Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Necrotizing enterocolitis (NEC) is an inflammatory bowel necrosis seen in infants who are either born extremely premature or are critically ill with multiple organ dysfunction syndrome (MODS). The intestinal injury in NEC begins in the mucosa and progresses outwards, and in severe cases, may result in transmural necrosis and perforation. , Unfortunately, despite major progress in most outcomes, this disease continues to be a leading cause of morbidity and mortality in neonates born between 22 and 28 weeks’ gestation. , The pathophysiology also remains unclear; epidemiologic studies show modest associations with a wide range of seemingly disparate genetic, ethnic, geographical, seasonal (more in April/May), temporal (decadal variations), and clinical factors. , , The clinical associations may span across the pre-, peri-, and post-natal periods, but it is unclear whether these events truly cause NEC or are just epiphenomena related to the overall severity of illness. These clinical variables include intrauterine growth retardation, placental abruption, prolonged rupture of membranes, chorioamnionitis, perinatal asphyxia, congenital cardiac defects, bacterial dysbiosis, patency of the ductus arteriosus and/or its treatment with non-steroidal anti-inflammatory drugs, histamine receptor 2 blockers, formula feedings, bovine fortifiers of human milk, certain viral infections, feed thickeners, and severe anemia and/or subsequent red blood cell transfusions. , , Thrombocytopenia been noted as a risk-factor for NEC in some reports, but the drop in platelet counts could just be a part of the MODS during NEC and not actually cause it. ,
We, , and others, , have described the inflammatory response in NEC lesions. Histopathologically, NEC lesions are marked mainly by intestinal inflammation marked by mucosal edema and leukocyte infiltration, and foci of infarction. Most of these infiltrating leukocytes are macrophages. The specific findings can range from mucosal injury to full-thickness bowel necrosis and perforation. The terminal ileum and colon are involved in most cases, although the entire gastrointestinal tract can be affected in severe cases. Gas-filled bubbles ( pneumatosis ) are seen in affected tissues in 50%; most of these lesions are seen in the submucosa but can extend to other layers in about 50%. , These changes can be seen both on the mesenteric and the antimesenteric sides. Most of the earlier studies, particularly those on older patients, suggested that pneumatosis represented dilated lymphatic vessels, but newer descriptions suggest gases entrapped in damaged tissues may be a more likely cause. Microthrombi and microhemorrhages are seen, although vascular thrombi are rare. As the gut heals, bowel wall thickening, fibrinous adhesions, and areas of stenosis can be seen. ,
