Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The neurological evaluation of the newborn comprises, as it does at other ages in pediatric medicine, the history, physical examination, and appropriate specialized studies. Appropriate neurodevelopmental follow-up is the critical next step in the neurological evaluation. The history is discussed best in the context of essentially every chapter of this book and is not repeated in detail here. Appropriate specialized studies (see Chapter 13 ) and neurodevelopmental follow-up (see Chapter 14 ) are discussed in separate chapters.
In this chapter, the focus is the neonatal neurological examination, because an organized approach to the infant is so critical and, in fact, is the cornerstone of the overall neurological evaluation. The approach is organized on the framework of the neurological examination of older infants and children but is supplemented and modified significantly for adaptation to the newborn. Too often an organized, systematic approach to the infant is omitted because of the morass of catheters, tubes, monitors, blindfolds, intravenous accoutrements, and the like surrounding the child. It is curiously paradoxical that these outward manifestations of our attempts to provide optimal therapy may interfere significantly with the careful clinical examination that is necessary for rational judgments regarding diagnosis, prognosis, and management. On the other hand, the examiner should remember that the sick newborn, especially the premature infant, often has only tenuous control of such critical functions as respiration and cardiovascular status, and that overly vigorous manipulation of the baby may have adverse consequences.
The single most important advice that I (JJV) can convey concerning the neonatal neurological examination is to stand there and look; don’t just do something. Examination of the infant requires patience, a careful eye, and minimal intrusion. Indeed, I am often asked to illustrate how I perform a neurological examination of the infant. The illustration, I fear, has disappointed many who expected that I performed a series of secret, all-revealing maneuvers. My examination of the infant is dominated by careful observation and very little of the poking, prodding, scratching, head-dropping maneuvers described in many classical writings. Most of my time is spent watching the infant, with some gentle touches, to assess level of consciousness, eye position and movement, facial symmetry and movement, head position, asymmetry of limb positions, onset of spontaneous movement, and so forth. Surely, of course, evaluation of tone and reflexes has a role, but most of my examination is performed by watching the infant carefully. It has been somewhat embarrassing for me at times to watch visitors or trainees watch me watch the infant, when I felt that they expected to see much more. To repeat: stand there and look; don’t just do something.
In the following section, the normal features of the neonatal neurological examination are outlined ( Table 12.1 , ). Before addressing the formal neurological examination, brief discussions of determination of gestational age and evaluation of the head are necessary.
| Level of Alertness |
| Cranial Nerves |
| Olfaction (I) |
| Vision (II) |
| Optic fundi (II) |
| Pupils (III) |
| Extraocular movements (III, IV, VI) |
| Facial sensation and masticatory power (V) |
| Facial motility (VII) |
| Audition (VIII) |
| Sucking and swallowing (V, VII, IX, X, XII) |
| Sternocleidomastoid function (XI) |
| Tongue function (XII) |
| Taste (VII, IX) |
| Motor Examination |
| Tone and posture |
| Motility and power |
| Tendon reflexes and plantar response |
| Primary Neonatal Reflexes |
| Moro reflex |
| Palmar grasp |
| Tonic neck response |
| Sensory Examination |
Estimation of gestational age is particularly important for several reasons. First, various aspects of the neonatal neurological evaluation change with maturation, and recognition of these changes is critical in assessing the observations. Second, certain disorders are particularly characteristic of infants that are born prematurely but are of average weight for gestational age, those born at term but are small for gestational age, and the like. Third, the same insult (e.g., hypoxia-ischemia) will have a different impact on various regions of the central nervous system (CNS), in large part as a function of the gestational age of the infant.
One of the more helpful pieces of information for estimating gestational age is the date of the mother’s last menstrual period (LMP), particularly in the smallest infants. However, it has been found that an early first trimester obstetrical ultrasound (up to and including 13-6/7 weeks of gestation) with the measurement of the crown-rump length (CRL) is the most accurate means of estimating gestational age with an accuracy of ±5 to 7 days. Unfortunately, often neither accurate LMP nor CRL is known precisely. Thus a variety of other measures have been used to estimate gestational age, including anthropometric measurements, such as birth weight and head circumference; certain external characteristics; neurological evaluation; radiological study of bone maturation; certain neurophysiological parameters, especially measurement of motor nerve conduction velocity or the electroencephalogram; and imaging determinations of sulcal development. All of these approaches have certain merits and limitations. Detailed discussion of the aspects of the physical examination useful for assessment of gestational age is available in multiple neonatal sources. Of the techniques evaluated, examination of certain external characteristics has been most convenient and generally effective. I have found four selected external characteristics to be particularly useful: the ear cartilage and its reflection in ear position, the amount of breast tissue, the characteristics of the external genitalia, and the creases of the plantar surface of the foot ( Table 12.2 ). In the first hours of life, although I routinely assess tone and posture, I do not use these measurements for the principal purpose of assessing gestational age but rather to assess neurological status, because in my experience these measurements are variable and are sensitive to exogenous factors, including the process of birth. However, after the first hours of life some find these evaluations useful for assessing maturation.
| GESTATIONAL AGE | ||||
|---|---|---|---|---|
| EXTERNAL CHARACTERISTIC | 28 weeks | 32 weeks | 36 weeks | 40 weeks |
|
Pinna soft, remains folded | Pinna slightly harder but remains folded | Pinna harder, springs back | Pinna firm, stands erect from head |
|
None | None | 1–2-mm nodule | 6–7-mm nodule |
|
Testes undescended, smooth scrotum | Testes in inguinal canal, few scrotal rugae | Testes high in scrotum, more scrotal rugae | Testes descended, pendulous scrotum covered with rugae |
|
Prominent clitoris, small, widely separated labia | Prominent clitoris, larger separated labia | Clitoris less prominent, labia majora cover labia minora | Clitoris covered by labia majora |
|
Smooth | 1–2 anterior creases | 2–3 anterior creases | Creases cover sole |
The external characteristics of the head to be evaluated include the size and shape (see later discussion) and the skin. The skin of the head should be examined carefully for the presence of dimples or tracts, subcutaneous masses (e.g., encephalocele, tumor, cephalhematoma, subgaleal collection/hemorrhage), or cutaneous lesions, all generally discussed elsewhere in this book. In this setting I will discuss only the significance of port-wine stains , congenital vascular abnormalities that are present at birth and persist into adulthood. At birth, these lesions are most often pale pink macular lesions that subsequently become dark red to purple and often nodular. They are categorized according to their dermatomal distribution ( Fig. 12.1 ). Their importance, apart from the significant cosmetic issue, relates principally to their association with abnormalities of choroidal vessels in the eye, which may result in glaucoma, and of meningeal and superficial cerebral vessels, which may result in cortical lesions with seizures and other neurological deficits (i.e., Sturge-Weber syndrome). The relations between the location of the port-wine stain and the incidence of glaucoma or the intracranial vascular lesion are shown in Table 12.3 . In one large series, the intracranial vascular lesion of Sturge-Weber syndrome occurred in 40% to 50% of children with total involvement of V 1. Notably, with partial involvement of V 1 , the risk was markedly lower, and none of the 64 children with involvement of V 2 or V 3 or both (but not V 1 ) developed either the intracranial lesion or glaucoma. The particular prognostic importance of involvement of V 1 (particularly the superior eyelid) has been confirmed in later series. Particular prognostic importance for involvement of half or more of a contiguous area of the hemiforehead has been shown recently. The disorder has been shown recently to be caused by a somatic mutation in a gene encoding a guanine nucleotide binding protein (GNAQ) . The optimal timing of therapy has been the subject of debate. Pulsed dye laser therapy of the skin lesion is most effective and best tolerated when used early in infancy.
