Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
By the end of this chapter the reader should:
Understand the principles of body composition in children and its assessment
Understand the scientific basis of nutrition
Understand the physiological basis of normal enteral nutrition and its variation throughout childhood
Know the constituents of a healthy diet at all ages, including breastfeeding and formula feeding in infancy
Know the constitution of infant feeds commonly used in health and disease
Know the principles and methods of dietary supplementation, e.g. calories, vitamins, minerals
Know the principles of nutritional management in childhood disease, e.g. neonates, sick children
Understand the assessment of faltering growth
Know about the assessment of being overweight or obese
The traditional measures of nutritional status and well-being during infancy and childhood are body weight and/or length or height, plotted on appropriate centile charts so that the individual child's growth can be compared with that of a child of the same age and gender from a ‘reference’ population.
Currently, five different growth charts are available in the UK (see Further reading for more information). The UK-WHO ‘0–4 years’ is the growth chart most commonly used to monitor growth in healthy infants and young children. In addition to this, a Neonatal and Infant Close Monitoring chart (NICM) has been designed to monitor growth in very preterm infants and those whose growth may be affected by early health problems or chronic disease. This chart has low lines to monitor unusually short or underweight children and provides assistance with correction for gestational age. Two different charts are now available for school-aged children: the ‘2–18’ chart, whose features include guidance on monitoring puberty, BMI and an adult height predictor; and the new Childhood and Puberty Close Monitoring chart (CPCM). This chart is for use in school-aged children with growth or nutritional problems and can be used until the age of 20. Separate charts are also available for children with Down's syndrome.
In May 2009, the new UK-WHO growth charts replaced the British 1990 (UK90) charts for children aged 0–4 years. These new charts combine data from the British 1990 reference at birth with data from the WHO Multicentre Growth Reference Study (MGRS; see Further reading ) from 2 weeks to 4 years. The MGRS was conducted to provide data on the way that infants should grow (a standard) rather than how they do grow (a reference). It is conceptually different from older growth references and is based on the premise that babies of all ethnicities grow similarly and to the same extent when receiving optimal nutrition – breastfeeding – and with no environmental constraints on growth. Therefore, the WHO MGRS collected anthropometric data from term infants exclusively breastfed for at least the first 4 months of life from the most advantageous backgrounds in six countries of differing ethnicity. By contrast, older growth charts (references) were based on data from a mixture of formula-fed and breastfed babies from a range of socio-economic backgrounds, generally in a single country or area.
Key differences between the new UK-WHO chart and the old UK90 chart are:
It is designed for term infants (the MGRS did not include preterm infants)
Birth measurements for term infants born between 37 and 42 weeks' gestation are plotted at a single point
A separate UK preterm chart is available, incorporating older UK90 data until term age, and WHO data from 2 weeks
No data are provided from 0–2 weeks, allowing for the expected dip in weight and regaining of birth weight up to 2 weeks
The 50th centile is de-emphasized (it is no longer printed in bold type)
When values for the same infants are plotted on both charts, the apparent growth trajectory can vary significantly, especially for weight and head circumference. Plotting infants on the new charts after the age of 6 months will give twice as many above the 98th centile for weight and only 1/200 below the 2nd centile. Indeed, this was one of the aims of the new growth standard, since breastfed infants tend to grow more slowly than formula-fed infants during the second 6 months of life and were often diagnosed as having growth faltering when older charts were used. However, the expected trajectory of weight gain on the new charts is harder to achieve in the period up to 6 months (perhaps because of the highly selected nature of the reference population). This has the potential to result in mothers stopping breastfeeding or supplementing with infant formula if they think their infant is not gaining weight normally. Clinicians need to be aware of the changes to the growth charts and the apparent impact this can have on a child's growth, in order to advise mothers appropriately.
‘Body composition’ refers to the amount of fat and fat-free mass in the body. There is increasing evidence that this is relevant both for nutritional management and for clinical outcome. For example, providing additional energy to chronically ill children whose linear growth is stunted may make them gain fat without improving linear growth or lean mass; similarly, underweight children with developmental handicaps may become fatter when given improved nutrition without beneficial effects on any clinical outcome. Theoretically, basing energy intake on lean mass, which is the metabolically active component, might be a more sensible approach.
