Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Despite the evolution of new technologies for assessing neonatal brain function, electroencephalography (EEG) remains one of the most valuable diagnostic and prognostic tools. It is considered the gold standard for distinguishing epileptic seizures from nonepileptic paroxysmal events and for detecting subclinical seizure activity in high-risk babies. In babies who are severely ill, EEG is a more efficient predictive test than the neurologic examination. Background patterns, more than the presence or absence of seizures, correlate significantly with the long-term outcome. The prognostic value of neonatal EEG has long been recognized in term and preterm infants, and its value can be increased by obtaining early recording (e.g., within the first 48 hours of life and prior to administration of antiepileptic drugs [AEDs]), prolonged recording to include samples of different activity states, and serial EEGs at short intervals to assess rapid changes that are likely to occur in high-risk infants. Different drugs currently used in the neonatal intensive care unit (NICU), especially if in the toxic range, can alter the EEG background. Those effects need to be considered in the interpretation of neonatal EEG. It is important to distinguish neonatal seizures from neonatal-onset epilepsies and epilepsy syndromes. Both benign and severe neonatal epilepsy syndromes exist. Benign familial neonatal seizures represent a neonatal syndrome with benign outcome, whereas early myoclonic encephalopathy (EME) and Ohtahara syndrome (OS) are severe epilepsy syndromes.
In the past decade, neonatal EEG has faced a phase of renewal due to increased possibilities of data elaboration and display because of digital acquisition allowing for continuous video-EEG (vEEG) monitoring. The use of vEEG has now entered everyday clinical practice in many NICUs, leading to improved diagnosis and prognostication ability, brain function assessment, and neonatal seizure diagnostic rate.
Conventional EEG with simultaneous video recording is now considered the standard of care in many institutions. It is used for routine examination, usually lasting 30 to 90 minutes, and well as for continuous monitoring. It is considered the gold standard for seizure diagnosis, seizure characterization, and quantification of seizure burden in newborns. It allows clinicians to determine the exact localization of seizure onset and of their propagation and can provide detailed information on brain maturation, acute or remote brain injury, prognosis, and response to AEDs. However, not every institution has ready access to it, or at least not on a 24-hour basis, and there is limited availability of expertise in performing and interpreting EEG in the neonatal period. On the other hand, with the development of new therapeutic strategies for hypoxic-ischemic encephalopathy (HIE), such as hypothermia, there has been great interest in generating alternative strategies for the continuous assessment of brain function in the neonate, including trend analysis of EEG recordings such as amplitude-integrated EEG (aEEG). aEEG is a simplified method of continuous cerebral function monitoring that has become common clinical practice in the NICU, as it can be easily interpreted by neonatologists and bedside nurses.
aEEG is an EEG-based bedside brain-monitoring tool. It displays one or two channels of EEG data after filtering, rectification, and smoothing, on a semilogarithmic scale. Studies have demonstrated reliability in assessing background activity and degree of HIE, with good correlation with outcome ; however, its use for seizure detection is debated, and it is generally agreed that it has to be considered as a screening, complementary tool , and that reference to conventional or “raw” EEG should be made in order to increase sensitivity and specificity. , In fact, aEEG has a number of limitations (e.g., seizures of short duration and focal seizures remote from the recording electrodes might be missed), and these must be taken into account. ,
The combined use of both of these techniques provides complementary benefits of (1) displaying aEEG on the bedside monitor to assist neonatologists in prompt decision making and (2) allowing full revision and interpretation by neonatal neurologists or neurophysiologists for subsequent diagnostic confirmation or useful refinements.
In this chapter we will focus on conventional EEG, its technical aspects and major indications, behavioral states, characteristics of physiologic and pathologic EEG patterns according to postmenstrual age (PMA), and principal electrographic features of common clinical disorders, including seizures. Finally, we will give an overview of the EEG patterns seen in neonatal-onset epilepsies and epileptic encephalopathy and provide information for early diagnosis.
Neonatal EEG recordings should cause the least possible distress to the newborn in order to reduce interference with behavior.
To properly assess the neonatal EEG, it is necessary to interpret it in light of the PMA at the time of the recording, the behavioral state, the clinical data including medication history, and the presence and depth of therapeutic hypothermia. Furthermore, the recording should include a complete cycle of wakefulness, active sleep, and quiet sleep in infants aged at least 30 weeks of conceptional age (CA), or include the most continuous and most discontinuous pattern and last at least 40 minutes in infants aged less than 30 weeks CA, in whom indeterminate sleep is the most consistent behavioral state. , The American Clinical Neurophysiology Society (ACNS) recommends the application of 11 scalp electrodes according to the International 10 to 20 System modified for neonates (Fp1, C3, T3, O1; Fp2, C4, T4, O2; Fz, Cz, Pz; Fig. 130.1 ).
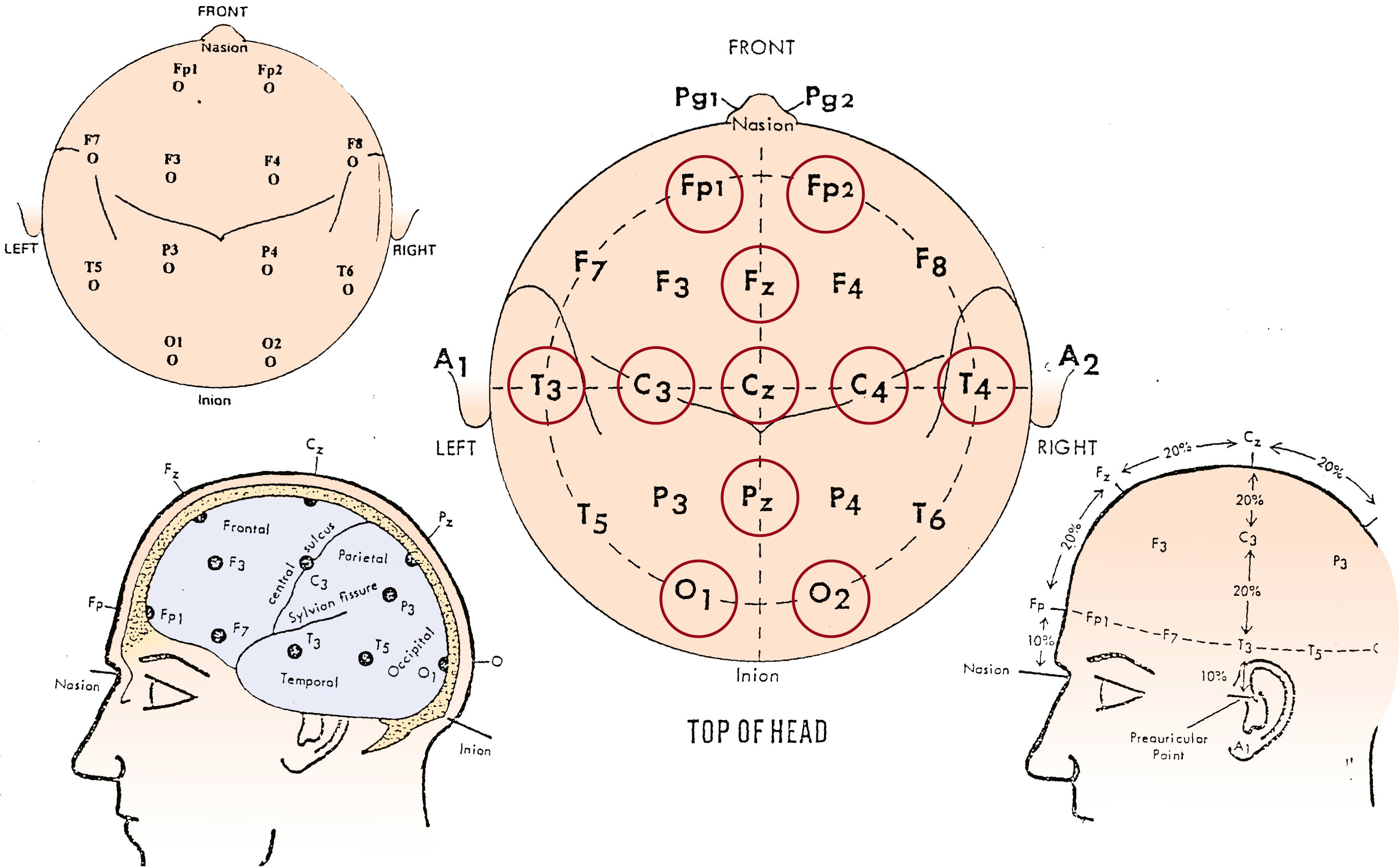
The standard recommended bipolar montages are the longitudinal and the transverse.