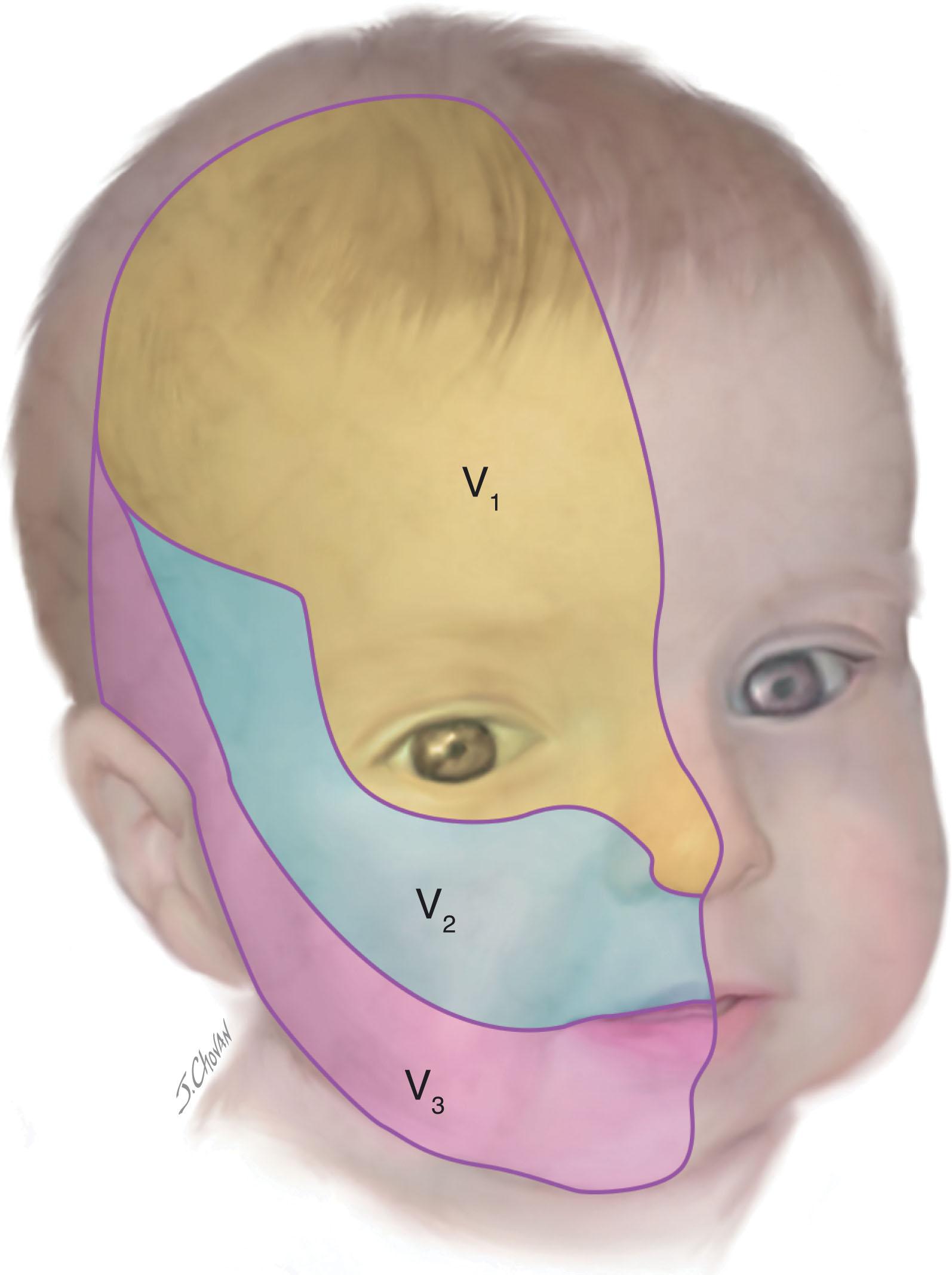
| LOCATION OF PORT-WINE STAIN (DERMATOMAL DISTRIBUTION) | TOTAL NUMBER | INTRACRANIAL VASCULAR LESION ± GLAUCOMA | GLAUCOMA ALONE | PORT-WINE STAIN ONLY |
|---|---|---|---|---|
| V 1 (total) alone | 4 | 2 | 1 | 1 |
| V 1 (total) with other dermatomes | 21 | 9 | 3 | 9 |
| V 1 (partial) with or without other dermatomes | 17 | 1 | 0 | 16 |
| V 2 alone | 29 | 0 | 0 | 29 |
| V 3 alone | 13 | 0 | 0 | 13 |
| V 2 + V 3 (unilateral or bilateral) | 22 | 0 | 0 | 22 |
Head circumference is a useful measure of intracranial volume and therefore also of volume of brain and cerebrospinal fluid. Less commonly, head circumference is significantly affected by the size of extracerebral spaces (subdural and subarachnoid) or by the intracranial blood volume. Scalp edema (caput succedaneum), subcutaneous infiltration of fluid from intravenous infusion, subgaleal collection/hemorrhage, and cephalhematoma have obvious effects as well. Nevertheless, measurement of head circumference remains one of the most readily available and useful means for evaluating the status of the CNS in the newborn period. Longitudinal measurements in particular provide valuable information.
Head circumference is influenced by head shape: the more circular the head shape, the smaller the circumference needs be to contain the same area and the same intracranial volume. Infants with relatively large occipital-frontal diameters often have larger measured head circumferences than those with relatively large biparietal diameters. This fact has important implications in evaluating the head circumference of an infant with a skull deformity such as craniosynostosis (see next paragraph). Overall significant changes of increase and decrease can occur in the first few days of life due to molding and extracranial swelling, both of which can vary based on presentation and mode of delivery. In premature infants, over the first 2 to 3 months of life, there is an impressive change in head shape that is characterized by an increase in occipital-frontal diameter relative to biparietal diameter ( Fig. 12.2 ). Because this alteration does occur over a matter of weeks, it usually does not cause major difficulties in the interpretation of head circumference, but it does remain a fact to be considered, especially in infants with unusually marked dolichocephalic change.
![Fig. 12.2, Change in head shape in premature infants. Measurements of AP/BP ratio (anterior-posterior [ AP ] and biparietal [ BP ] diameters) and drawings of head shape (vertex view) of an infant, born at 28 weeks of gestation, made at (A) 1 week, (B) 5 weeks, and (C) 11½ weeks. Fig. 12.2, Change in head shape in premature infants. Measurements of AP/BP ratio (anterior-posterior [ AP ] and biparietal [ BP ] diameters) and drawings of head shape (vertex view) of an infant, born at 28 weeks of gestation, made at (A) 1 week, (B) 5 weeks, and (C) 11½ weeks.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/NeurologicalExaminationNormalandAbnormalFeatures/1_3s20B9780443105135000127.jpg)
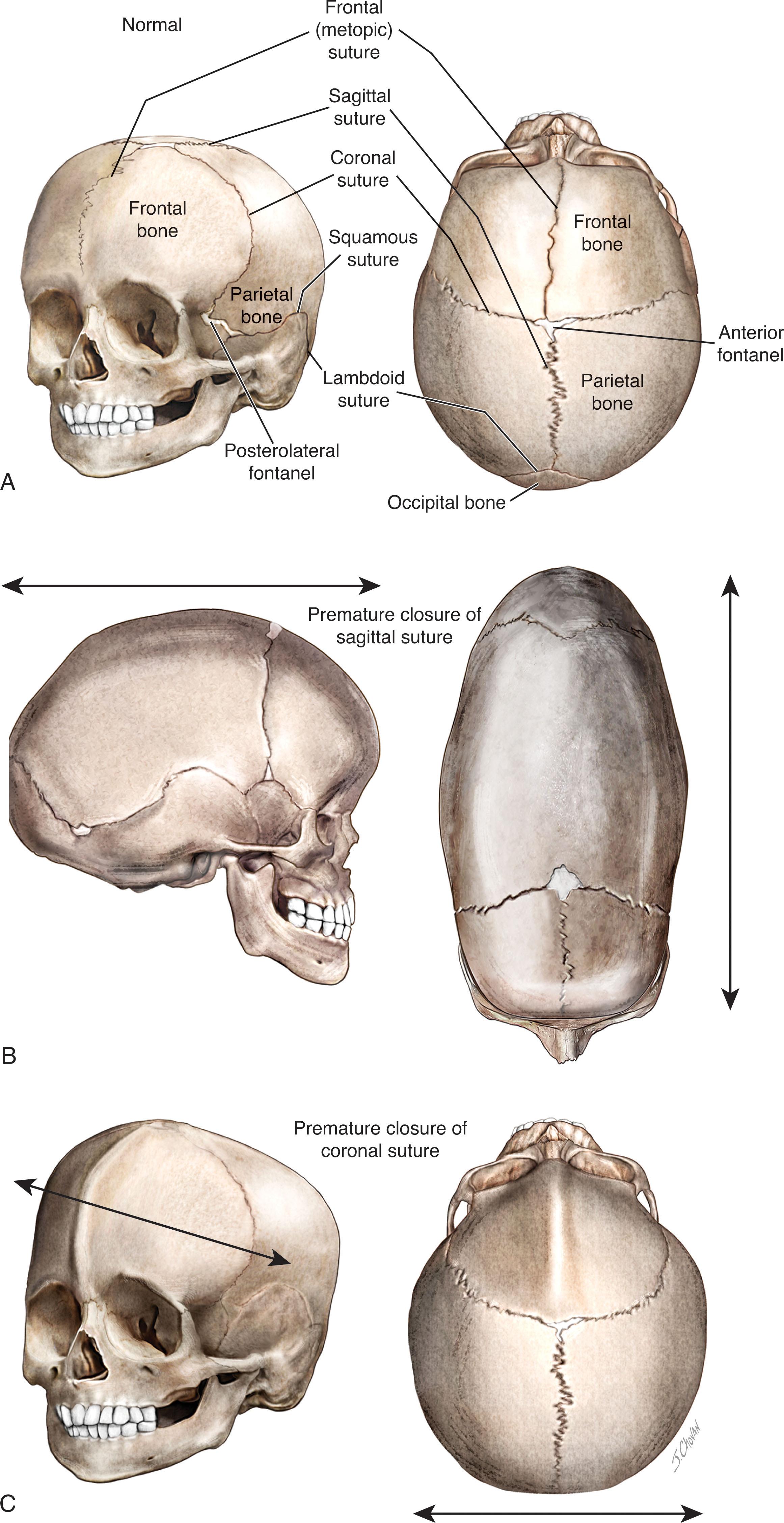
Craniosynostosis , premature closure of cranial suture(s), may affect one or more cranial sutures ( Table 12.4 ). Simple sagittal synostosis is most common and accounts for 50% to 60% of cases. In a large neurosurgical series coronal synostosis was next most common and accounted for 20% to 30% of cases ( Table 12.4 ). In less selective series, metopic synostosis is second most common (23% to 25%). The diagnosis can be suspected by the shape of the head; with synostosis of a suture, growth of the skull can occur parallel to the affected suture but not at right angles ( Fig. 12.3 ). The “keel-shaped” head of sagittal synostosis is termed dolichocephaly or scaphocephaly; the wide head of coronal synostosis brachycephaly; and the tower-shaped head of combined coronal, sagittal, and lambdoid synostosis acrocephaly . The initial evaluation traditionally has been skull radiography, although recent work indicates that cranial ultrasonography is as effective as skull radiography (except for assessment of the metopic suture) and avoids radiation. For infants requiring intervention, 3D computed tomography reconstructions are used for surgical planning. Approximately 10% to 15% of cases of cranial synostosis are familial or represent complex syndromes, the major features, genetics and neurological outcome of which are summarized in Table 12.5 . Bilateral coronal synostosis is a common feature of these syndromes. Most syndromic craniosynostoses are related to mutations in the fibroblast growth factor receptor pathway. The outcome in the more common single-suture craniosynostosis is generally favorable, although at least 40% of cases later exhibit learning, behavioral, and other developmental deficits. Among the subjects with single-suture craniosynostosis, the most neurodevelopmentally vulnerable are those with coronal and lambdoid fusions. The neurological outcome in syndromic craniosynostosis in general is more unfavorable than the outcome in nonsyndromic cases. The importance of early correction of synostosis for optimal cosmetic appearance and the other aspects of management are discussed in standard textbooks of neurosurgery. In large series of nonsyndromic cases, outcome has been better for infants operated on early in the first year than later in infancy. Surgical approaches have been reviewed elsewhere.