Body Mass Index (BMI = weight/height 2 ) is widely used as a measure of ‘adiposity’ or fatness. However, this index is a composite of fat and fat-free tissue and does not provide information about the amount of fat or fat-free tissue in the body. In groups or populations, it is reasonable to regard BMI as a proxy for adiposity, since fat is the most variable component of body composition. However, this is not the case for individuals; children with the same BMI can have very different amounts of fat and fat-free mass. Indeed, the relative amount and accretion rates of fat and lean tissue differ between boys and girls.
Historically, the use of more detailed body composition measurements in clinical practice has been limited due to methodological problems in obtaining reliable measurements and to a lack of reference data for the paediatric age group. UK reference data for body composition are now available for children aged 5 and above, so individual children can be given a centile or SD score for fat and lean mass in the same way as for weight, height or BMI.
A number of different techniques are available to measure body composition ranging from simple measures such as skinfold thicknesses, waist circumference or mid upper arm circumference (see Fig 33.5 ) to more complex methods suitable only in a research setting. These methods are outlined below, and the advantages and disadvantages of individual methods are further summarized in Table 13.1 .
| Technique | Assumptions made | Reference data | Availability * | Advantages/disadvantages |
|---|---|---|---|---|
| Skinfolds – raw | Constant skin protein content | Y | +++ | For: simple measure of regional fat |
| Against: no information on lean mass | ||||
| Skinfolds ∝ whole body fat | N | +++ | For: simple and quick | |
| Against: population specific, poor accuracy in individuals and groups | ||||
| Body mass index | Var weight = var fat | Y | +++ | For: simple and quick |
| Against: measures nutritional status not body composition | ||||
| Waist circumference | Waist ∝ central fat | Y | +++ | For: simple, quick, robust measure of abdominal fat |
| Against: not so accurate as measure of internal visceral fat | ||||
| Bioelectric impedance analysis (BIA) | Conductivity ∝ body water | N | ++ | For: simple and quick |
| Against: population specific, poor accuracy in individuals and groups | ||||
| DXA (dual X-ray absorptionometry) | Constant attenuations of FFM and F | Y | ++ | For: accurate for limb lean and fat |
| Against: radiation exposure, whole body bias ∝ size, sex, fatness | ||||
| Densitometry † | Constant D ffm and D f | N | + | For: acceptable two-component technique |
| Against: effects of disease on lean mass reduce accuracy | ||||
| Isotope dilution | Constant H ffm | N | + | For: only technique acceptable in all age groups |
| Against: delayed results, inaccurate if disease affects H ffm | ||||
| MRI | Electromagnetic properties | N | + | For: accurate for regional AT |
| Against: expensive, limited availability, measures AT not fat |
* Availability: +, low; ++, medium; +++, high.
† Densitometry by air displacement plethysmography (BodPod). Abbreviations: AT, adipose tissue; D f , density of fat; D ffm , density of fat-free mass; D min , density of mineral; F, fat; FFM, fat-free mass; H ffm , hydration of fat-free mass; Var, variability.
‘Two-component’ models divide the body into fat mass (FM) and fat-free mass (FFM). Body composition is either ‘predicted’ (for example, by combining several skinfold thickness measurements in a prediction equation, or using bioelectric impedance ( Fig. 13.1C ) to predict body water and hence FFM) or ‘measured’ (for example, measuring body density (usually by air displacement plethysmography), body water using a stable isotope such as deuterium as a tracer, or by dual X-ray absorptionometry (DXA; Fig. 13.1B )). The main limitation of these two-component models is the need to assume a constant hydration of fat-free mass. In fact, hydration has been shown to vary with age and is altered by obesity as well as in many acute and chronic diseases.