Macrophages constitute 60% to 70% of the leukocytes in these inflammatory lesions, although there can be some variability between individual patients and sometimes even between noncontiguous lesions in tissues excised from the same patient. , , , These changes were seen as indicative as a sign of chronic inflammation. However, in our murine models, macrophage, not neutrophil, infiltration was an initiating sign of inflammation in NEC-like injury. , , , , Pender et al . have also noted increased CD68 + macrophages in NEC lesions. Managlia et al . noted the role of inflammatory activation of monocytes in experimental NEC. In another study, Tanner and coworkers collated the information in existing studies of NEC and also emphasized the role of macrophages. In preterm infants, peritoneal macrophages also show similar immaturity with hyperinflammatory characteristics. Most NEC lesions also show 20% to 40% neutrophils, but the total number of lymphocytes (about 10%) does not seem to change over unaffected bowel loops.
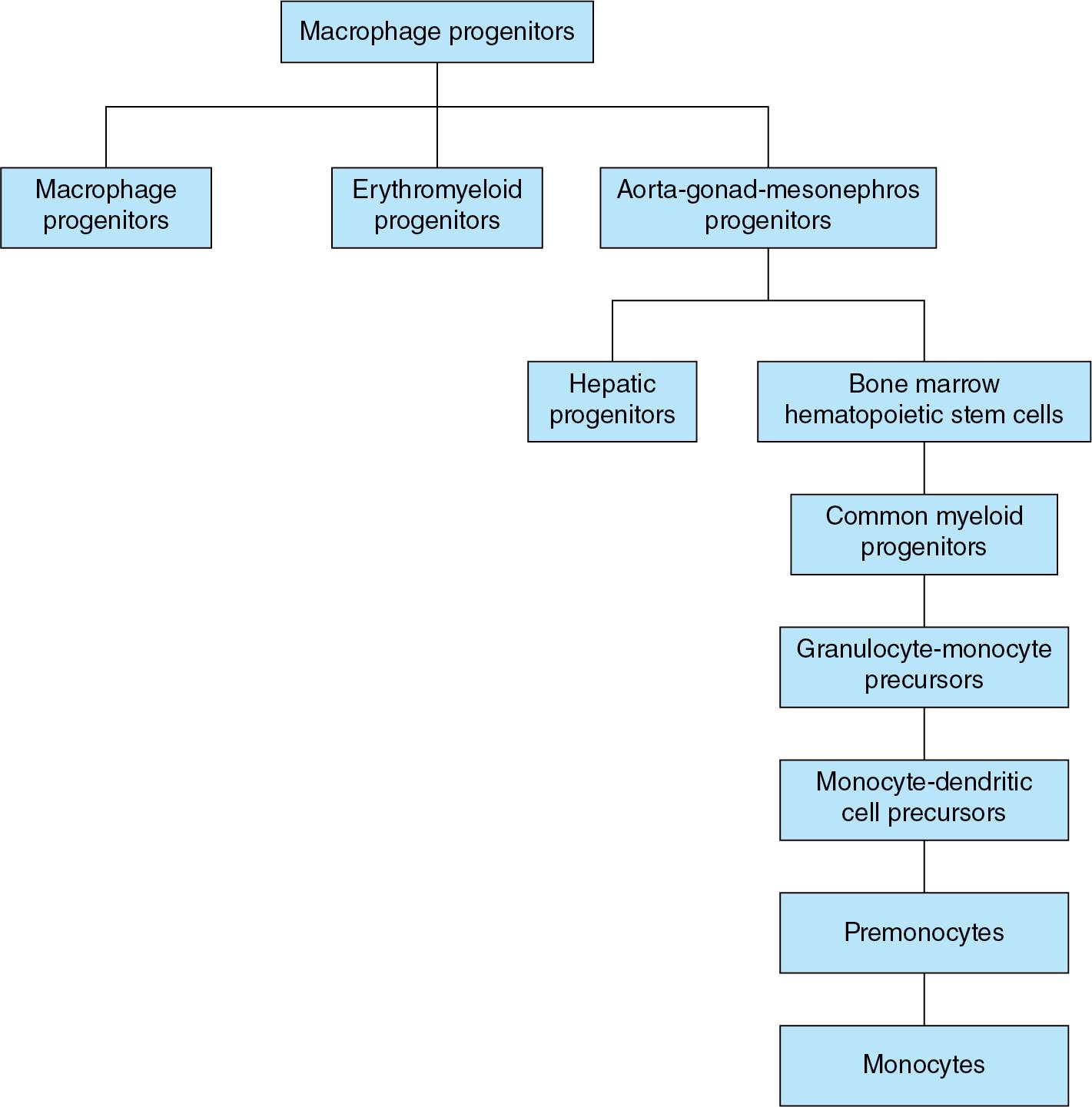
The name “macrophages” or “big eaters” came from the Greek words, makros or large, and phagein or eat. These cells have large nuclei with abundant cytoplasm that is sometimes vacuolated. Functionally, macrophages are highly motile, phagocytic, and can also modulate the function of other immune cells. During development, macrophages develop either in (1) the yolk sac from primary progenitors on day 18 or the erythromyeloid progenitors (EMPs) on day 30 ; and (2) the aorta-gonad-mesonephros (AGM) zone, from CD34 + CD45 + hematopoietic stem cells (HSCs) ( Fig. 2.1 ). On day 32, these HSCs migrate and differentiate in either the (1) liver during 8 to 32 weeks’ gestation or (2) bone marrow. The stages of differentiation can be identified more clearly in the marrow. HSCs mature into common myeloid progenitors, granulocyte-monocyte precursors, common monocyte and dendritic cell (DC) precursors (MDP), premonocytes, and monocytes. After birth, liver HSCs migrate to the bone marrow. HSCs mature into common myeloid progenitors (CMPs) and then differentiate either directly into tissue macrophages or pass through the aforementioned stages to differentiate into monocytes. There are 3 subsets of monocytes: (1) nearly 90% are the classical CD14 + + inflammatory monocytes; (2) 10% are the non-classical CD16 + + type that patrol the vasculature to assess endothelial integrity, and produce cytokines at intermediate levels; and (3) a few cells that express CD14, CD16, and MHC-II; present antigens; and might activate T lymphocytes.