| SUTURES | PERCENT OF CASES a |
|---|---|
|
56 |
|
25 |
|
13 |
|
12 |
|
4 |
|
2 |
|
13 |
| NAME OF SYNDROME | CRANIUM | OTHER MAJOR FEATURES | GENETICS | NEUROLOGICAL OUTCOME |
|---|---|---|---|---|
| Antley-Bixler | Brachycephaly with multiple synostosis, especially of coronal suture | Maxillary hypoplasia, radio humeral synostosis, choanal atresia, arthrogryposis | Autosomal recessive | Intelligence probably normal |
| Apert | Brachycephaly with irregular synostosis, especially of coronal suture | Midfacial hypoplasia, syndactyly of fingers and toes, broad distal phalanx of thumb and big toe | Autosomal dominant (usually new mutation) | Intellectual disability or borderline intelligence common |
| Baller-Gerold | Synostosis of variable sutures, including metopic with trigonocephaly | Radial dysplasia with absent thumbs | Autosomal recessive | Intellectual disability common |
| Carpenter | Acro-brachycephaly with synostosis of coronal, sagittal, and lambdoid sutures | Lateral displacement of inner canthi, polydactyly and syndactyly of feet | Autosomal recessive | Intellectual disability common |
| Crouzon | Acrocephaly (tower-shaped) with synostosis of coronal, sagittal, and lambdoid sutures | Ocular proptosis (shallow orbits) and maxillary hypoplasia | Autosomal dominant (variable expression) | Intellectual disability occasional |
| Greig | High forehead with variable synostosis | Hypertelorism, polydactyly and syndactyly of fingers and toes | Autosomal dominant | Mild intellectual disability occasional |
| Muenke | Brachycephaly with coronal synostosis (unilateral or bilateral), macrocephaly | Midface hypoplasia, hypertelorism, hearing loss | Autosomal dominant | Normal intelligence usual |
| Opitz | Trigonocephaly with synostosis of metopic suture | Upward slant of palpebral fissures, epicanthal folds, narrow palate, anomalies of external ear, loose skin, variable polydactyly or syndactyly of fingers | Autosomal recessive | Intellectual disability common |
| Pfeiffer | Brachycephaly with synostosis of coronal and/or sagittal sutures | Hypertelorism, broad thumbs and toes, partial syndactyly of fingers and toes | Autosomal dominant | Normal intelligence usual |
| Saethre-Chotzen | Brachycephaly with synostosis of coronal sutures | Prominent ear crus, maxillary hypoplasia, partial syndactyly of fingers and toes | Autosomal dominant (variable expression) | Intellectual disability uncommon |
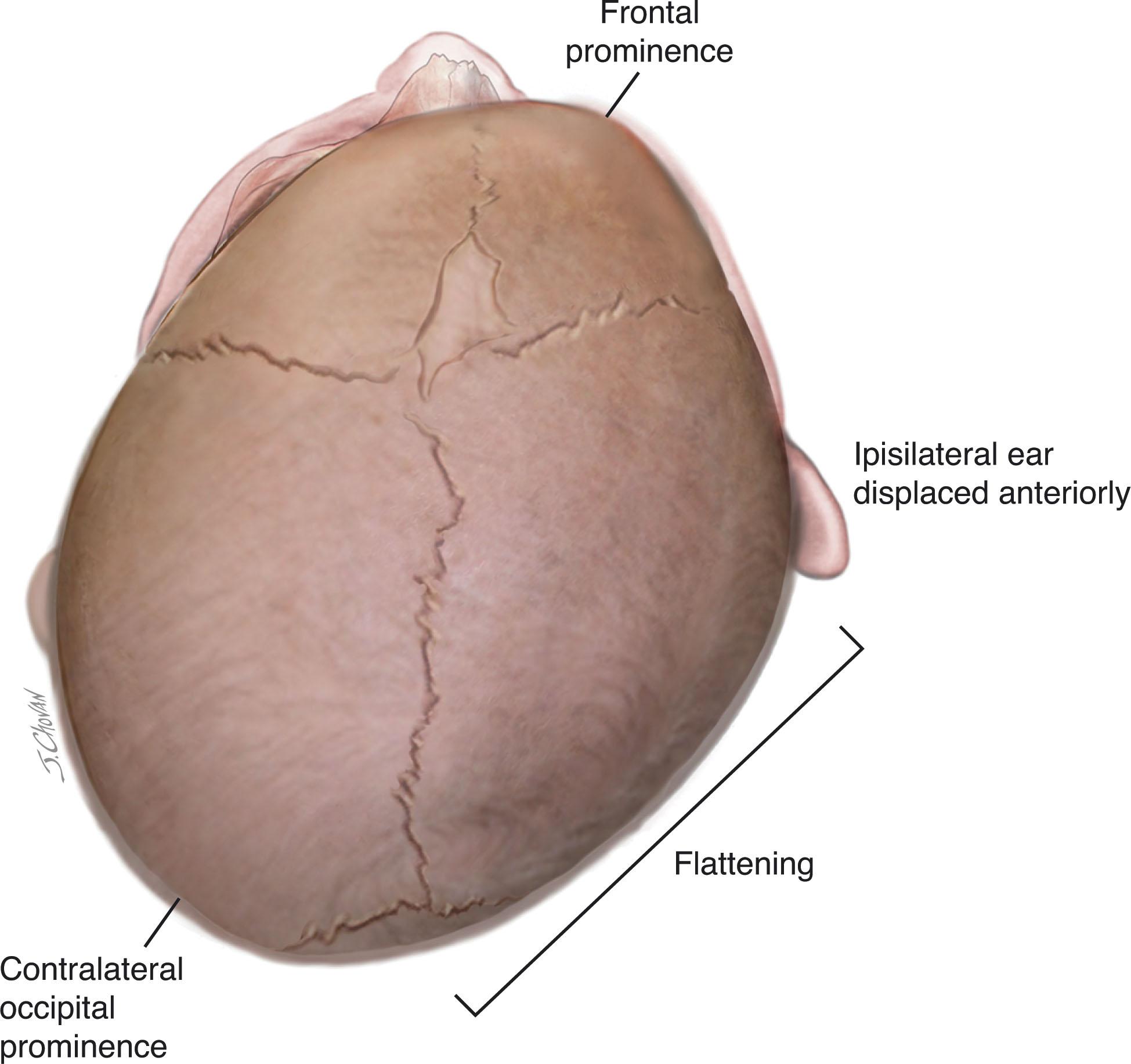
Positional or deformational plagiocephaly has become a frequent clinical issue in recent years. Plagiocephaly (“oblique head,” from the Greek) refers to a head appearance in which the occipital region is flattened and the ipsilateral frontal area is prominent (i.e., anteriorly displaced) ( Fig. 12.4 ). In positional or deformational plagiocephaly, caused by external molding forces, the ipsilateral ear is also displaced anteriorly and the contralateral face may appear flattened. Torticollis may be associated and cause a head tilt. Deformation plagiocephaly may be present at birth , secondary to intrauterine restriction to head movement as with multiple gestation, abnormal uterine lie or neck abnormality (e.g., torticollis), or it may evolve over the first weeks to months of life , usually secondary to supine sleeping position as part of the “Back to Sleep” program. Differentiation of deformational plagiocephaly from the rare unilateral lambdoid synostosis, which can also cause occipital flattening, is usually readily made clinically; in the latter the anterior displacement of the frontal area is usually less, the ear is posterior, not anterior, and is displaced inferiorly, and facial deformity is rare. Minimization of plagiocephaly in the neonatal intensive care unit (NICU) can be achieved by the use of simple positioning devices. Management of deformational plagiocephaly consists of parental counseling regarding head positioning with the infant supine, supervised time in the prone position, various exercises, and skull-molding helmets if necessary ( Fig. 12.4 ).
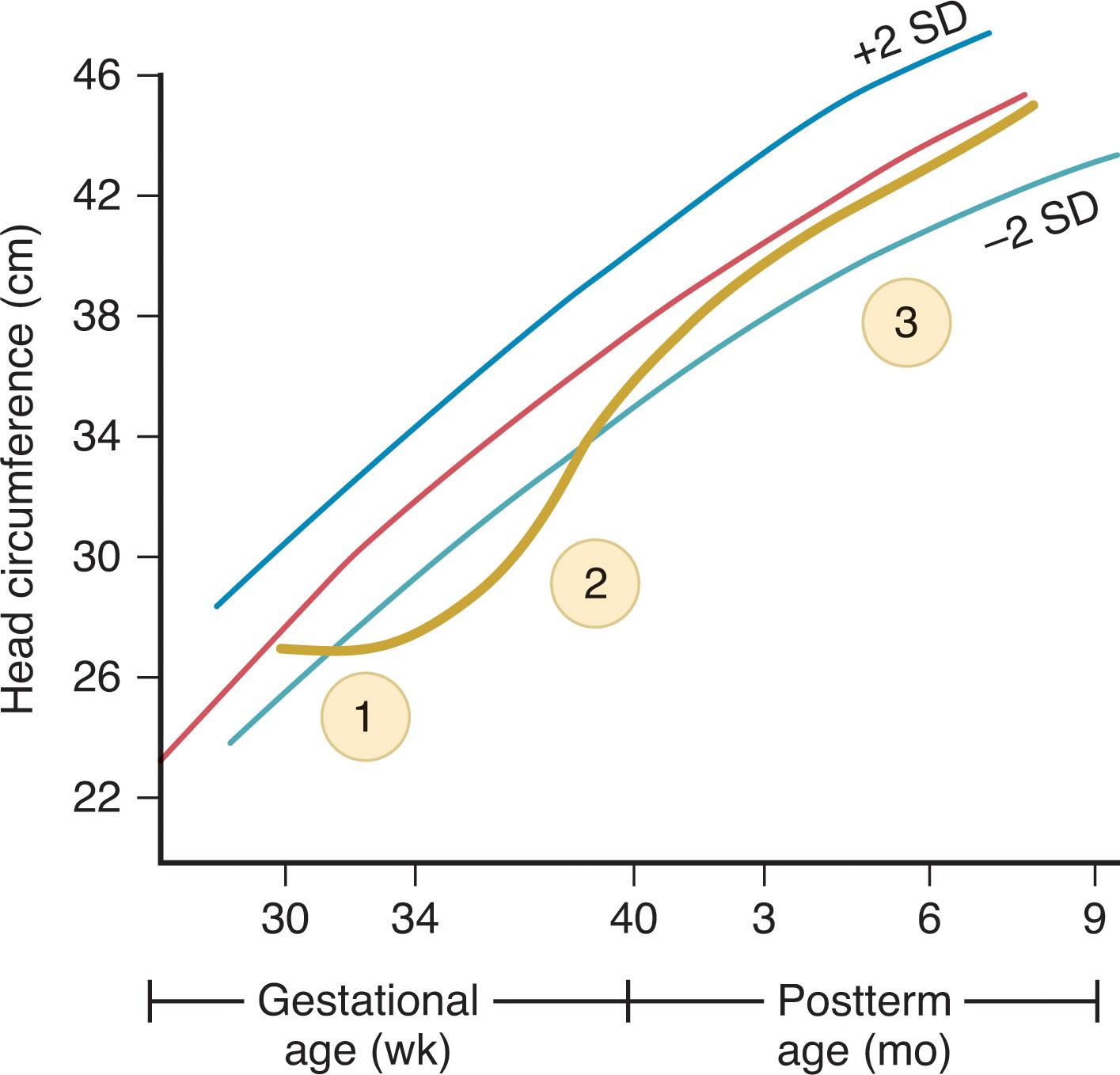
Interpretation of the rate of head growth in premature infants is often difficult, in part because normal postnatal rates have been difficult to define conclusively (in contrast to normal rates of intrauterine growth, as plotted on most standard charts) and in part because commonly occurring systemic diseases and nutritional deprivations in the neonatal period may interfere with brain and head growth.