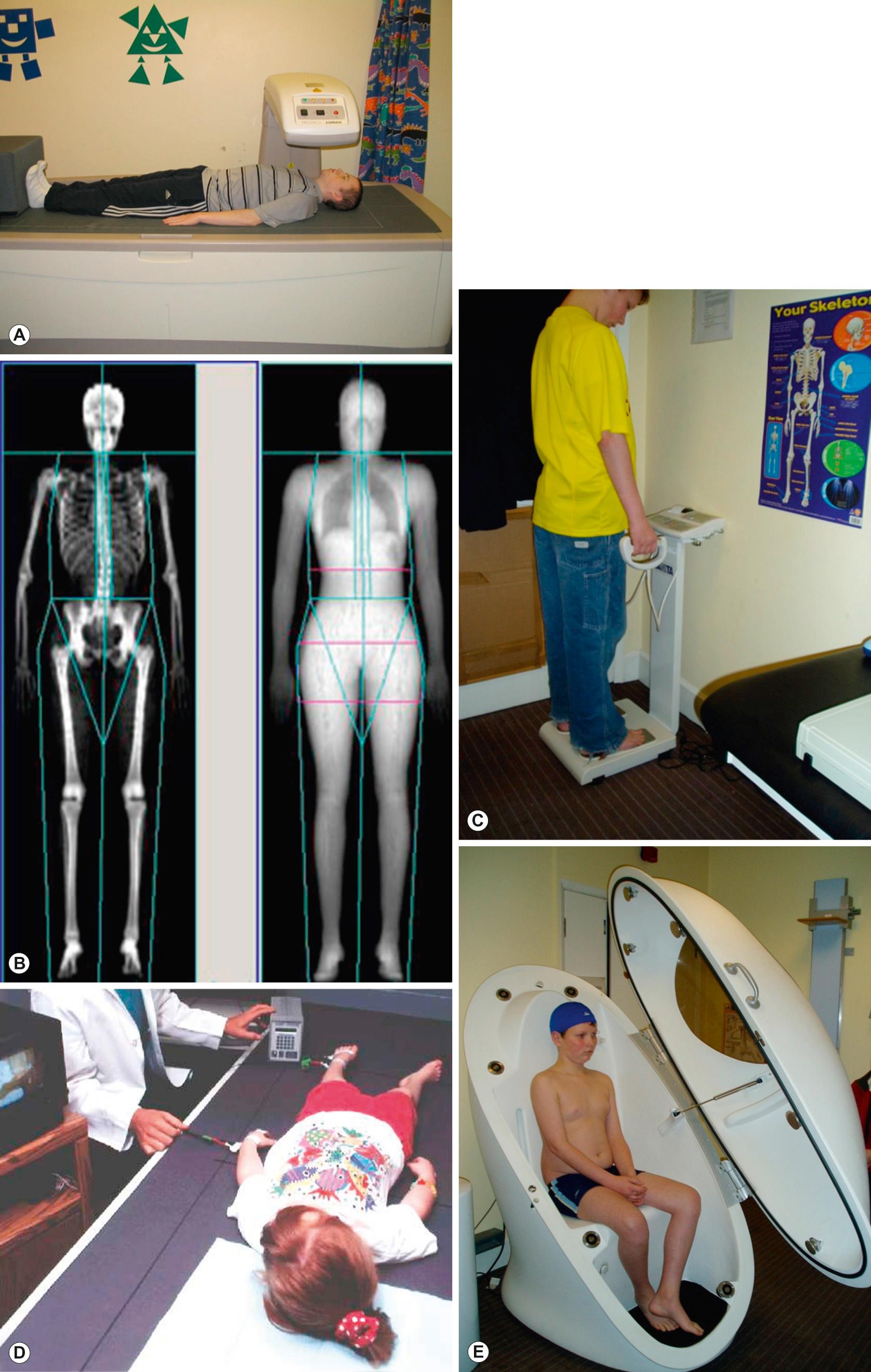
More sophisticated models (‘three- and four-component’ models) reduce the number of assumptions made by performing more measurements. In the three-component model, the body is divided into fat mass, fat-free mass and water; whereas in the four-component model, fat-free mass is further divided into mineral mass and protein mass. The four-component model is regarded as the gold standard for measuring body composition in vivo since it makes the fewest assumptions. However, it requires the subject to undergo air displacement plethysmography ( Fig. 13.1E ), a DXA scan and a measurement of total body water using stable isotopes, so is not suitable outside a research setting. Furthermore, it is currently almost impossible to accurately measure body volume in children between the ages of about 8 months and 4 years.