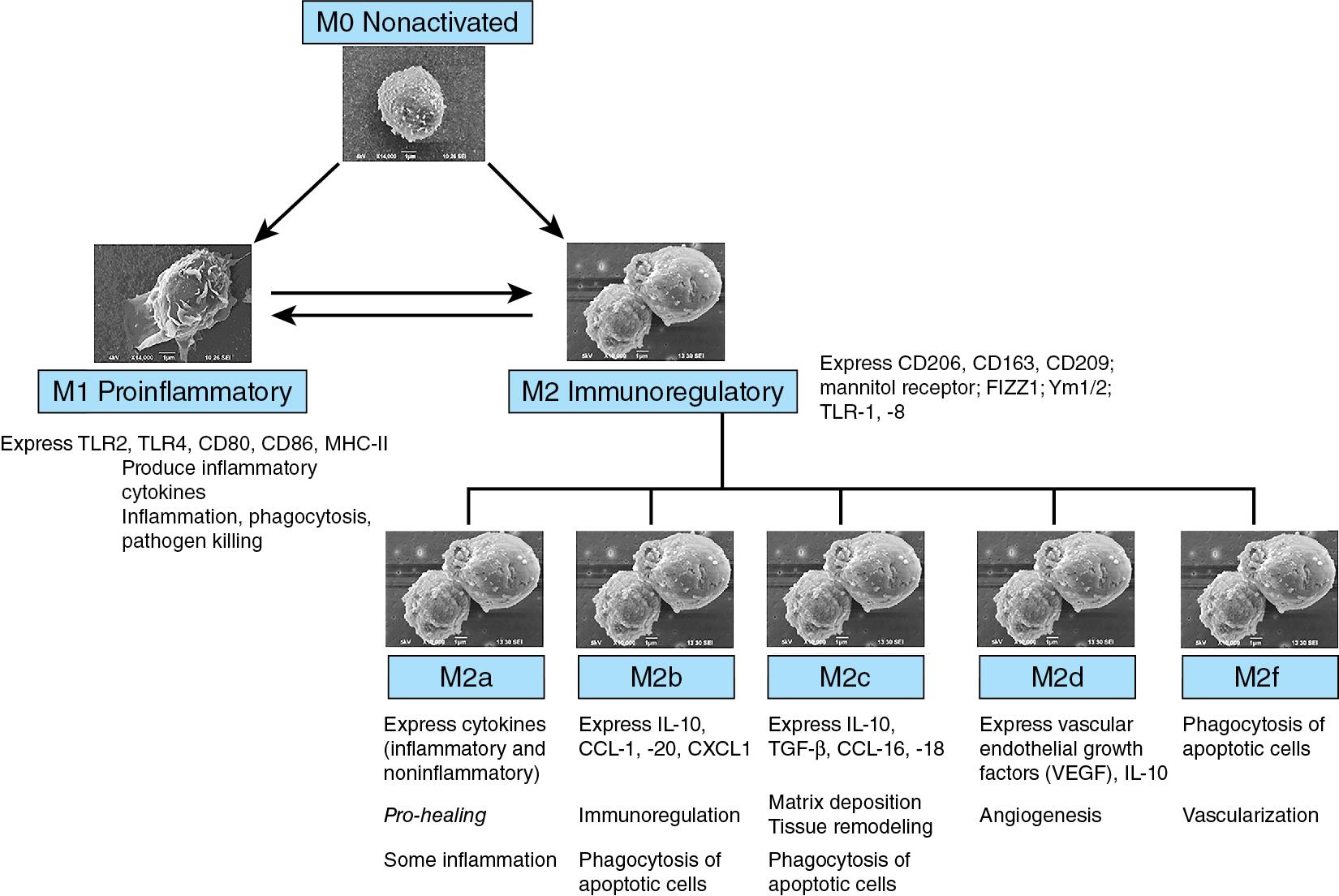
There are two major subgroups of macrophages that can be differentiated by surface markers, tissue localization, intracellular signaling, and function. The proinflammatory M1 macrophages are regulated by cytokines such as the tumor necrosis factor (TNF) and interferon-γ, bacterial lipopolysaccharide (LPS), and the granulocyte-macrophage colony-stimulating factor (GM-CSF). These cells express surface markers such as CD54, CD80, CD86, and CD197. , M1 macrophages quickly “inhibit” and kill pathogens as a host defense mechanism. The second class is comprised of the immunoregulatory M2 macrophages responds to interleukin-4 (IL-4), IL-10, IL-13, and IL-21, and to glucocorticoids. M2 macrophages express scavenger receptors such as CD163, CD204, and the mannose receptor, CD206. , These macrophages are active in immunoregulation, maintain tissue integrity following injuries and in chronic infections, and promote angiogenesis. There is some variability in this nomenclature, although the heterogeneity in the M2 macrophages is being recognized (M2a, M2b, M2c, M2d, and M2f) ( Fig. 2.2 ). ,
In the intestine, EMPs fade away at birth. Macrophages differentiate either from primary progenitors or from circulating monocytes. Unlike the non-inflammatory macrophages in the mature intestine, these cells are still in the process of tissue-specific differentiation and may resemble those in other tissues with inflammatory M1 and various M2 polarization. ,
Macrophages show considerable functional diversity. In premature infants, macrophages express high levels of CD11b, chemokine receptors CCR1, CCR2, CCR5, CXCR1, CXCR2, molecules such as CD115, triggering receptors expressed on myeloid cells (TREM), and 6-sulfo N -acetyllactosamine (LacNAc) glycans. Neonatal macrophages respond both to exogenous chemicals, microbial products, and microparticles ; and endogenous stimulants such as cytokines; oxidized lipids; reactive oxygen (ROS) and nitrogen species (RNS); metabolic products, heat-shock proteins (HSPs), and damage-associated molecular patterns (DAMPs).
MDMs and mDCs show important differences. Ex vivo, monocytes differentiate into macrophages upon exposure to M-CSF, and into mDCs following treatment with GM-CSF and IL-4. Both macrophages and DCs share the phenotypic markers CD11b, CD11c, CD80, CD86, CD163, CD209, and MHC-II. However, macrophages typically express CD68 (macrosialin) and CD33 (siglec-3) with some specificity; DCs may express intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN/CD209), CD1c, and CD141.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here