The rate of head growth in premature infants has been the subject of a variety of reports. In the healthy premature infant , there is a minimal amount of change in the head circumference in the first days of life; indeed, a small amount of head shrinkage with suture overriding has been documented. Head shrinkage reaches a peak at approximately 3 days of life, usually averages 2% to 3% of the head circumference at birth, and correlates closely with postnatal weight and urinary sodium losses. In view of these facts and the overriding of sutures, it has been suggested that the head shrinkage relates to water loss from the intracranial compartment.
A longitudinal study of 41 premature infants (of <1500 g birth weight) with favorable neurological outcome at age 2 years (as assessed by neurological examination and the Bayley Mental Developmental Scale) defined the rates of head growth shown in Table 12.6 . Thus after a period of decreasing head circumference in the first week, head growth increased by a mean of approximately 0.50 cm in the second week, 0.75 cm in the third week, and 1.0 cm per week thereafter in the neonatal period. Approximately similar data have been obtained in larger, more recent studies, although rates of 0.75 cm/week were documented in the last 6 to 8 weeks before term. Slower rates of head growth were observed in infants with serious systemic disorders and subsequent neurological impairment. More rapid rates of head growth in the first 6 weeks suggest hydrocephalus (e.g., after intraventricular hemorrhage), as detailed in Chapter 28 . It is important to recognize that “sick” preterm infants with systemic disease often will exhibit a “normal” acceleration of head growth (i.e., “catch-up” head growth) after recovery from their illness. However, the smallest infants (<1000 g birth weight) generally do not exhibit as rapid growth as premature infants >2000 g and may not catch up even by 2 years of age. Additionally preterm infants born small for their gestational age often do not exhibit as rapid head growth or as effective catch-up as infants born average for their gestational age.
| POSTNATAL WEEK | RATE OF HEAD GROWTH (CM/WEEK) |
|---|---|
| First | –0.60 |
| Second | 0.50 |
| Third | 0.75 |
| After third | 1.0 |
The importance of duration of neonatal caloric deprivation (<85 kcal/kg/day) on head growth in the neonatal period was shown initially in a study of 73 preterm infants (mean gestational age 30 ± 2 weeks) ( Fig. 12.5 ). Three phases of head growth were defined: an initial period of growth arrest or suboptimal head growth, followed by a period of catch-up growth, and terminated by a period of growth along standard curves. The duration of the period of growth arrest or suboptimal growth was directly related to the initial period of caloric deprivation and to the duration of mechanical ventilation, and the period of catch-up growth was directly related to the duration of the preceding caloric deprivation. The rate of head growth along standard curves was between the mean and 1 standard deviation below the mean for all infants except those calorically deprived the longest (4 to 6 weeks), in whom values were more than 1 standard deviation below the mean. Indeed, such infants calorically deprived for more than 4 weeks had developmental scores below normal ranges at 1 year of corrected age. The deleterious effect of postnatal caloric deprivation is worse for preterm infants born small for their gestational age.
The potential value of determination of neonatal head growth in preterm infants for prediction of neurodevelopmental outcome and for delineation of the relation of such head growth to effective nutrition and body growth has been shown particularly effectively in multiple studies. The overall theme has been a positive relation between better weight gain, linear growth, head growth, and neurodevelopmental outcome, even after control for such confounders as evidence for brain injury. The critical issues regarding the effects of nutrition in the neonatal period and infancy on brain growth and neurological outcome are discussed in more detail in Chapters 7 , 8 and 20 .
The formal neonatal neurological examination should begin with assessment of the level of alertness. The level of alertness is perhaps the most sensitive of all neurological functions, as it is dependent on the integrity of several levels of the CNS (see later). Several terms have been used by others to describe this aspect of neurological function, such as state and vigilance . It is important to recognize that the level of alertness in the normal infant will vary, depending particularly on time of last feeding, environmental stimuli, recent experiences (e.g., painful venipuncture), and gestational age. Before 28 weeks of gestation, it is difficult to identify periods of wakefulness. Persistent stimulation leads to eye opening and apparent alerting for time periods measured principally in seconds. At approximately 28 weeks, however, there is a distinct change in the level of alertness. At that time, a gentle shake will rouse the infant from apparent sleep and will result in alerting for several minutes. Spontaneous alerting also occasionally occurs at this age. Sleep-waking cycles are difficult to observe clinically but can be shown electrophysiologically. By 32 weeks, stimulation is no longer necessary; frequently the eyes remain open, and spontaneous roving eye movements appear. Sleep-waking alternation, as defined by clinical observation, is apparent. By 36 weeks, increased alertness can be observed readily, and vigorous crying appears during wakefulness. By term, the infant exhibits distinct periods of attention to visual and auditory stimuli, and it is possible to study sleep-waking patterns in detail.
Olfaction, a function subserved by the first cranial nerve, is evaluated only rarely in the newborn period. In a study of 100 term and preterm infants, Sarnat observed that all normal infants of more than 32 weeks of gestation responded with sucking, arousal-withdrawal, or both to a cotton pledget soaked with peppermint extract. Eight of 11 infants of 29 to 32 weeks of gestation, but only 1 of 6 infants of 26 to 28 weeks of gestation also responded. Activation of the orbitofrontal, olfactory cortex was detected by near-infrared spectroscopy in full-term newborns exposed to vanilla or maternal colostrum in the first weeks of life.
More sophisticated techniques have demonstrated olfactory discriminations in newborns. Using habituation-dishabituation techniques and recordings of respiration, heart rate, and motor activity, Lipsitt and coworkers demonstrated detection and discrimination among a variety of odorants. Mediation of discriminations at a higher level than the periphery was shown by the observation that infants, initially habituated to mixtures of odorants, exhibited dishabituation when presented with the pure components of the mixtures. A particularly interesting demonstration of olfactory discrimination in the infant involved discrimination of the odor of breast pads of the infant’s mother from unused pads or those of other nursing mothers. Infants consistently adjusted their faces and gazes toward the pads of their own mothers. A recent study with a similar paradigm and using functional magnetic resonance imaging (MRI) concluded that infants born from 32 weeks could process low-concentration maternal breast odors at a cortical level. Later work involving coupling of stroking with different odorants demonstrated complex associative olfactory learning in the first 48 hours of life. That olfactory discrimination develops in utero is suggested by the demonstration of neonatal preference for the odors of amniotic fluid. Finally, nutrient (breast milk or formula) odor exposure via a pacifier was shown to stimulate nonnutritive sucking during gavage feeding of premature newborns.
Visual responses, the afferent segment of which is subserved by the second cranial nerve, exhibit distinct changes with maturation in the neonatal period. By 26 weeks, the infant consistently blinks to light. By 32 weeks, light provokes eye closure that persists for as long as the light is present (dazzle reflex of Peiper). A series of behaviors associated with visual fixation can be identified by 32 weeks of gestation and can be shown to increase considerably over the next 4 weeks. By 34 weeks, over 90% of infants will track a fluffy ball of red wool. At 37 weeks, the infant will turn the eyes toward a soft light. By term, visual fixation and following are well developed. For testing of visual fixation and following, I have found most useful as a target a fluffy ball of red yarn or a red ball. Opticokinetic nystagmus, elicited by a rotating drum, is present in the majority of infants at 36 weeks and is present consistently at term.
The anatomical substrate for visual fixation and for following a moving object in the newborn may not be primarily the occipital cortex, as usually thought. Thus two studies of newborn infants with apparent absence of occipital cortex secondary to maldevelopment (holoprosencephaly) or destructive lesion (congenital hydrocephalus, ischemic injury) suggest that these abilities are mediated at subcortical sites. Experimental studies in subhuman primates have defined such a subcortical system involving retina, optic nerves and tract, and pulvinar and superior colliculus—the so-called collicular visual system. Visual abilities beyond the ability to track a moving object, such as visual discriminatory skills (see next paragraphs), however, do require the geniculocalcarine cortical system. Recent work using diffusion-based MRI provides evidence for maturation of the geniculocalcarine tract as critical for higher-level visual discriminations after 32 weeks of gestation.
Elegant studies have provided important information about neonatal visual acuity, color perception, contrast sensitivity , and visual discrimination . Through use of the opticokinetic nystagmus response to striped patterns of varying width, it has been demonstrated that the newborn exhibits at least 20/150 vision. Using a visual fixation technique, Fantz showed that the newborn attended to stripes of 1/8-inch width. Visual acuity in premature infants with birth weight of 1500 to 2500 g studied at approximately 38 weeks of gestation is similar to that of term infants. Although studies of color perception in the newborn period often have not rigorously distinguished brightness and color, newborn infants clearly will follow a colored object. Color vision is demonstrable by at least as early as 2 months of age. Contrast sensitivity increases dramatically between 4 to 9 postnatal weeks.