Because the current gold standard for correct state classification and differential diagnosis of paroxysmal events is represented by polygraphic vEEG, the following noncerebral signals should also be simultaneously recorded: (1) electrooculogram, (2) electromyography, (3) electrocardiogram, and (4) pneumogram; , in addition, a video camera should be synchronized with the EEG. Along with the information derived from polygraphic vEEG recording, careful annotations from the attending technologist are very useful in describing the patient’s activity (e.g., eye opening/closing, subtle movements, hiccup, sucking), handling by ward personnel, beginning and end of drug administration, and artifacts when difficult to remove. With digital tracings it is also important to remember the importance of sampling frequency, which is recommended to be at least of 256 Hz.
EEG recording should be considered as an important part of the neurologic evaluation of the critically ill newborns admitted to the NICU, as these patients constitute a group at high risk of neurologic sequelae in whom assessment of brain function exclusively by means of neurologic examination might prove challenging, for example because of iatrogenic paralysis. EEG should be considered for concerns about the neonate’s neurologic status or the possibility of seizures, and when specific risk factors exist (e.g., fetal distress, central nervous system infection, HIE, preterm birth, intracranial hemorrhage, cardiac surgery). The literature has widely demonstrated that only a small percentage of neonatal seizures have a clinical correlate, , and therefore vEEG monitoring is recommended to diagnose subclinical siezures. According to the ACNS, monitoring should be performed for a period of 24 hours, although some research has proven an increased risk in the first 36-hour period, and some centers monitor for longer durations.
In the presence of suspicious clinical events, EEG is necessary in the differential diagnosis of abnormal movements, such as myoclonic jerks, lip-smacking, pedaling, or boxing, and other paroxysmal events or fluctuating autonomic signs to exclude or confirm a diagnosis of neonatal seizures and possibly avoid unnecessary prescription of anticonvulsant therapy. When this constitutes the indication for conventional EEG, its duration should primarily depend on the ability to record multiple typical clinical events.
Once the diagnosis of neonatal seizures is confirmed, prolonged or continuous monitoring is used to assess seizure burden and diagnose neonatal status epilepticus, , which has therapeutic and prognostic significance. EEG is mandatory to monitor effectiveness of antiseizure drugs (ASDs) as well as in the differential diagnosis between true response and uncoupling, which has been reported in 58% of newborns with persistent seizures after either phenobarbital or phenytoin. Once the true response, which is absence of EEG seizures, has been achieved, it is recommended to continue EEG monitoring until the patient has been seizure-free for 24 hours; it is also advisable to monitor for seizure recurrence during and after discontinuation of ASDs.
EEG helps assess the functional extent of brain injury by evaluating the degree of abnormality of background EEG patterns and, especially with serial recordings, predict neurologic outcome according to its dynamic evolution over time. Proper evaluation of background activity includes recording of wakefulness, active sleep, and quiet sleep and usually requires at least 1 hour for recording, or longer in case of disruption of sleep rhythms after the acute phase of encephalopathy. This has been extensively studied in the context of acute HIE but holds true for different etiologies. For example, in newborns with meningitis, the degree of background abnormality and the presence of seizures have been demonstrated to be outcome predictors. In the context of HIE, even a brief EEG assessment can be useful in predicting neurologic outcome and short-term risk of neonatal seizures in the following 18 to 24 hours, depending on the background activity. However, several studies conducted during hypothermia treatment for HIE demonstrated that continuous EEG monitoring provides the most accurate information. , Monitoring of a burst-suppression (BS) pattern with conventional EEG is also advisable when the aim is to quantify interburst intervals (IBIs), for example in the course of metabolic encephalopathies, in which progressive shortening of IBI has been correlated with medical correction of hyperammonemia, or when the BS is drug-induced in the context of treatment of refractory status epilepticus. In this second scenario, conventional EEG would also allow for the detection of ongoing seizure activity. In preterm newborns, EEG can help in determining the most likely timing of a brain insult by distinguishing between acute- and chronic-stage abnormalities, whereas with serial EEGs beginning immediately after birth the degree of severity can also be estimated.