A recent study comparing results from different body composition measurement techniques against the four-component model in children with a variety of underlying illnesses concluded that DXA and bioelectric impedance analysis (BIA) were likely to be the most accurate and feasible methods in clinical practice, although it is unlikely that a single method could be used in all patients on all occasions; DXA requires the child to be taken to the scanner and to lie still for a few minutes, whereas BIA is not suitable in children with significant abnormalities in hydration.
Historically, the main objective in feeding infants and children was meeting nutritional needs, preventing nutritional deficiencies and facilitating adequate growth and development. However, the increasing evidence that early nutrition has biological effects, with important implications for short-term health, clinical course and prognosis as well as for later health outcomes, has led to a shift in focus. Until recently, nutritional recommendations and practice were underpinned largely by observational or physiological studies, or by small clinical trials designed to test for the effects of specific products on nutritional status, growth and tolerance. However, the past 20 years have seen the application of randomized trials to nutritional interventions. These trials examine both short-term and long-term efficacy and safety and are increasingly regarded as an essential component of the development and testing of novel nutritional products by regulatory bodies.
The concept that there are sensitive periods in early life when insults or stimuli may have long-term or even lifetime effects has been defined as ‘programming’. Evidence that nutrition could act as a programming agent was shown in a variety of animal species by McCance and Widdowson. For example, in the early 1960s, they showed that malnutrition during an early ‘critical period’ in rats permanently impaired their growth. In this experiment, rat pups that were malnourished during the suckling period by putting them in large litters showed later growth deficits which were not reversible even with an unrestricted food supply after weaning; whereas a similar period of malnutrition later in the growth period produced only a temporary effect on weight gain, which was rapidly reversed when adequate food was supplied. Since then, animal studies in a wide range of species, including non-human primates, have shown that nutrition during critical periods in early life can programme outcomes such as changes in metabolism, endocrine function, gut function, size, body fatness, blood pressure, insulin resistance, blood lipids, learning, behaviour and longevity. Over the past few years, findings from randomized intervention studies in human infants with later follow-up have confirmed that health outcomes in human infants, as in other species, can be programmed by early diet. Early diet has been shown to have long-term effects on blood pressure, insulin resistance, blood lipids, obesity risk, bone health, atopy, cognitive function and brain structure. The size of the effect on some outcomes is large enough to be of public health significance; for example, in the case of cardiovascular risk factors (blood pressure, blood lipids, insulin resistance), the programming effects of early nutrition are greater than non-pharmacological interventions in adult life, such as exercise and weight loss. The implication of these findings is that it is no longer sufficient to think about nutrition only in terms of short-term adequacy or outcomes; potential effects on later health must also be considered.
Nutritional requirements throughout infancy and childhood vary more than at any subsequent stage of life, due to changing requirements for growth and organ development. Hence, the potential for inadequate or excessive nutrition is also greatest during this period.
Recommendations for nutrient intakes in the UK are provided as Dietary Reference Values (DRVs) for boys and girls (0–3, 4–6, 7–9, 10–12 months, 1–3 years, 4–6 years, 7–10 years), and for males and females (11–14 years, 15–18 years, 19–50 years, 50+ years). There are separate recommendations for pregnant and breastfeeding women.
In North America, recommendations for the intake of specific nutrients are provided in the Dietary Reference Intakes (DRIs; see Further reading for more information), a set of recommendations intended to provide guidance for evaluating nutrient intakes and planning diets for both individuals and population groups. Recommendations are provided for the following life stage groups: Infancy (first 6 months, second 6 months), Toddlers (1–3 years), Early childhood (4–8 years), Puberty/adolescence (9–13 and 14–18 years), Young adult/middle age (19–30 and 31–50 years), Adulthood and older age (51–70 and 70+ years), and pregnancy and lactation.