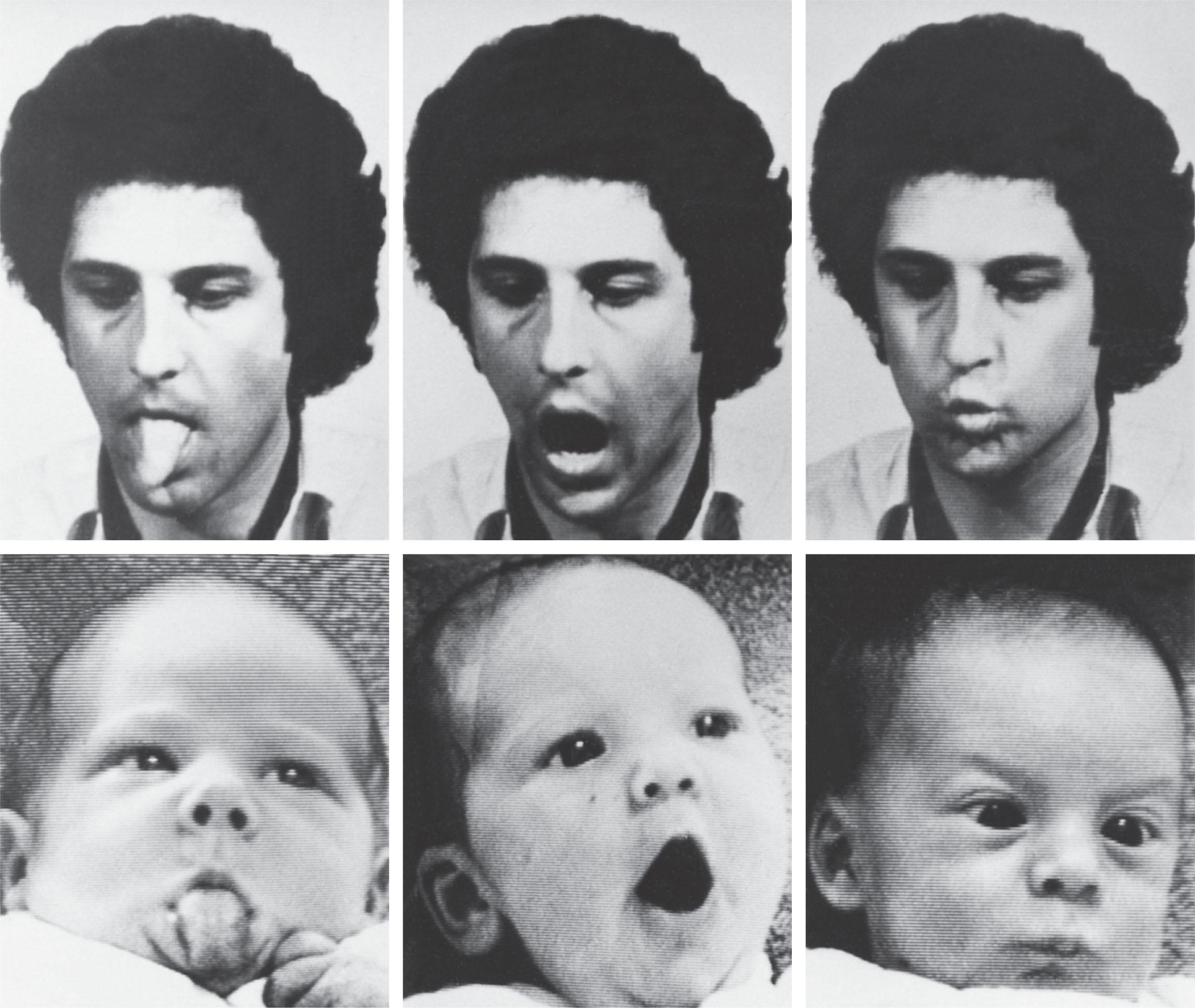
Discrimination of a rather complex degree has been demonstrated for newborn infants. Infants as young as 35 weeks of gestation exhibit a distinct visual preference for patterns, particularly those with a greater number of and larger details. Curved contours are favored over straight lines. Preference for novel patterns becomes apparent at 3 to 5 months. Preference for patterns with facial resemblance develops between approximately 10 and 15 weeks of age, and promptly thereafter there is discrimination according to facial features. The degree of contrast has a direct effect on preferences. Binocular vision and appreciation of depth also appear by approximately 3 to 4 postnatal months. Binocular visual acuity increases most rapidly during the same interval. These higher-level visual abilities may reflect a change in the major anatomical substrate from subcortical to cortical structures. Moreover, two functional MRI studies of infants from the first days of life do show some evidence for activation of the visual cortex with visual stimulation; subcortical structures could not be addressed because of small anatomical size. Full-term infants in the first days of life also have been shown to imitate facial gestures ( Fig. 12.6 ). Additionally, imitation of finger movements, especially involving the left hand, has been demonstrated in healthy term infants. Thus striking changes in cortically mediated visual function occur in the first weeks and months of postnatal life. It is noteworthy that this is a period for rapid axonal growth, with beginning myelination of the optic radiation and rapid dendritic development with synaptogenesis in visual cortex (see Chapters 7 and 8 ).
The funduscopic examination in the newborn period is facilitated considerably by the aid of a nurse and patience on the part of the examiner. The optic disc of the newborn lacks much of the pinkish color observed in the older infant and has a paler, gray-white appearance. This color and the less prominent vascularity of the neonatal optic disc may make distinction from optic atrophy difficult. Retinal hemorrhages have been observed in 20% to 40% of all newborn infants, with no necessary association with obvious perinatal difficulties, concomitant CNS injury, or neurological sequelae ( Table 12.7 ). A relationship to vaginal delivery is apparent; in one study, 38% of infants delivered vaginally exhibited retinal hemorrhages in contrast to 3% of those delivered by cesarean section. The hemorrhages are most commonly bilateral (59%) and predominantly intraretinal and in the posterior pole. The lesions generally resolve completely within 7 to 14 days.
| RETINAL HEMORRHAGES | |
|---|---|
| PERINATAL FACTOR | AFFECTED (%) |
|
|
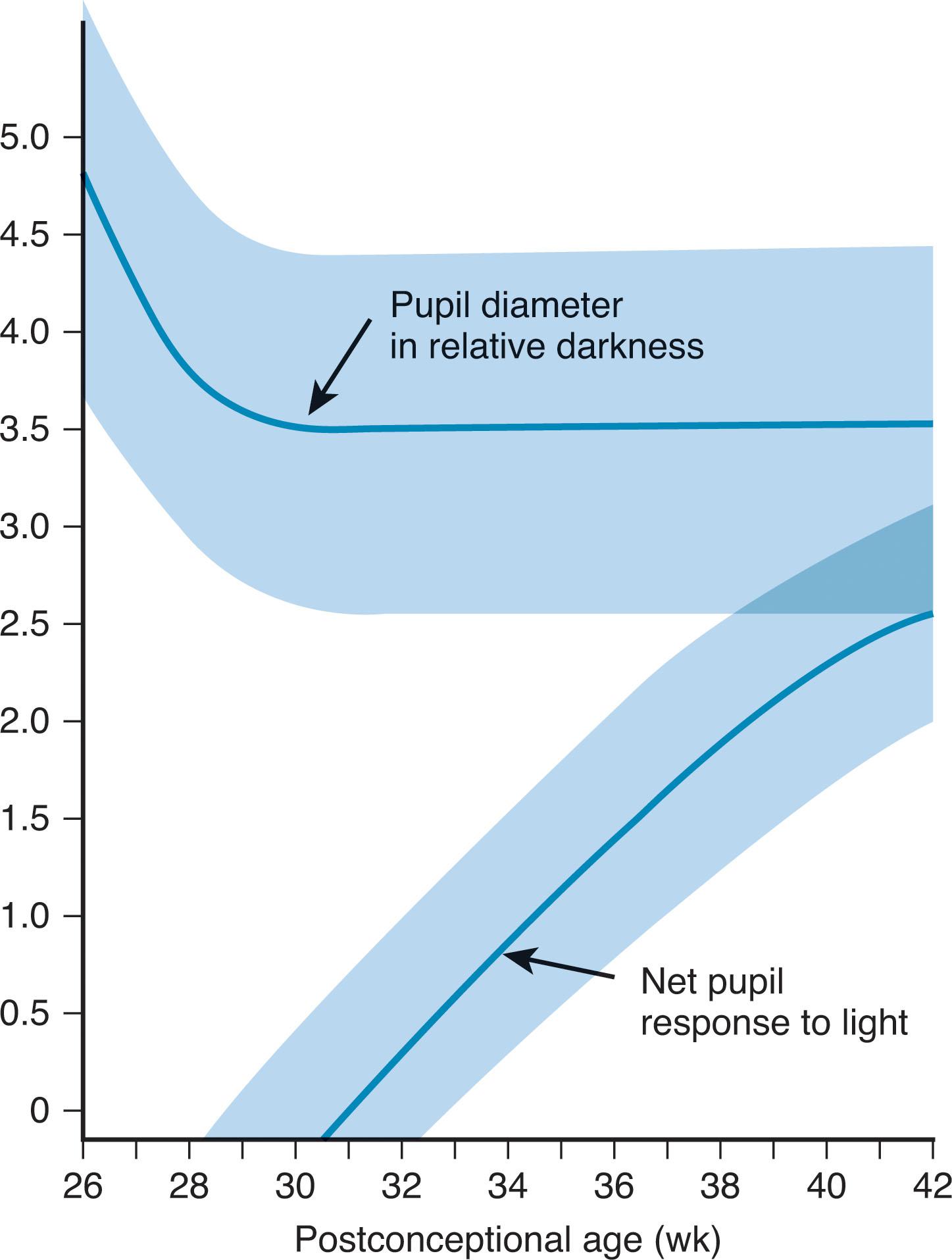
The pupils are sometimes difficult to evaluate in the newborn, especially the premature baby, because the eyes are often closed and resist forced opening, and the poorly pigmented iris provides poor contrast for visualizing the pupil. The size of the pupils in the premature infant of more than 28 weeks of gestation and of the full-term infant is approximately 3.5 mm. In premature infants of less than 28 weeks of gestation, pupillary size is larger and approximates 4.5 to 5.0 mm at 26 weeks of gestation. Reaction to light begins to appear at approximately 30 weeks of gestation, but it is not present consistently until approximately 32 to 35 weeks. The amplitude of the pupillary response increases markedly between 30 weeks and term ( Fig. 12.7 ). The afferent arc of this reflex leaves the optic tract before the lateral geniculate nucleus and synapses in the pretectal region of midbrain before innervating the Edinger-Westphal nucleus of the oculomotor nerve, the efferent arc of the reflex.