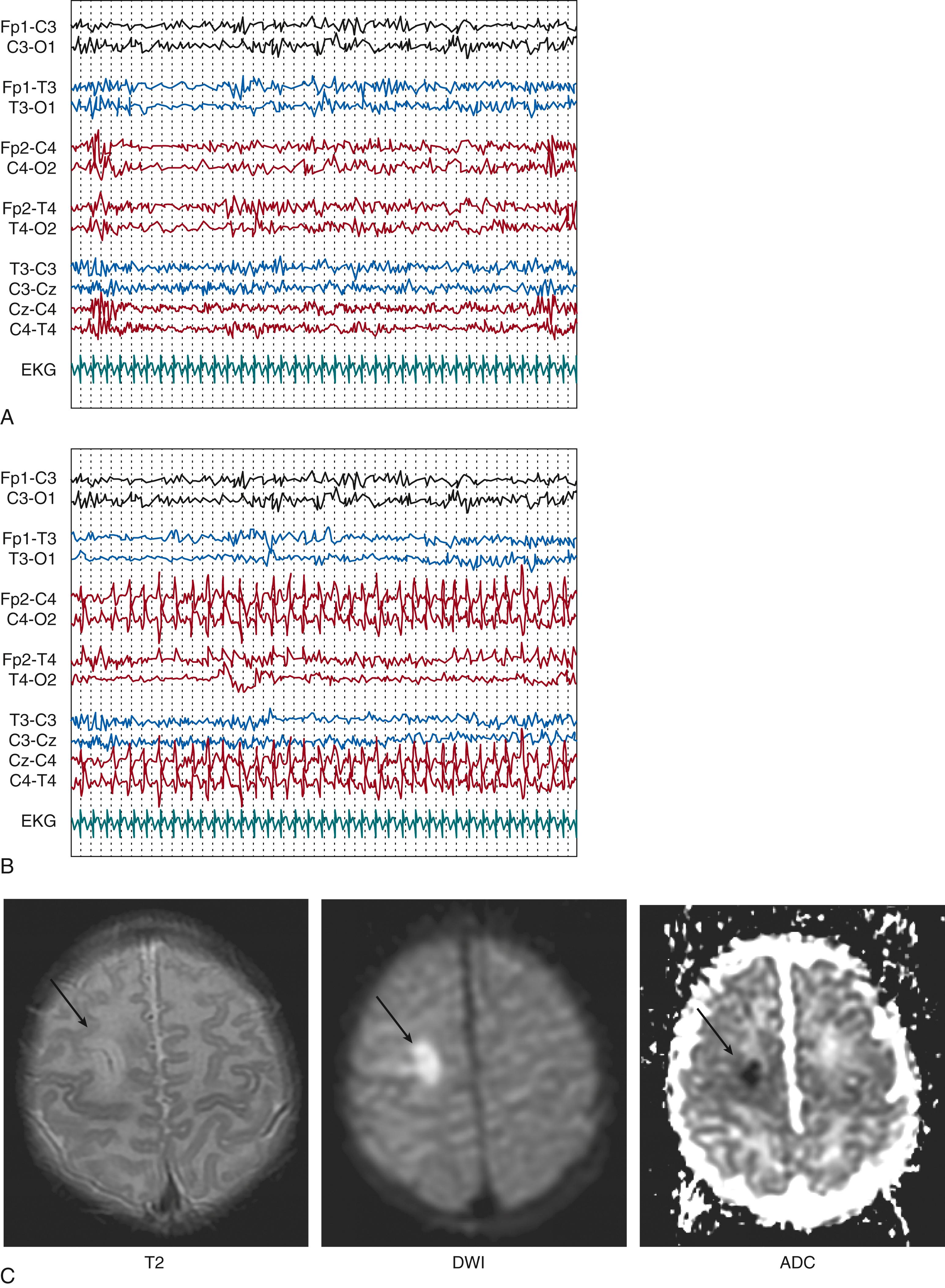
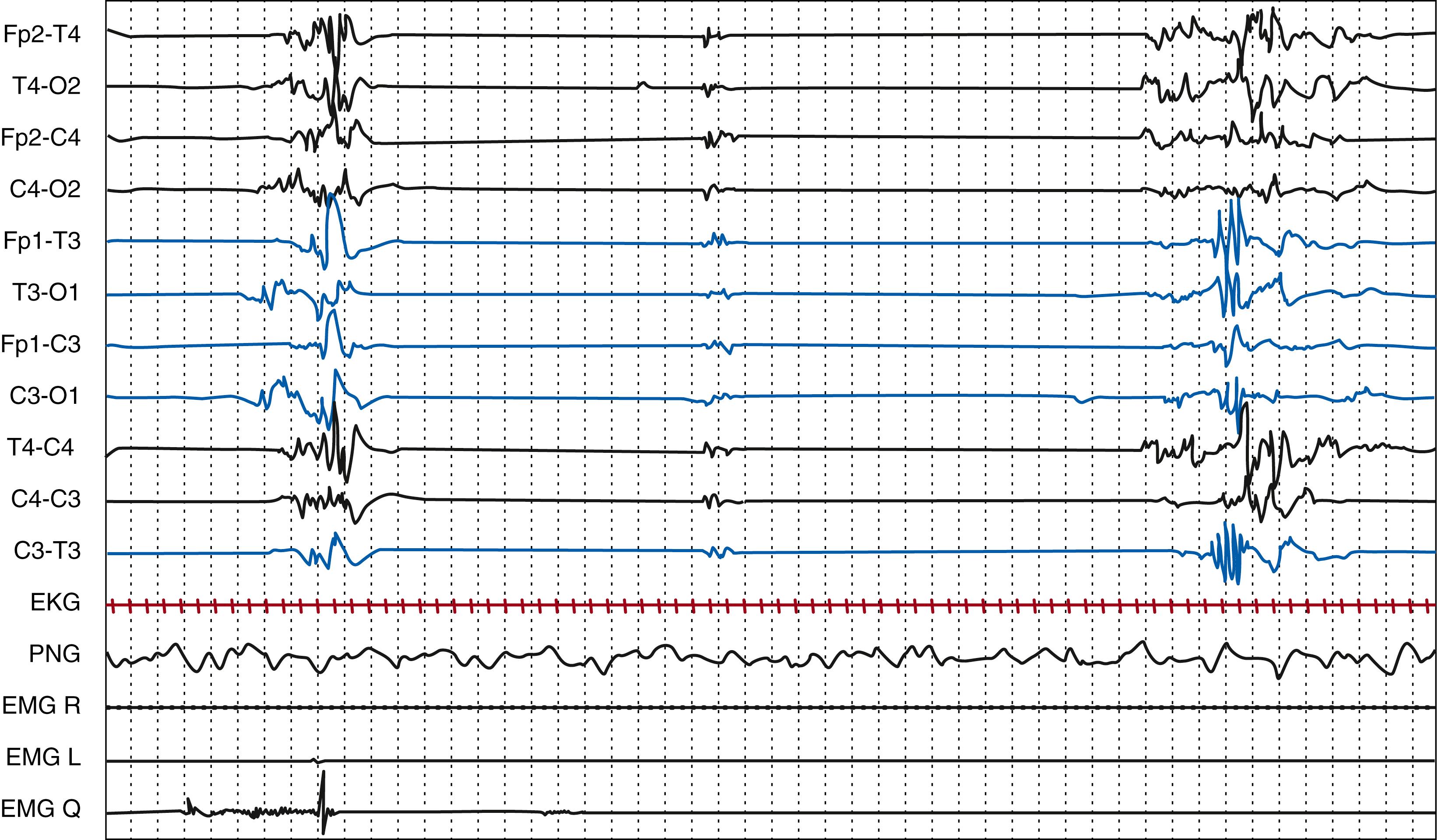
vEEG can allow for the early detection of electroclinical features that can suggest a focal cerebral injury, for example in the context of perinatal ischemic stroke or in case of subarachnoid or subdural hemorrhage. EEG might point to a focal structural etiology by identifying focal electrographic seizures, focal sharp waves, or focal attenuation of background activities, which would prompt neuroimaging ( Fig. 130.2 ). A study on perinatal arterial ischemic stroke confirmed suppression over the infarcted side, together with the presence of unilateral theta bursts intermixed with sharp or spike waves. Seizure patterns characteristically consisted of focal sharp/spikes/polispikes at 1 to 2 Hz and a predominance of electrographic-only seizures. Even if other methods for detection of seizures in the newborn such as aEEG are becoming readily available, vEEG remains the gold standard for the diagnosis of neonatal-onset epilepsies and epileptic encephalopathies. Finally, EEG monitoring can contribute to NICU decisions to continue medical care, offer supportive care, or, in countries where this decision is applicable, withdraw care.
