lung has aerated and respiratory mechanics have stabilized, but they are not routinely used in the delivery room when respiratory mechanics are rapidly changing and respiratory function is very difficult to manage in a safe and effective way.
The movement of air into the lung at birth is primarily determined by airway resistance and the applied pressure gradient, as defined by F = ΔP/R; where F is flow, ΔP is the pressure gradient and R is airway resistance, which includes the resistance to moving liquid across the distal airway wall. As flow equals volume (V) divided by time (T), the factors determining the movement of air (inflation volume) into the lung can be defined as V = (ΔP × T)/R. As such, the main controllable factors determining inflation volume are the applied pressure gradient (ΔP) and time (T) over which the pressure is applied (inflation time). Although increasing the inflation pressure can overcome the high initial resistance, as the resistance decreases with lung aeration, there is a high risk of overinflating and injuring the lung if the pressure is not simultaneously reduced. Alternatively, increasing the inflation time using a slower, sustained inflation (SI) allows lower inflation pressures to be used. Although the initial flow of gas into the lung is slow, it rapidly increases as more of the airways aerate and the resistance decreases ( ). Theoretically, this approach has multiple advantages, as during a sustained inflation the lung’s end inflation volume is self-limiting and determined by the inflation pressure, which can be much lower than the pressure required to initially aerate the lung with a shorter inspiratory time.
As different lung regions aerate at different rates, a SI allows more lung regions to aerate during a single inflation ( , ). This has important implications for lung injury, because during the subsequent inflation, air will rapidly flow into and expand aerated lung regions first due to the much lower airway resistance. Therefore if the inflation time is short (as occurs with iPPV), the entire tidal volume will only enter aerated regions, potentially causing overexpansion and injury in those regions with little further lung aeration ( , ). Furthermore, as gas exchange only occurs when the distal gas exchange regions aerate, there is no reason to terminate the inflation to allow exhalation when these regions are liquid filled. These explanations underpin the rationale for providing a SI for the first inflation after birth, but the benefits described in animal studies have not been replicated in humans ( ; ). Although the reasons are unclear, in animal studies the SI was applied with an endotracheal tube, whereas in all human studies, the SI was applied noninvasively, usually with a face mask (including the SAIL trial). This is a major point of difference (see later), and studies that are restricted to comparing a SI with conventional ventilation in intubated infants may possibly reveal results that are as clear cut as the animal studies.
What are the adverse effects of higher levels of PEEP or sustained inflation during resuscitation?
High levels of PEEP or a prolonged sustained inflation can decrease venous return and reduce cardiac output.
Recent studies have suggested that a stepwise PEEP recruitment maneuver (up to 20 cmH 2 O) that extends over 2 to 3 minutes can achieve better postmaneuver lung mechanics than an SI ( ). This suggestion is consistent with the concept that lung aeration is a function of applying an elevated pressure over an extended period, and the results show significant improvements in lung mechanics ( , ). However, applying this maneuver ignores the cardiovascular consequences of applying high elevated airway pressures that increase intrathoracic pressures for an extended period. Simple physics dictates that as soon as intrathoracic pressures exceeds central venous pressure, then all venous return to the heart will cease and, as such, cardiac output must decrease (see later). Furthermore, in the aerated lung, high PEEP levels reduce PBF, and this effect on PBF is not completely reversed following the reduction in PEEP ( ). Although this adverse effect of increased intrathoracic pressure on PBF is applicable to both a sustained inflation and PEEP recruitment maneuver, a sustained inflation does not influence the time related increase in PBF, perhaps because sustained inflation is only 10 to 30 seconds long ( ). However, the PEEP recruitment maneuver can take 2 to 3 minutes ( , ), and it is unclear how it influences the increase in PBF at birth. There is also a need to be cautious of any rebound in cardiac output, as per a Valsalva maneuver that may occur post maneuver.
Whether a sustained inflation or a PEEP recruitment strategy is the most effective approach for aerating the lung remains unclear, and more studies are required. In particular, there is much debate about what is the most appropriate starting pressure and duration of the sustained inflation. However, evidence from animal studies indicate that these are not the correct starting points ( ), particularly as a “one-size-fits-all” approach is unlikely to be successful in different infants ( , ). Targeting a set inflation volume instead of a fixed inflation time and using a ramped pressure increase, which is then held constant once gas starts to move into the lungs, may be more appropriate ( ; ; ). Measurement of CO 2 levels in the expired air can then indicate whether a second inflation is needed. This approach is easy to implement in animals, but its application in humans will depend on the use of sophisticated approaches to monitor newborns immediately after birth ( ).
At 60 min of life the infant’s respiratory rate is 120/min. There is intermittent grunting. The inspiratory oxygen concentration is 100% to achieve a saturation of 90%.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here