Particular attention should be paid to eye position, spontaneous eye movements, and movements elicited by the doll’s eyes maneuver , and, as needed, vertical spin, or caloric stimulation, as well as to a variety of abnormal eye movements (see later discussion). These oculomotor functions are subserved by cranial nerves III, IV, and VI and their interconnections within the brainstem. In most premature and some full-term infants, the eyes are slightly dysconjugate at rest, one or the other being 1 to 2 mm out. (This is demonstrated readily by observing the light reflected off each pupil with the light source in the midline at approximately 2 feet from the face.)
As early as 25 weeks of gestation, full ocular movement with the doll’s eyes maneuver can be elicited. Because interfering ocular fixation is not well developed at this stage, elicitation of lateral eye movements with the doll’s eyes maneuver is much easier in the small premature than in the full-term infant. Another convenient means of eliciting oculovestibular responses is to spin the baby held upright; the eyes will deviate in a direction opposite to the spin. Rapid maturation of this response with development of nystagmus and eye deviation occurs in the first 2 postnatal months. Additionally, at 30 weeks of gestation, caloric stimulation with cold water will lead to deviation of the eyes toward the side of the stimulated ear. Spontaneous roving eye movements are common at approximately 32 weeks. The tracking movements of the full-term and older infants at first are rather jerky and do not become smooth and gliding until approximately the third month of life.
Subserved by cranial nerve V (the trigeminal nerve), facial sensation is examined best with pinprick. The resulting facial grimace begins on the stimulated side of the face. If the infant has a facial palsy, this response will be impaired and may be attributed mistakenly to involvement of the trigeminal nerve or nucleus. The strength of masseters and pterygoids is also dependent on the motor function of the trigeminal nerve. This strength is assessed by evaluation of sucking and by allowing the infant to bite down on the examiner’s finger.
The parameters of interest are the position of the face at rest, the onset of movement, and the amplitude and symmetry of spontaneous and elicited movement. Facial motility is subserved by cranial nerve VII. With the face at rest, attention should be paid to the vertical width of the palpebral fissure, the nasolabial fold, and the position of the corner of the mouth. Examination of the face should never be restricted to observation of elicited movements (e.g., crying) because the quality of spontaneous facial movement is of greatest importance in the assessment of cerebral lesions. Subtle lesions at all central levels are best detected by close observation of the onset of movement.
The eighth cranial nerve, via its connections in the brainstem and cerebral cortex, subserves auditory function. By 28 weeks, the infant will startle or blink to a sudden, loud noise. As the infant matures, more subtle responses become evident, such as cessation of motor activity, change in respiratory pattern, opening of mouth, and wide opening of eyes. The relation of such responses to the development of hearing has been the subject of considerable study and controversy, but it is likely that these responses represent the presence of at least some auditory function. Inability to elicit these responses is related usually to the failure to test in a quiet surrounding, while the baby is alert and not agitated or very hungry, and to ensure that the ear canals are free of the often copious vernix. In most cases, an infant who does not respond on the initial examination will respond when retested under more favorable conditions. More detailed evaluation of auditory function, including electrophysiological measurements (e.g., brainstem auditory evoked responses; see Chapter 13 ), certainly is indicated if behavioral responses are consistently absent.
More sophisticated studies have provided insight into neonatal auditory acuity, localization , and discriminations . Using the occurrence in the newborn of cardiac acceleration in relation to sound intensity, Steinschneider demonstrated a threshold for cardiac acceleration of about 40 decibels. Auditory localization has been shown by demonstrating loss and recovery of habituation to an auditory stimulus by changing the locus of the stimulus. Auditory-visual coordination in localization was shown by exposing the infant to his mother speaking before him through a soundproof glass screen, her voice transmitted via a stereo system. When the stereo system was in balance (i.e., the voice came from straight ahead), the infant was content, but if the voice appeared to come from a location different from that of the face, the infant became very upset. Maturation of connections between brainstem auditory nuclei (superior olivary nucleus, nucleus of lateral lemniscus, inferior colliculus), sensory nuclei, and the facial nerve nucleus has been studied by measurement of the amplitude of the blink response to glabellar tap when the tap is preceded by an auditory tone.
Through the use of heart rate patterns and a habituation-dishabituation model, it has been possible to demonstrate auditory discriminations in 3- to 5-day-old newborn infants on the basis of intensity, pitch, and rhythm ( Table 12.8 ). These findings are of particular interest in view of information suggesting that intensity and pitch discriminations may be mediated at subcortical levels, whereas cortical levels are required for discrimination of temporal patterns. Discrimination of synthetic speech sounds according to phonemic category and of tonal sounds of different frequencies was demonstrated in newborns in the first days of life. Discrimination of real and computer-simulated cries by newborn infants was shown by observing much restlessness and crying in infants stimulated by the real cry and considerably less such behavior in those stimulated by the computer-simulated cry. Moreover, results of other studies indicate a preference of the newborn for human voice rather than nonhuman sounds and particular preference for the mother’s voice rather than another human voice. Finally, 2- to 4-week-old infants can learn to recognize a word that their mothers repeat to them over a period of time (2 weeks) and will “remember” the word up to 2 days without intervening presentations.
|
Studies based on optical topography or functional MRI show that newborns in the first days of life respond to normal speech with activation of the temporal regions preferentially in the left hemisphere. These interesting observations demonstrate that the newborn brain exhibits the cortical organization to process speech and the regional specification for the left hemisphere for language. Similarly, a magnetoencephalographic study using a paradigm based on sound discrimination and important in auditory cognitive function demonstrated positive responses in newborns shortly after birth.
The importance of auditory input in the neonatal period after premature birth for subsequent language development has been shown by recent studies that show poorer language development and cortical folding in temporal areas in infants cared for in single-patient rooms versus open wards. A role for maternal vocal involvement was shown in a subsequent study; infants with high maternal involvement in both single-patient rooms and open wards had higher language (and cognitive) scores than those infants with low maternal involvement.
The importance of the nature of the auditory input was shown by a recent study of the effects of different varieties of music exposure on cortical connectivity. Thus music exposure in preterm infants had, at term equivalent age, lasting learning effects on music processing, with an increased connectivity between primary auditory cortex and brain regions involved in several aspects of music processing (e.g., temporal and cingulate cortex, basal ganglia).
The underlying physiology of these effects of auditory experience on language development relates in part to the striking development of the auditory system during the premature period. Connections between the cochlea and the brainstem are established by 24 to 25 weeks and connections to temporal lobe and auditory cortex by 30 to 31 weeks.
Sucking requires the function of cranial nerves V, VII, and XII; swallowing, cranial nerves IX and X; and tongue function, cranial nerve XII. The importance of tongue function, particularly the “stripping” action of the medial tongue, has been demonstrated in ultrasonographic and fiberoptic studies of neonatal feeding. The act of feeding requires the concerted action of breathing, sucking, and swallowing. Not surprisingly, the brainstem control centers for these actions, termed pattern generators , are closely situated. Sucking and swallowing are coordinated sufficiently for oral feeding as early as 28 weeks; this finding, perhaps, is not surprising because swallowing is observed in utero as early as 11 weeks of gestation. The development of rooting at approximately 28 weeks is a relevant complementing feature. At this early age, however, the synchrony of breathing with sucking and swallowing is not well developed, and thus oral feeding is difficult and, in fact, dangerous. By 34 weeks of gestation, however, the normal infant is able to maintain a concerted, synchronous action for productive oral feeding. However, maturation continues rapidly, and linkage of breathing, sucking, and swallowing is not achieved fully until 37 weeks of gestation or more. Moreover, even in the healthy term infant, coordination of swallowing and breathing rhythms are not optimal in the first 48 hours of life.
The gag reflex, subserved by cranial nerves IX and X, is an important part of the neurological evaluation in this context. A small tongue blade or a cotton-tipped swab can be used to elicit the reflex. Active contraction of the soft palate, with upward movement of the uvula and of the posterior pharyngeal muscles, should be observed.
Function of the sternocleidomastoid muscle is mediated by cranial nerve XI. Because the function of the muscle is to flex and rotate the head to the opposite side, it is difficult to test in the newborn, especially in the premature infant. One useful maneuver with the full-term infant is to gently extend the head over the side of the bed with the child in the supine position. Passive rotation of the head reveals the configuration and bulk of the muscle, and function sometimes can be estimated if the infant attempts to flex the head.
Function of tongue is mediated by cranial nerve XII. The parameters of interest are the size and symmetry of the muscle, the activity at rest, and the movement. Tongue movement is assessed best during the infant’s sucking on the examiner’s fingertip. The important role of the tongue in oral feeding was discussed in relation to sucking and swallowing.
Taste is evaluated only rarely in the neonatal neurological examination. This function is subserved by cranial nerves VII (anterior two-thirds of tongue) and IX (posterior one-third of tongue). The newborn infant is very responsive to variations in taste and is capable of sharp discriminations. Lipsitt and coworkers utilized various parameters of sucking behavior, not only to define gustatory discriminations but also to study learning processes in the newborn. An apparatus that allows control of the fluid to be obtained by sucking, as well as measurement of duration and frequency of sucking, has been used to demonstrate that, when presented with a sweet fluid (e.g., 15% sucrose), the infant sucks in longer bursts and with fewer rest periods than when presented with water or a salty fluid. When sucking the sweet fluid, the heart rate increased. It was presumed from these data that the newborn infant “hedonically monitors oral stimuli and signals the pleasantness of such stimuli with the heart rate as an indicator response.” The preference of the newborn for sweet has been confirmed by others, as has an aversion for sour and bitter.