The term behavioral states describes a series of physiologic and behavioral variables that when interpreted together provide the basis for identification of wakefulness and/or sleep states required for the correct interpretation of EEG. The ACNS Critical Care Monitoring Committee established that a behavioral state should be considered as present when its features are recognizable for at least 1 minute.
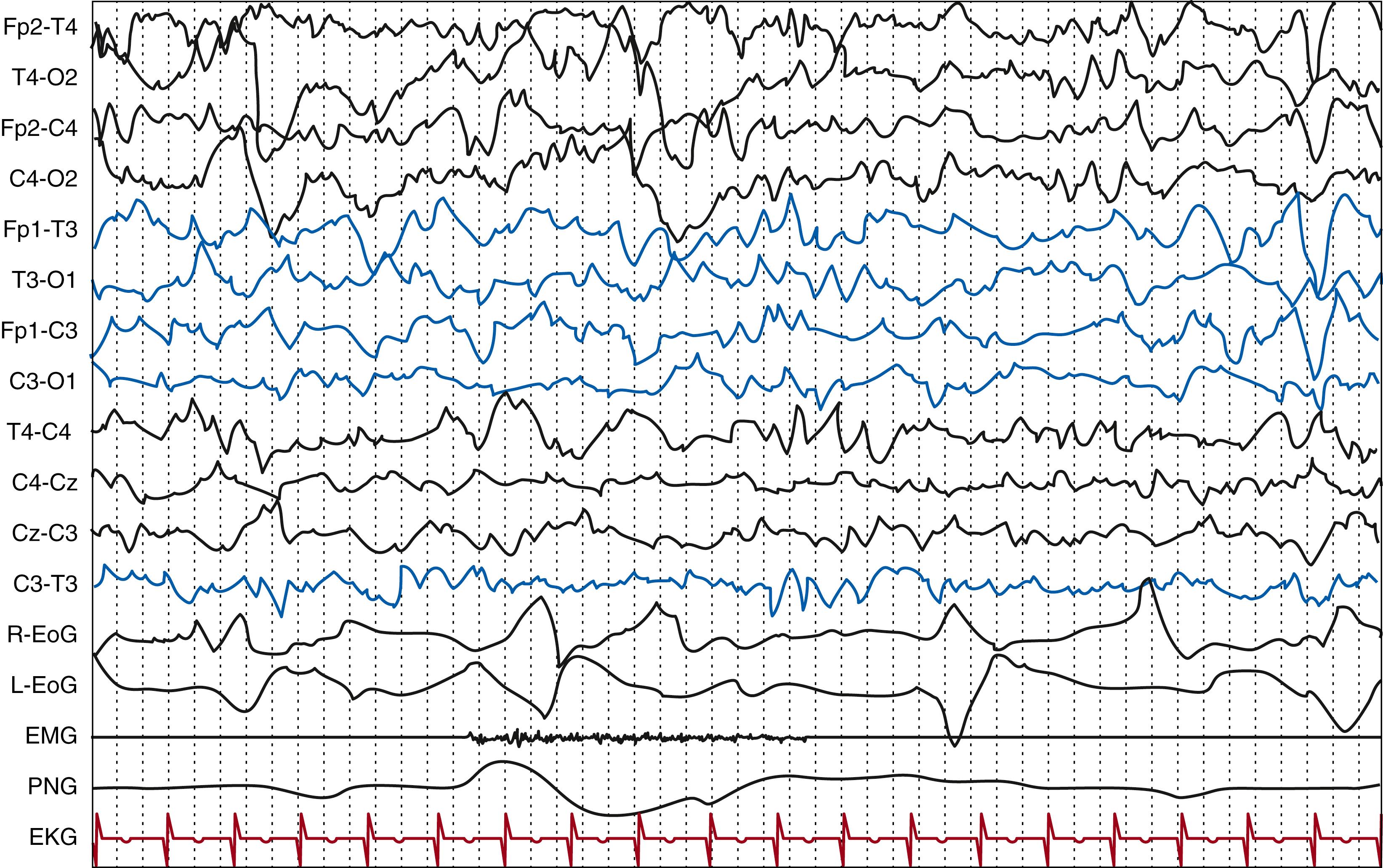
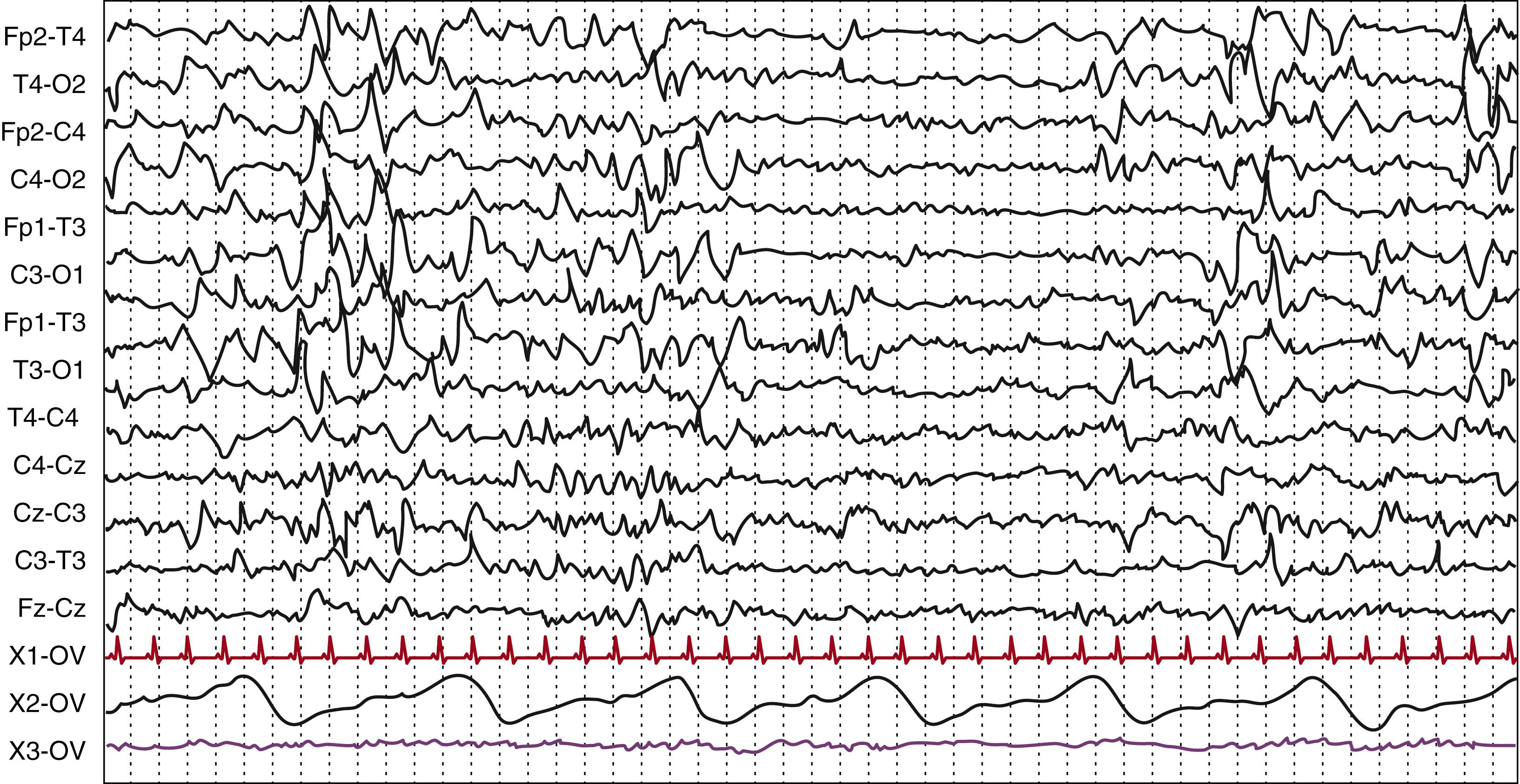
Wakefulness is defined by the presence of eyes open, a condition that is not possible before 24 weeks PMA, when eyelids are still fused together. In the preterm newborn, at around 32 to 34 weeks PMA, other polysomnographic signs (e.g., irregular respiratory pattern, phasic or tonic chin electromyographic (EMG) activity, the presence of body movements) can reliably contribute to the wakefulness definition. In the healthy term infant, wakefulness is defined by the association of the above-stated behavioral condition with the presence of the activité moyenne as the background activity ( Fig. 130.3 ). This is a typical EEG pattern of the full-term newborn corresponding to wakefulness or to active sleep state characterized by continuous electric activity with prevalent delta-theta frequencies, with an amplitude between 25 and 50 μV, with superimposed beta activity.
Two different types of eyes-open wakefulness can be recognized: active (distinguished by the presence of agitation) and quiet wakefulness.
To recognize sleep states, it is necessary to combine data from observation of the clinical state of the newborn together with EEG and polygraphic information, especially linked to the presence of rapid eye movements (REMs), which represent a hallmark of REM sleep (also known as active sleep in the newborn). Periods in which the various characteristic physiologic parameters cannot be unequivocally identified correspond to indeterminate or undifferentiated sleep , which is also called transitional when separating two periods of well-identifiable sleep states. The first appearance of the sleep-wake cycle has been reported in neonates between 25 and 30 weeks PMA. By 30 weeks gestation the concordance between electrographic and clinical indicators of behavioral states begins to arise, although this is not consistent. Between 24 and 27 weeks PMA, neonates typically remain in an indeterminate sleep, characterized by few ocular movements and poorly variable heart rate. More recently, it has been argued that criteria developed in term or near-term neonates are not applicable to extremely preterm newborns and that a differentiation between states based on REM and EEG activity alone should be used instead, as body movements are not reliable in defining sleep states in this population. Following this approach, rudimentary REM (high-voltage, more continuous EEG activity) and non-REM (more discontinuous EEG) sleep states can be recognized as early as 25 weeks CA.