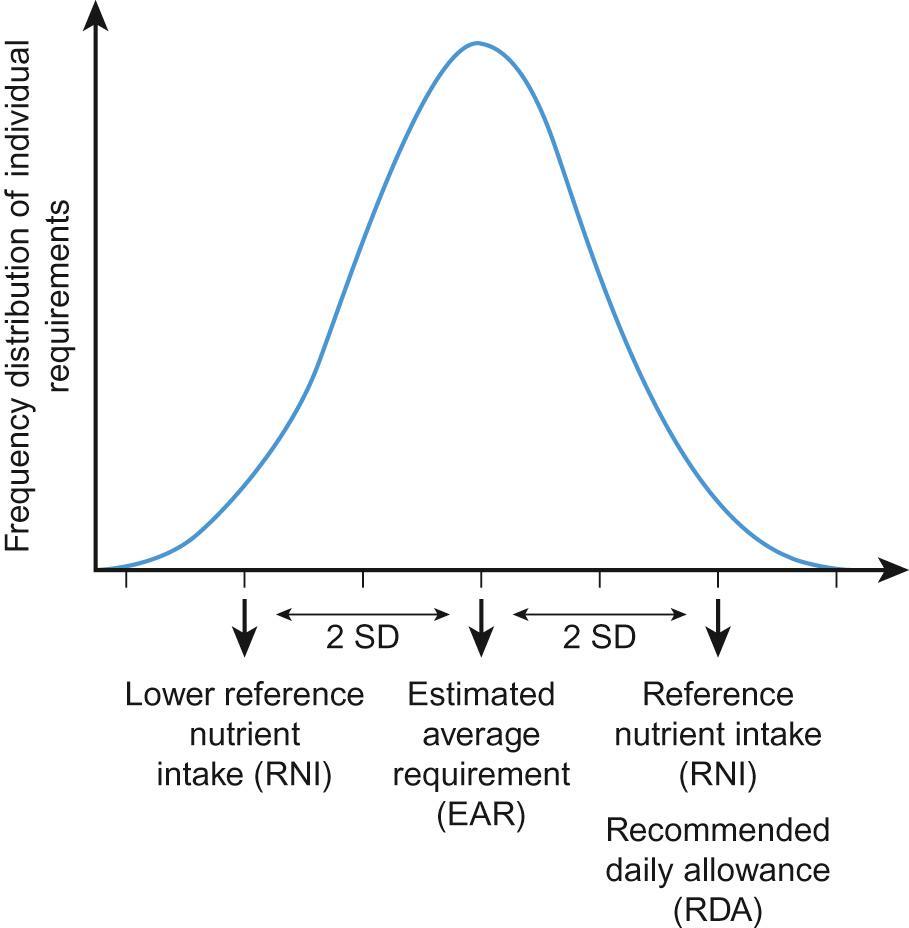
The DRVs and DRIs consist of different estimates, summarized and defined in Table 13.2 and Figure 13.2 . They share a number of common features, which are important to consider when interpreting and using the recommendations:
Recommendations are made for healthy populations. They are not appropriate for malnourished or sick populations, or for those with pre-existing deficiencies.
Where available, data on the effect of nutrients on chronic health or disease are considered, so recommendations are not just based on preventing deficiency.
Compared to older recommendations, there is a greater emphasis on the distribution of nutrient requirements in a population, rather than on a single value.
Where data exist, upper levels of intake are given, taking into account the potential for harmful effects, which is considered important given the increased use of food fortification and supplements.
| UK – Dietary Reference Values | North America – Dietary Reference Intakes |
|---|---|
| Estimated average requirement (EAR): The average requirement for a nutrient 50% of population require more and 50% require less |
Estimated average requirement (EAR): Average daily nutrient intake level estimated to meet the requirements of half the healthy population |
| Reference nutrient intake (RNI): The amount of a nutrient needed to meet the requirements of 97.5% of the population |
Recommended dietary allowance (RDA): The average daily nutrient intake sufficient to meet the requirements of 97.5% of the population |
| Lower reference nutrient intake (LRNI): The amount of a nutrient that is sufficient only for the 2.5% of the population with the lowest requirements |
|
| Safe intake: Used where there is insufficient evidence to set EAR, RNI or LRNI. The amount at which there is judged to be no risk of deficiency, but below the level where there is a risk of undesirable effects. |
Adequate intake (AI): Used where there is insufficient evidence to set EAR/RDA. The average daily nutrient intake based on observed or experimental approximations or estimates of intake by apparently healthy people that are assumed to be adequate. |
| Tolerable upper intake level (UL): Highest daily nutrient intake likely to pose no risk to almost all individuals |
The data used to formulate the recommendations for different nutrients varies in quality and quantity, and is often inconsistent over the different age groups specified. The types of data used include clinical, dose response, balance studies, depletion–repletion studies, and observational studies (for example, recording the intakes of apparently healthy individuals or those with clinical signs of deficiency); combined with theoretical estimations based on requirements for maintenance and growth, taking into account bioavailability, absorption and excretion. For ethical reasons, many of these approaches are difficult or impossible in infants and children and, consequently, many recommendations for toddlers and children are based on data extrapolated from adults, with consequent limitations.