The major features of the motor examination to be evaluated in the neonatal period are muscle tone and the posture of limbs, motility and muscle power, and the tendon reflexes and plantar response. The postnatal age and level of alertness of the infant have an important bearing on essentially all of these features. Most of the observations to be described next are applicable to an infant of more than 24 hours of age and in an optimal level of alertness, unless otherwise indicated.
Muscle tone is assessed best by passive manipulation of limbs with the head placed in the midline. Moreover, because tone of various muscles will in part determine the posture of the limbs at rest, careful observation of posture is valuable for the proper evaluation of tone. Some investigators have devised various maneuvers of passive manipulation of limbs (e.g., approximation of heel to ear, hand to opposite ear [scarf sign], or measurement of angles of certain joints, such as the popliteal angle) to attempt to quantitate tone. These maneuvers have not been particularly useful for me and are not discussed in detail.
Saint-Anne Dargassies and coworkers have described an approximate caudal-rostral progression in the development of tone, particularly flexor tone, with maturation. At 28 weeks, there is minimal resistance to passive manipulation in all limbs, but by 32 weeks, distinct flexor tone becomes apparent in the lower extremities. By 36 weeks, flexor tone is prominent in the lower extremities and is palpable in the upper extremities. By term, passive manipulation affords appreciation of strong flexor tone in all extremities.
The posture of the infant in repose reflects these changes in tone to some extent. In my experience, these postures are apparent principally when the infant is in a slightly drowsy state. The alert infant at these various gestational ages is more active and motile, and fixed postures or so-called preference postures are difficult to define. This fact has been documented well by Prechtl and coworkers and by others. Nevertheless, the very quiet infant at 28 weeks often lies with minimally flexed limbs, whereas by 32 weeks there is distinct flexion of the lower extremities at the knees and hips. By 36 weeks, flexor tone in the lower extremities results in a popliteal angle of 90%, and there is consistent and frequent flexion at the elbows. By term, the infant assumes a flexed posture of all limbs. Fisting, usually bilateral, is the predominant hand posture. The evolution of hip (and knee) flexor tone with maturation is reflected in the developmental increase in pelvic elevation when the infant is in the prone position.
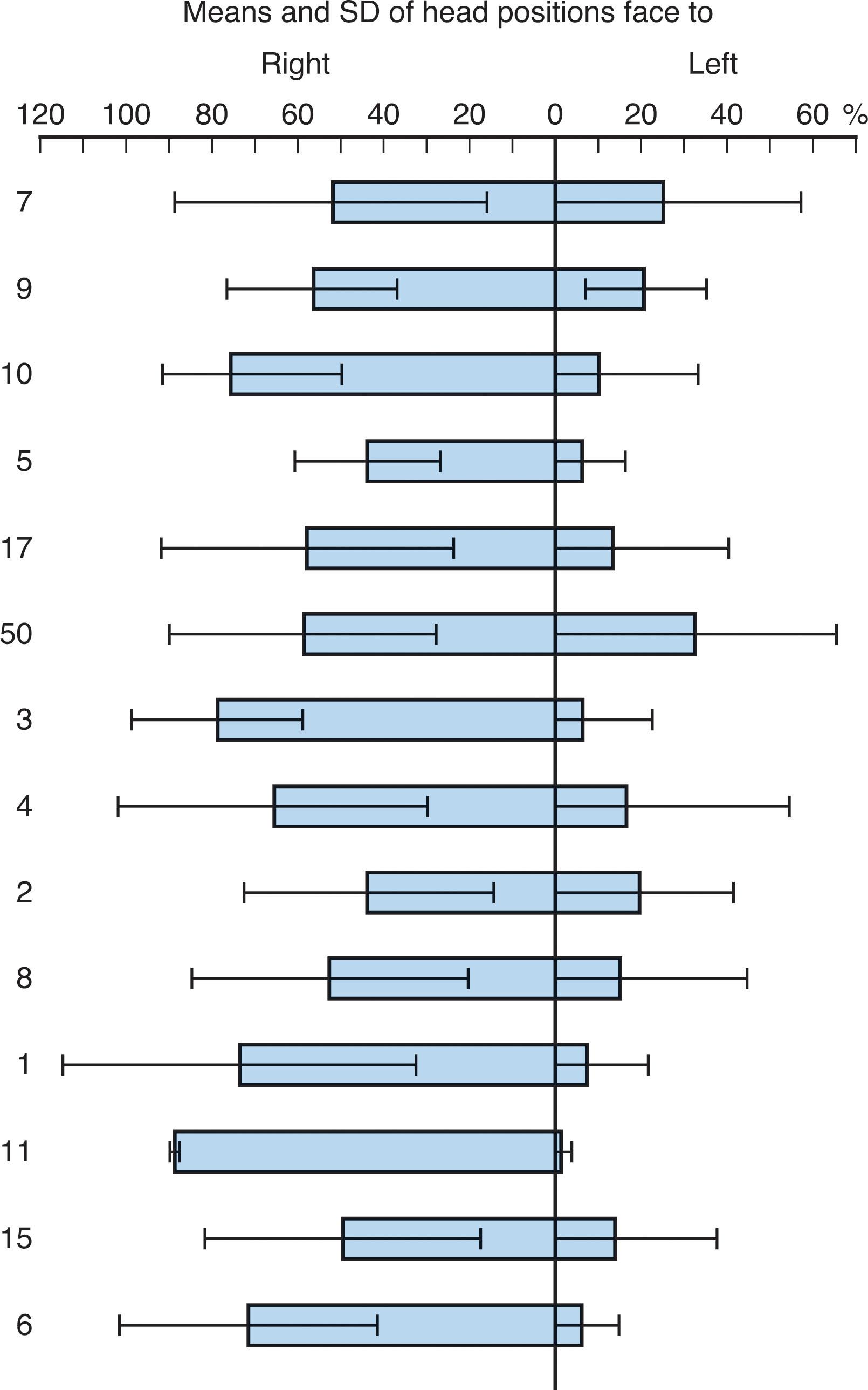
A consistent and interesting aspect of posture in newborn infants is a preference for position of the head toward the right side. Prechtl and coworkers demonstrated head position toward the right side 79% of the time versus 19% toward the left and 2% toward the midline ( Fig. 12.8 ). In one study, this preference increased with gestational age, whereas in another it decreased. The head orientation preference may be less prominent in the first 24 hours of life. This preference has not been attributable to differences in lighting, nursing practices, or other factors, but appears to reflect a normal asymmetry of cerebral function at this age. Notably the left hemisphere, particularly the frontal region, mediates movement of head to the right. As noted earlier the left hemisphere appears dominant for speech perception in the newborn.
The quantity, quality, and symmetry of motility and muscle power are the parameters of interest. Prechtl and coworkers combined videotape and electrophysiologic methods to describe the postnatal development of motor activity in the term infant. In the first 8 weeks, movements with a writhing quality predominate; in the period from 8 to 20 weeks “fidgety” movements are prominent, and after the latter period, rapid large-amplitude antigravity and intentional movements (“swipes” and “swats”) are prominent. In general, preterm infants exhibited similar patterns of motor development when they attained comparable postmenstrual ages, albeit with minor delays in tone and quality of movements. Prechtl and others emphasized that the quality of spontaneous movements in preterm and term infants is of major importance vis-á-vis the status of the CNS.
Saint-Anne Dargassies, using less sophisticated techniques, described the developmental changes in motility in the preterm infant. I find her observations especially useful. At 28 weeks, movements tend to involve the entire limb or trunk and may have a slow rotational component or a fast, large-amplitude characteristic. By 32 weeks of gestation, movements were seen to be predominately flexor, especially at the hips and knees, often occurring in unison. Although head turning is present, neck flexor and extensor power are negligible, as judged by complete head lag on pull to sit or when the infant is held in the sitting position. By 36 weeks, the active flexor movements of the lower extremities are stronger and often occur in an alternating rather than symmetrical fashion. Flexor movements of the upper extremities are prominent. For the first time, definite neck extensor power can be observed. When the infant is supported in the sitting position, the head is lifted off the chest and remains upright for several seconds. By term, the awake infant is particularly active if stimulated with a gentle shake. Limbs move in an alternating manner, and neck extensor power is still better. Neck flexor power becomes apparent; when the infant is pulled to a sitting position with firm grasp of the proximal upper limbs, the head is held in the same plane as the rest of the body for several seconds.
The importance of a fixed developmental program in motor development is suggested by the similarities in such development when comparing (at the same postmenstrual age) the fetus, the premature infant, and the term infant, albeit with minor exceptions. The similarities outweigh the rather small differences.