Sleep onset in the neonatal period is marked by the appearance of active sleep, which is characterized by the presence of REM, small and large body movements, irregular respiration, and, in the term infant, continuous activity on the EEG. Beyond 35 weeks CA, two different types of active sleep can be recognized: active sleep 1 (AS 1), which precedes quiet sleep and is characterized by high-amplitude mixed pattern, and active sleep 2 (AS 2), which follows quiet sleep and is characterized by a low-voltage irregular pattern. Active sleep in the preterm infant is initially characterized by a tracé discontinu (discontinuous tracing) on the EEG (predominating before 28 weeks CA), and movements tend to include segmental myoclonus or generalized myoclonic and tonic posturing. Continuous EEG activity is observed by 34 weeks CA. By 28 to 31 weeks CA, periods of concordance can be recorded (eyes closed, REM, irregular respirations, body movements, and continuous EEG).
In the preterm, quiet sleep is initially characterized by a tracé discontinu (emerging at around 28 weeks CA and recorded only during quiet sleep by 34 to 36 weeks CA). The pattern of quiet sleep is fully developed at 36 to 37 weeks CA and is defined by absent REM, increased EMG tone, regular respiration, absent body movements and possibly chin movements, occasional sucking activity, and generalized myoclonic startles. , At term, two different types of quiet sleep activity can be distinguished: tracé alternant (TA) and high-voltage slow (HVS) pattern, the latter of which is first recorded at 36 weeks CA and replaces TA at 44 to 45 weeks CA. Finally, by 4 to 8 weeks post-term, sleep spindles appear. ,
At term, a complete sleep and waking cycle typically lasts 3 to 4 hours with a sleep cycle of 50 to 60 minutes, in contrast to 30 to 50 minutes in preterm infants less than 35 weeks CA. In extremely preterm infants born at 24 to 26 weeks CA, the duration of those periods varies widely from approximately 9 to 55 minutes. The percentage of time the newborn spends in quiet sleep increases with age: at 27 to 34 weeks CA, 40% to 45% of sleeping time is spent in active sleep, 25% to 30% in quiet sleep, and 30% in indeterminate sleep, whereas at term, approximately 50% to 60% of a sleep cycle is spent in active sleep, 30% to 40% in quiet sleep, and 10% to 15% in transitional sleep. , Quiet sleep is an important state to record because of the higher frequency of EEG abnormalities and because an increase in indeterminate sleep at the expense of quiet sleep may be a sign of encephalopathy, even in the presence of a normal waking or active sleep tracing.
To acknowledge the presence of a liability of the background activity in infants with disrupted EEG features that make recognition of definite specific sleep states impossible, the ACNS has defined the condition of unspecified state changes in which a cycling between different EEG patterns can be recognized due to modifications in the amount of discontinuity, voltage, or frequencies of a minimum duration of 1 minute each.
The major characteristics of neonatal EEG comprise its reactivity, the temporal organization (defined by continuity, symmetry, and synchrony), and spatial organization (mainly defined by the presence of physiologic features constituting maturational hallmarks, which characteristically appear at a certain CA, peak to a period of maximum expression, and then progressively decline).