Recommended nutrient intakes for infants are based on the estimated nutrient intakes of healthy breastfed infants growing normally during the first 6 months, and from 6–12 months on the nutrient intakes of infants who are receiving breast milk alongside complementary foods. Given the wide variation in breast milk intake and milk composition over time, between and within individuals, this approach has obvious limitations; and this is likely to be even more problematic in older infants, where the type of complementary foods consumed is also very variable.
Where data are considered to be sufficient in quality and quantity, an estimated average requirement (EAR) is determined; defined as the nutrient intake estimated to meet the requirements of half the population in a particular age group. From this figure, a recommended daily allowance (RDA) is calculated as the average nutrient intake sufficient to meet the requirements of nearly all subjects (97.5%; calculated as the mean + 2SDs). If the data are insufficient to establish an EAR, then an adequate intake (AI; US) or safe intake (UK) is provided. Recommended nutrient intakes for infants <12 months are provided as adequate intakes, reflecting the lack of data sufficient to determine an EAR for this population.
Important points to consider when using these recommended intakes in infants and children include:
Recommendations are provided for individual nutrients rather than foods. In practice, it may be difficult or impossible to meet the theoretical requirements for all nutrients simultaneously using real foods for certain age groups; this is particularly the case during the complementary feeding period when recommended requirements for iron are very high and it is difficult to provide sufficient iron from foods without exceeding recommended intakes for other nutrients such as protein.
Recommendations do not generally take into account the bioavailability of the nutrient from different foods. This is particularly important for nutrients such as iron, where the absorption of haem iron (e.g. from meat) is much better than that of non-haem iron (e.g. from fortified cereals or supplements). Iron absorption is also affected by other foods and drinks; for example, vitamin C will increase absorption, whereas phytates (from cereals) or tea will decrease it.
The recommendations for energy intake in infants and children (see Further reading ) require special consideration. EAR values are provided, since an RDA/RNI (representing the mean + 2SDs) would provide excess energy for the majority of the population. Historically, recommendations were based on observed energy intakes in different populations, but this approach has obvious flaws for populations experiencing increasing rates of overweight and obesity. The development and refinement of the doubly-labelled water technique, which uses stable isotopes to measure total energy expenditure (TEE) non-invasively in free-living subjects, has allowed the development of recommendations based on energy expenditure. New EARs for energy intakes were produced in 2011.
The main components of TEE are the basal metabolic rate (BMR), which makes up 40–70%, and physical activity, which makes up 25–50% in the majority of individuals and is the most variable component of energy expenditure. Infants and children have an additional energy requirement for new tissue deposition. The energy costs for growth make up about 35% of the total energy requirement during the first three months of life, about 17.5% in the next three months and reduce further over the next six months to only 3% at 12 months. Energy for growth falls to less than 2% of daily requirements in the second year, remains between 1 and 2% until mid-adolescence, and gradually disappears by 20 years of age.
The natural biological food for a human infant is breast milk. Breastfeeding is the ‘gold standard’ for infant nutrition, and mothers should be encouraged and supported to breastfeed their infant. Although the beneficial effects of breast milk and breastfeeding (and the detrimental effects of not breastfeeding) are widely cited, there are considerable limitations of the evidence available on this topic, which apply both to the composition of breast milk and health effects of breastfeeding. These issues are relevant to the design of optimal breast milk substitutes for use when mothers cannot or choose not to breastfeed. One must also remember that the majority of infants in countries such as the UK receive an infant formula at some point even if they are initially exclusively breastfed, as the use of cows' milk as the main drink is not recommended before 12 months.