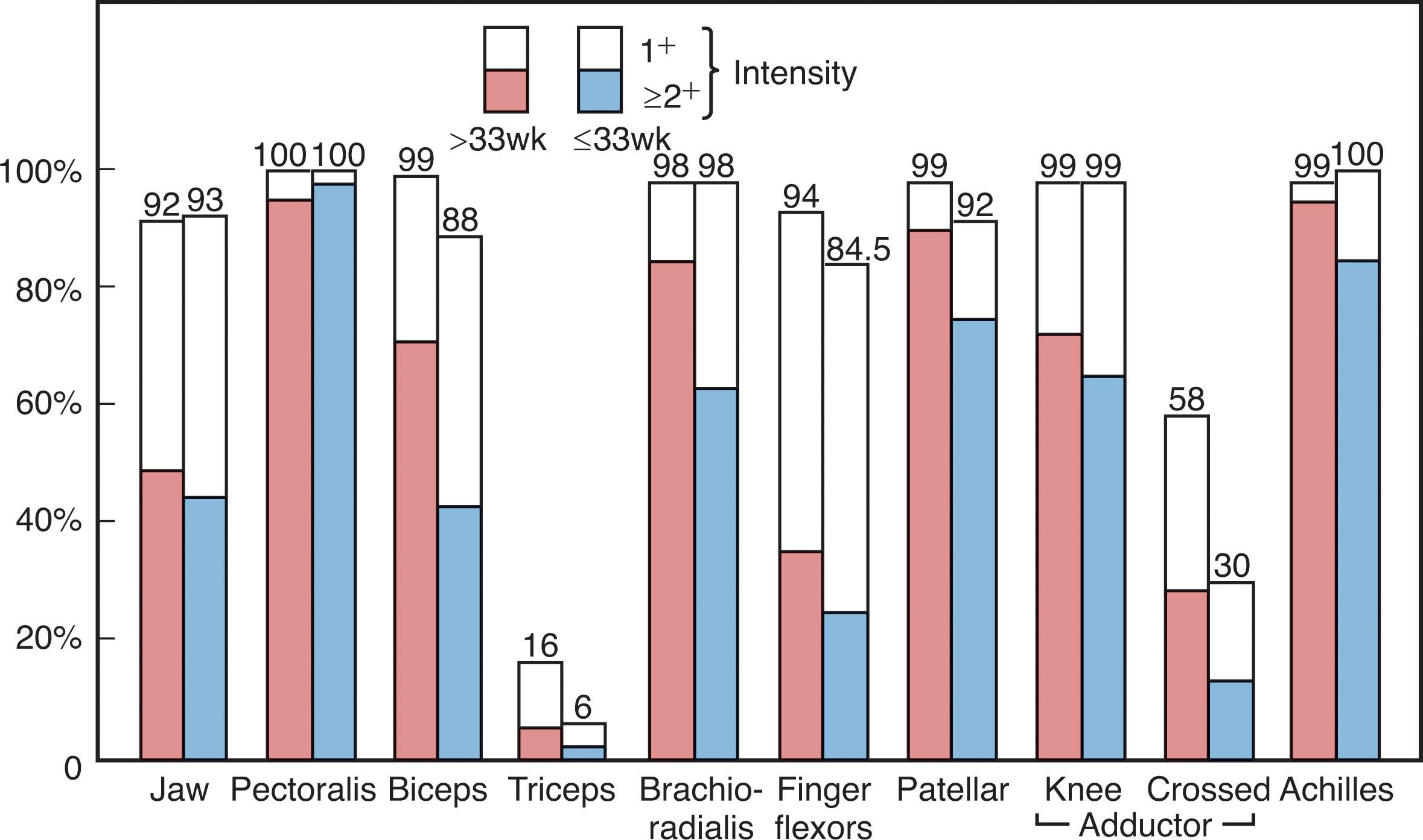
Tendon reflexes readily elicited in the term newborn are the pectoralis, biceps, brachioradialis, knee, adductor, and ankle jerks. I have considerable difficulty obtaining triceps jerks in term infants. Most of these reflexes are elicitable but less active in preterm infants ( Figs. 12.9 and 12.10 ). I prefer a small circular reflex hammer of the “Queen’s Square” type. The reflexes are elicited readily by tapping the examiner’s finger placed over the tendon of the designated muscle ( Fig. 12.9 ). An exception is the ankle jerk, which I prefer to elicit by tapping a finger placed over the distal plantar surface of the foot—the tap stretches the Achilles tendon and elicits the reflex. The knee jerk is often accompanied by crossed adductor responses (i.e., adduction off the opposite thigh), which should be considered a normal finding in the first months of life (<10% of normal infants demonstrate crossed adductor responses after 8 months of age). The adductor jerk also is often accompanied by a crossed adductor response.
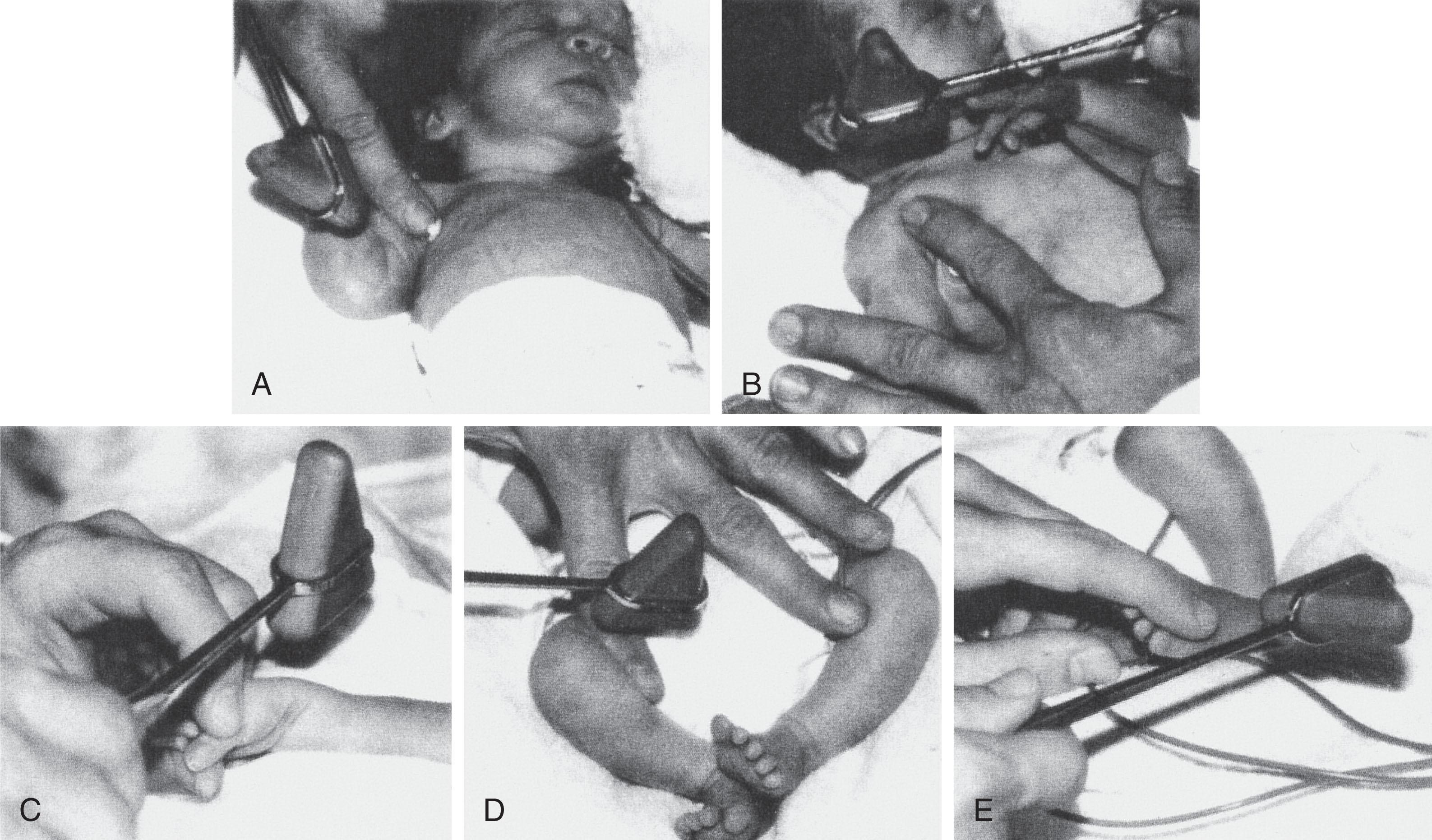
Ankle clonus of 5 to 10 beats also should be accepted as a normal finding in the newborn infant, if no other abnormal neurological signs are present and the clonus is not distinctly asymmetrical. Ankle clonus usually disappears rapidly, and the existence of more than a few beats beyond 3 months of age is abnormal.
The plantar response is usually stated to be extensor in the newborn infant. This result clearly relates to the manner in which the response is elicited. Using drag of thumbnail along the lateral aspect of the sole, Hogan and Milligan observed bilateral flexion in 93 of 100 newborn infants examined. We observed a similar result in 116 (94%) of 124 infants. In contrast, Ross and associates, using drag of pin or pinprick, observed a predominance of extensor responses, with flexion in only about 5% of patients.
When evaluating the neonatal plantar response, it is necessary to consider at least four competing reflexes leading to movements of the toes. Two reflexes that result in extension are nociceptive withdrawal (often accompanied by triple flexion at hip, knee, and ankle) and contact avoidance (elicited best by stroking the dorsum of the foot, which often occurs inadvertently when holding the foot to elicit the plantar response). Two responses that lead to flexion are plantar grasp and positive supporting reaction (both elicited by pressure on the plantar aspect of the foot). Because of these competing reflexes and the relative inconsistency of responses, I have considered the plantar response to be of limited value in the evaluation of the newborn infant when attempting to determine the presence of an upper motor neuron lesion (with involvement of the corticospinal tracts in a spinal cord lesion, the extensor plantar response is more consistent).
Many primary neonatal reflexes have been described in the classic writings on the neonatal examination. I have found useful the Moro reflex, the palmar grasp, and the tonic neck response ( Table 12.9 ). In general, I find these reflexes to be more valuable in assessment of disorders of the lower motor neuron, nerve, and muscle than of the upper motor neuron.
| AGE (WEEKS OF GESTATION; MONTHS POSTNATAL) | |||
|---|---|---|---|
| NEONATAL REFLEX | ONSET | WELL ESTABLISHED | DISAPPEARS |
| Moro reflex | 28–32 weeks | 37 weeks | 6 months |
| Palmar grasp | 28 weeks | 32 weeks | 2 months |
| Tonic neck response | 35 weeks | 1 month | 6 months |
The Moro reflex, elicited best by the sudden dropping of the baby’s head in relation to the trunk (the falling head should be caught by the examiner), consists of opening of the hands and extension and abduction of the upper extremities, followed by anterior flexion (“embracing”) of the upper extremities and an audible cry. Hand opening is present by 28 weeks of gestation, extension and abduction by 32 weeks, and anterior flexion by 37 weeks. Audible cry appears at 32 weeks. The Moro reflex disappears by 6 months of age in normal infants.
Palmar grasp is clearly present at 28 weeks of gestation, is strong at 32 weeks, and is strong enough and associated with enough extension of upper extremity muscles to allow the infant to be lifted from the bed at 37 weeks. The palmar grasp becomes less consistent after about 2 months of age when voluntary grasping begins to develop.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here