Reactivity represents the ability of the functional activity of the brain to modify in response to internal and external stimuli. This includes changes in continuity, amplitude, and/or frequencies accompanying changes in behavioral state and changes in background activity after a provocative stimulus. Reactivity is PMA- and state-dependent and should be distinguished from clinical reaction to stimulation. It is best evaluated during quiet sleep and is detectable, although still variable, from 28 to 29 weeks PMA, becoming more consistent at 30 to 31 weeks PMA, when stimulation during quiet sleep provokes the transient appearance of a continuous activity, whereas if performed during active sleep, it elicits a decrease in amplitude. At 35 to 36 weeks CA, reactivity can be more clearly visualized as a general transient decrease in background activity during active sleep and in the appearance of a high-amplitude continuous slow wave activity if performed during quiet sleep. At term, auditory, tactile, or painful stimuli can induce either attenuation or reinforcement of the background EEG activity.
The first hallmark of an EEG is its degree of continuity. Continuity is defined as the uninterrupted presence of electrical activity, with minimal amplitude of 25 μV and less than 2 seconds of voltage attenuation below this value, lasting at least 1 minute. , Extremely preterm newborns (24 to 28 weeks PMA) have almost exclusively discontinuous tracings ( Fig. 130.4 ). Continuity is present in active sleep beginning at approximately 28 weeks PMA. By 32 weeks of gestational age, continuous EEG dominates over discontinuous activity, and it is constant in both sleep states in healthy term newborns (at least 39 weeks PMA). One experimental study found normal EEG continuity to correlate with measures of cortical sulcation and surface and longer periods of inactivity in the first day of life to relate to less cortical surface and lower total brain volume.
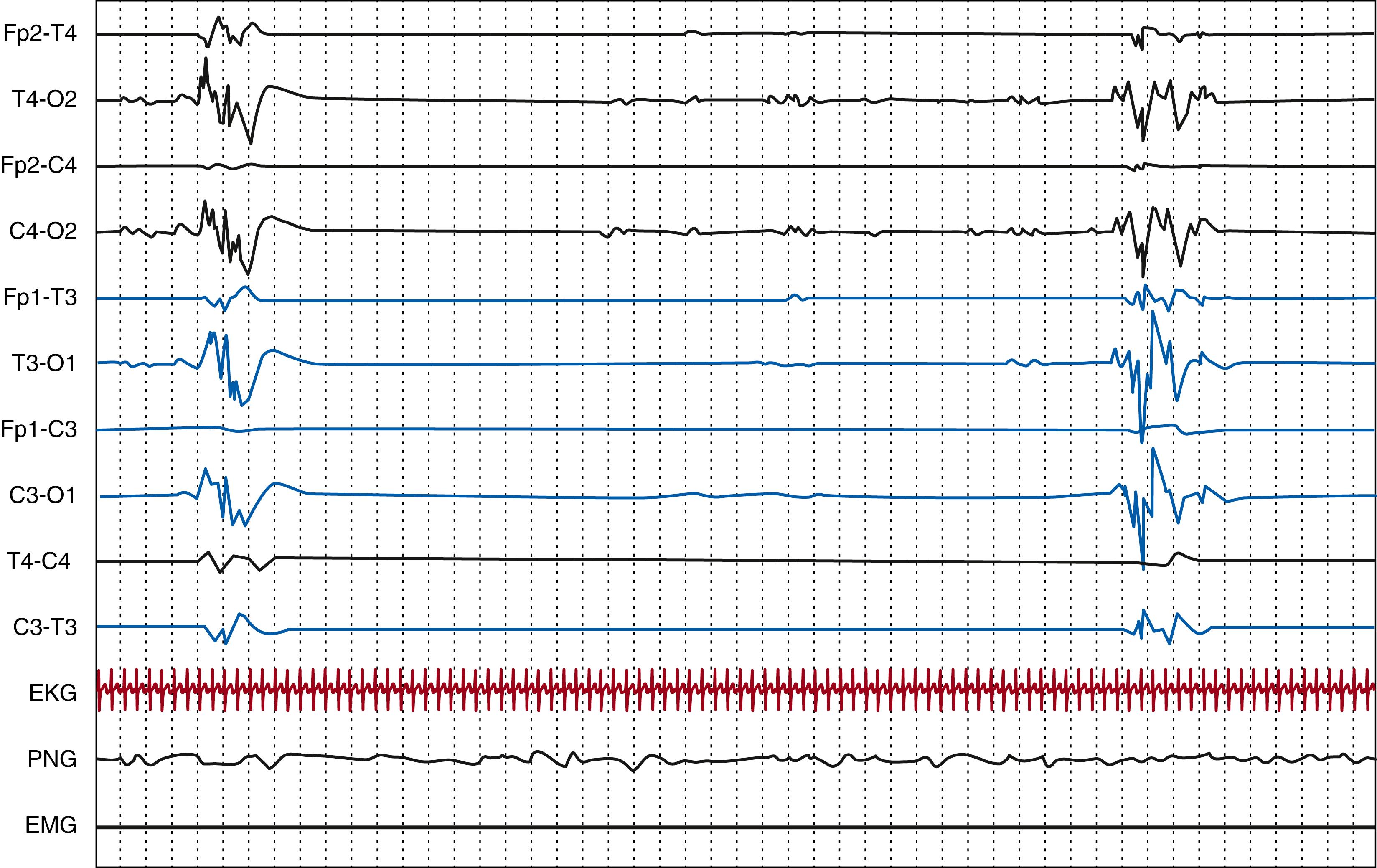
In the normal neonatal EEG in term infants, amplitude, frequencies, and distribution of transients should be equally represented on both hemispheres. In other words, the EEG should be symmetrical. However, in premature newborns, asymmetry is not considered pathologic unless it exceeds 50% over one hemisphere and is persistent.
Synchrony, defined as the onset of bursts of activity that occur nearly simultaneously between hemispheres, changes with PMA in a nonlinear fashion, being almost complete for high-amplitude bursts up to 28-weeks PMA, when it transiently decreases until approximately 30 weeks PMA and finally gradually increases to become almost complete at term. , From 35 to 43 weeks PMA, asymmetry, asynchrony, and transient flattening, frequently unilateral, can be seen at the onset of quiet sleep with no pathologic implications. One proposed operational definition implies a delay longer than 1.5 seconds between bursts of identical waveforms.
Physiologic features, which are the main element determining spatial organization, will be described separately.
The evolution of EEG patterns in the immature brain is the expression of ongoing cornerstone maturational processes. In fact, during early phases of development, the increase in the complexity of EEG waveforms parallels that of gyration, whereas spatial synchrony is related to the growth in thalamocortical and corticocortical connections. Early electrical activity in neuronal networks has a fundamental role in trophism and guidance for activity-dependent neuronal wiring. , Through experimental models, it has been possible to demonstrate that spontaneous and intermittent (“discontinuous”) activity is highly conserved during maturation in different species and is already present before sensory mechanisms become active. , Furthermore, the development of a more continuous tracing is temporally linked to the maturation of γ-aminobutyric acid (GABA) receptors toward an inhibitory function. ,
The background EEG activity of the term newborn is characterized by a continuous tracing, corresponding to activité moyenne in wakefulness, to the “mixed frequency tracing” during AS 1, and to the “low-voltage irregular pattern” in AS 2. Activité moyenne consists of irregular delta-theta activity, generally with amplitudes of 25 to 50 μV (but up to 100 μV), being higher over the posterior regions. Rhythmic, mainly theta, activity is usually superimposed, especially over the central regions, whereas irregular alpha and beta rhythms up to 30 μV are superimposed over all areas. , During “low-voltage irregular pattern,” EEG is dominated by low-voltage fast theta over all scalp regions, together with slower components.