The composition of breast milk has long been used as the basis for determining infant nutrient requirements during the first 6 months of life, and as a basis for regulations on the permitted composition of breast milk substitutes (infant formulas). Whilst this makes sense theoretically, in practice there are a number of problems associated with this approach, summarized in Table 13.3 . Recognizing these problems, in recent years, there has been a greater focus on trying to achieve the performance (health and developmental outcomes) of breastfed infants rather than simply trying to mimic the composition of breast milk, which is in many ways impossible.
| Issue | Consequence |
|---|---|
| Breast milk composition varies between mothers and changes over the course of lactation, during a day, during a feed | It is difficult to define a single reference concentration for many nutrients |
| The method used to obtain breast milk samples may affect the results (e.g. fore-vs hindmilk, hand- vs pump-expressed, single versus pooled samples) | |
| The composition of expressed milk may differ from that of the energy suckled by the infant direct from the breast | This probably resulted in an overestimation of human milk, which was then applied to infant formulas |
| Breast milk contains many bioactive substances (hormones, growth factors, etc.) | These are difficult or impossible to mimic in an infant formula |
| The milk from different mammals varies considerably not just in composition but in the configuration or quality of fat or protein, which can affect outcomes | Infant formulas may contain the same total fat or protein concentration as human milk, but there may still be significant differences in the type of nutrients, e.g. stereo-isomeric differences in triglycerides influence fat and calcium absorption; different protein composition (e.g. α-lactalbumin, β-lactoglobulin) can affect growth |
Data on the effectiveness and safety of any intervention can be obtained from observational studies or from randomized controlled trials (RCTs). Randomized trials are regarded as the ‘gold standard’ in terms of demonstrating causal relationships between intervention and outcome and underpinning clinical practice. They are now accepted as the preferred approach for evaluating nutritional interventions. Observational studies, such as birth cohorts, have the advantage that the sample sizes are generally large and results can often be obtained rapidly. However, because the type of infant feeding is not randomly assigned, these studies can show associations between infant feeding and health outcomes but they cannot demonstrate causation.
Whilst accepted as the gold standard, there are a number of problems when it comes to conducting RCTs in the field of infant nutrition. The main one is that healthy term infants cannot be ethically or feasibly randomized to be breastfed or formula-fed, which prevents the use of an RCT for assessing the effect of any intervention against the ‘gold standard’ for infant feeding. It is also difficult in practice to randomize breastfeeding mothers to different breastfeeding practices – for example, altering the duration of exclusive breastfeeding (EBF). Given these problems, it is perhaps not surprising that there have been only two experimental studies designed to examine the health effects of breastfeeding (one in preterm infants randomized to banked donor breast milk versus preterm infant formula in the 1980s in the UK; the other a cluster-randomized trial of a breastfeeding promotion intervention in healthy term infants in Belarus), and three published experimental studies examining health effects of the duration of EBF (two in Honduras, one in Iceland). All other data on the health effects of breastfeeding come from observational studies.
Lack of randomization to the exposure variable (in this case, breastfeeding) inevitably introduces significant methodological issues, particularly in the case of infant feeding studies where the choice of feeding mode is strongly related to social and demographic factors such as educational achievement and socio-economic status, which are in turn related to many of the health outcomes of interest. The issue of confounding, together with a number of other methodological problems, have been recognized for many years and publications from the 1980s by Bauchner and Kramer highlighted many of these problems and proposed potential solutions. Despite greater awareness of these issues, there is still a lack of consistency both in the extent to which they are addressed and in the methods used to do so. This makes meta-analyses difficult or impossible. These issues are summarized in Table 13.4 .
| Ascertainment of feeding behaviour (where are data obtained from?) | Often retrospective Different sources of data (e.g. mother, health professional ) |
| Definition of breastfeeding | Definitions are very variable, especially for exclusivity Range from ‘ever/never’ to detailed prospective records |
| Outcomes | Assessment not always blind Lack of consistent definitions or measurement methods |
| Control for confounders | Variable definition and collection of data on confounders Direction of confounding differs in different populations and with time |
| Reverse causality | Alteration in feeding behaviour is a response to outcome rather than the cause (e.g. mothers with a family history of atopy may breastfeed for longer to prevent the infant developing atopy, but if the infant then develops atopy, this may be attributed to breastfeeding |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here