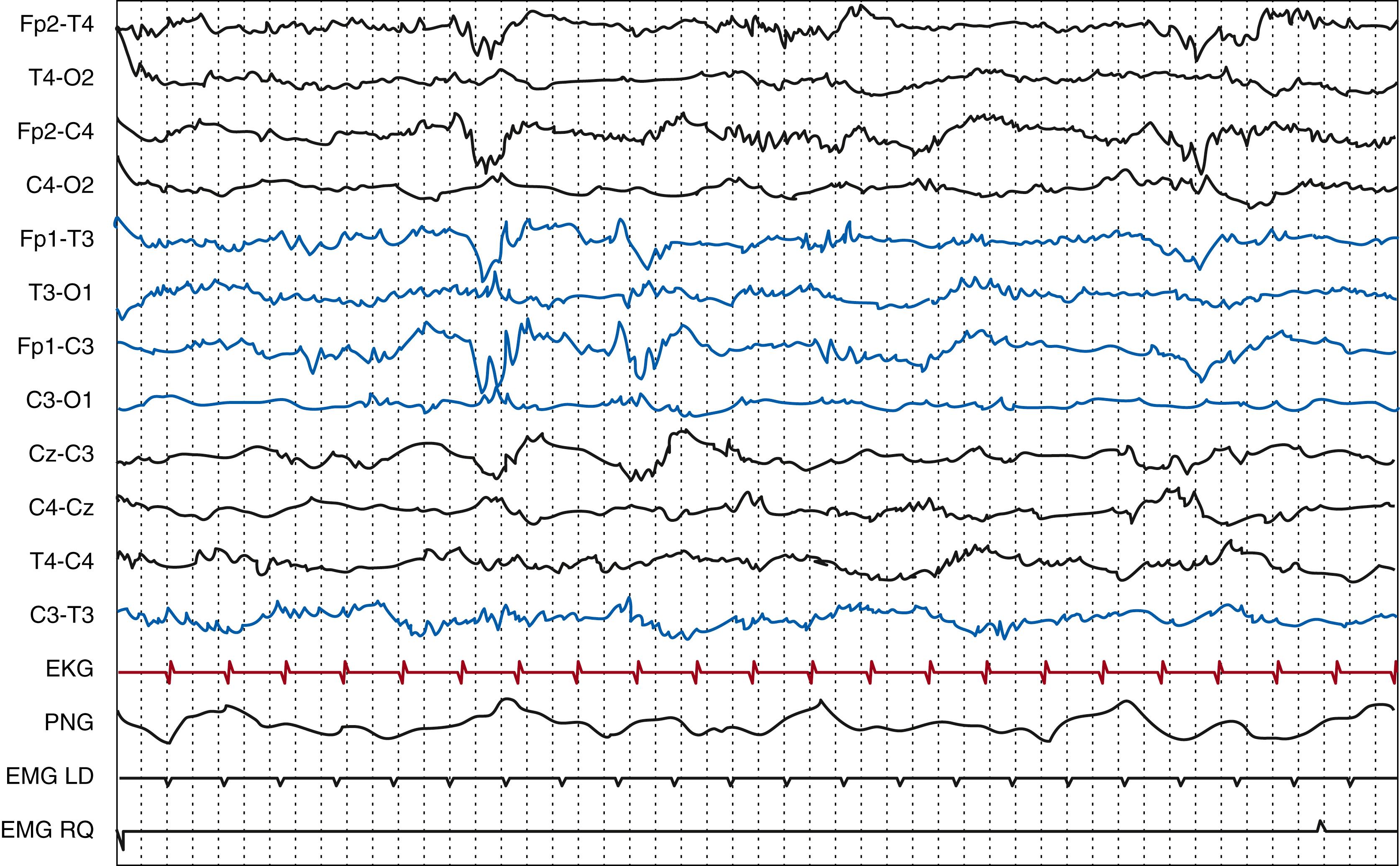
During quiet sleep, the EEG of the term infant can either show a tracé alternant (alternating tracing) or to “high-voltage slow” patterns. The former usually predominates between 37 weeks PMA, when it first appears, and 44 weeks PMA, and is characterized by bilateral synchronous and symmetrical bursts of delta waves of 50 to 150 μV over a continuous theta activity of 25 to 50 μV, in which the two approximately show the same duration, from 3 to 8 seconds ( Fig. 130.5 ). This pattern is reactive to stimuli and alternates with the continuous pattern of active sleep. It can be distinguished from the pathologic BS pattern by the presence of HVS wave activity alternating with periods of low voltage, which is higher compared with the very low, suppressed, interburst activity of the BS pattern. The HVS pattern (or tracé lent continu ) first appears at 38 weeks PMA and is characterized by a continuous delta activity predominating over occipital areas with an amplitude between 50 and 150 μV ( Fig. 130.6 ).
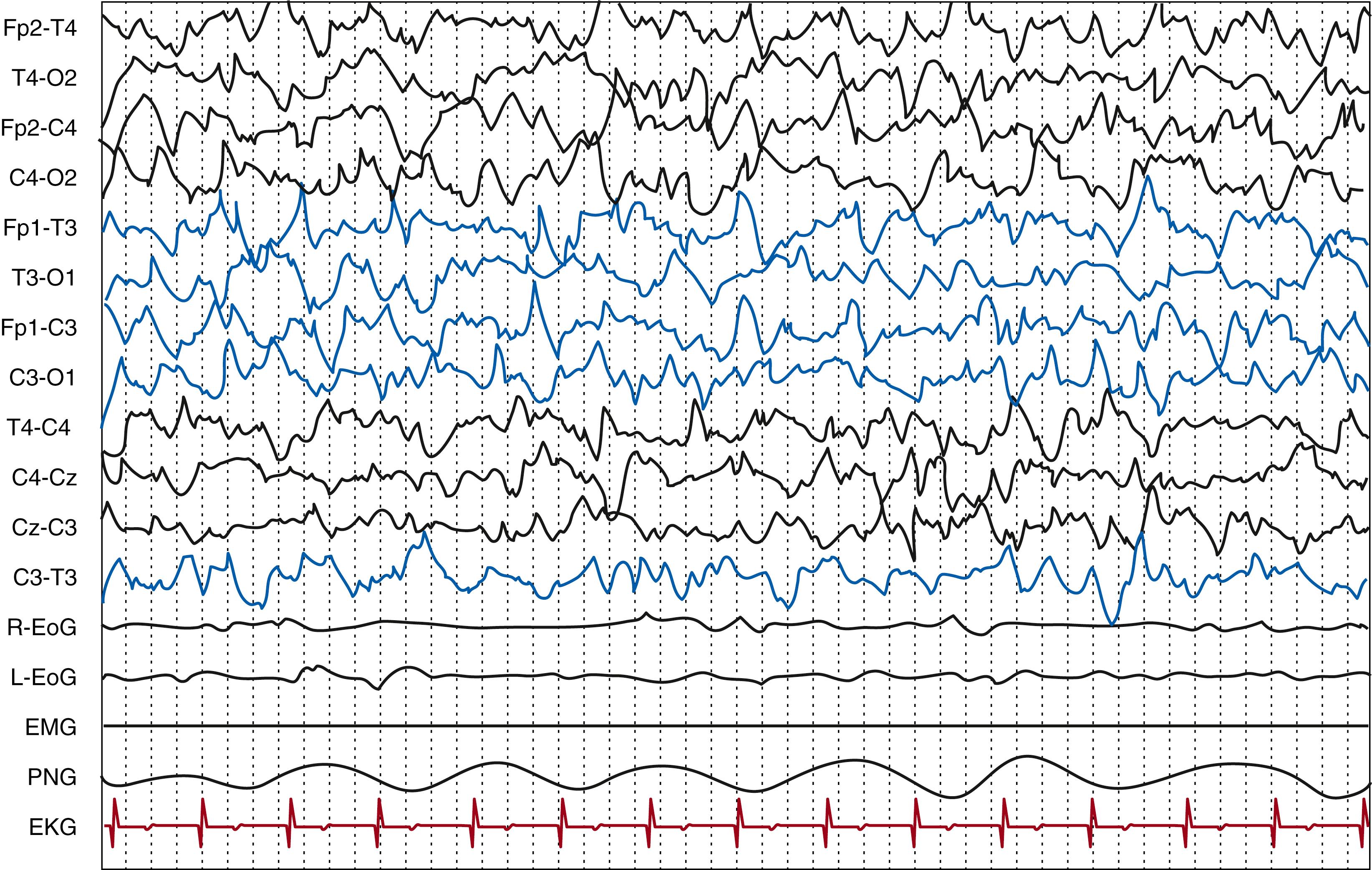
The EEG undergoes relevant changes in preterm babies, with gradual transition from a discontinuous to a more continuous tracing, a decrease in amplitude of ongoing activities, and a reduction in the total amount of delta waves with the appearance of a polyrhythmic pattern. With advancing PMA, the percentage of time showing discontinuous background patterns significantly diminishes. Hayakawa and colleagues found a mean percentage of discontinuous activity of 69% at 21 to 22 weeks PMA and of 48.4% at 25 to 26 weeks PMA. At 32 weeks PMA, discontinuous activity corresponds to 50% and by approximately 35 weeks PMA the EEG is continuous during the awake state. This increase in the length of periods of activity and reduction in the length of periods of inactivity can be seen in relation to a maturation in the excitatory synapses; a reduced drive from subcortical areas responsible for the abrupt bihemispheric inhibition of cortical activity is also related. This is also considered to play a prominent role in the high synchrony of the bursts before 30 weeks PMA, when it constitutes an important maturational criterion. , It has been shown that neuronal networks seem to express a different development in posterior and frontal areas, as frontally prominent networks emerge only in term newborns.
Discontinuous tracing, which is the hallmark of preterm EEG, is defined as an EEG pattern of bursts of physiologic activity appropriate for the gestational age separated by intervals of variably defined amplitudes (<25 μV 10 or 10 μV 66 ), with a duration of more than 3 seconds. The duration of both the bursts and the intervals of lower amplitude vary according to gestational age; they not appear to be consistent in the different studies. There is a growing interest in understanding early neuronal activity functioning, which is profoundly different from what is found in the mature brain. , , In a study of preterm infants of less than 30 weeks PMA, the maximum duration of IBI significantly correlated with the degree of cortical folding.
Although EEG features in near-term neonates are better known, it is difficult to describe normal features in extremely preterm neonates due to the poor concordance between studies regarding the amplitude of bursts and duration of IBI, the number of electrodes on which activity must be present, and the limited number of healthy subjects without brain injury or neurotropic medications.
Among the most precocious recordings reported was that of Hayakawa and coworkers, who described the characteristics of EEGs undertaken at 21 to 22 weeks PMA and found a mean and a maximum IBI duration of 25.8 and 18.4 seconds, respectively (with wide ranges), and a mean burst duration of 4.3 seconds. However, the whole sample size was small and the differences between the group of preterm infants of 21 to 22 weeks and preterms of 23 to 24 weeks PMA were not statistically significant, although there were statistical differences when those were compared with preterm infants aged 25 to 26 weeks PMA.
Between 24 and 27 weeks PMA, bursts constitute close to 35% of the recording. At 28 to 29 weeks, longer reported bursts can last up to 10 minutes. IBIs represent approximately 45% of the recording at 24 to 27 weeks, and the cutoff for the longest discontinuity considered physiologic is 60 seconds or less at 24 weeks, and it decreases to 20 seconds or less at 30 weeks.
In younger newborns, amplitudes are higher (100 to 300 μV) and frequencies are lower (0.5 to 1 Hz), with some superimposed faster rhythms of 5 to 9 Hz. Maturation mainly consists of a progressive displacement of delta waves to the central-occipital areas. Those are initially mainly synchronous and smooth, then increasingly superimposed by fast activities. Occipital delta waves are recorded as soon as 25 weeks PMA, whereas frontal delta waves are the least frequent in this extremely low PMA. Rudimentary REM and non-REM sleep states have been identified as early as 25 weeks PMA, provided that concordance among EEG, eye movements, and body movements is not consistent: rudimentary REM sleep is associated with a high-voltage more continuous EEG activity, whereas non-REM sleep is characterized by more discontinuous epochs of recordings. ,
Theta bursts are initially diffuse, then predominate over the occipital and then temporal areas. Two different types of theta bursts have been recognized between 26 and 28 weeks CA: high or very high rhythmic and sharp waves over the temporal areas and lower amplitude bursts predominantly located over the occipital regions, also known as sharp theta rhythm on the occipital areas of prematures (STOP) . , The maturation of theta rhythms from 26 to 28 weeks PMA seems to reflect the posterior-anterior gradient in cerebral maturation that has been documented with magnetic resonance imaging (MRI). Temporal theta rhythms are mainly recorded during sleep. ,
From 24 to 27 weeks PMA, temporal slow waves occur either isolated or in short sequences, more often unilaterally, whereas occipital and central slow waves appear monophasic, smooth, or with superimposed fast rhythms ( Fig. 130.7 ). Frontal slow waves are also present at this stage, although less abundant, either with a sharp and rapid morphology or slower and smoother. At 24 to 25 weeks, short bursts of synchronous HVS waves are also observed, disappearing before 28 weeks. At 28 to 29 weeks, the temporal slow waves are of intermediate amplitude, whereas the occipital ones show the highest voltages and the lowest frequency, and there are abundant central slow waves of low amplitude and duration of greater than 1 second. Waves of higher frequency are also superimposed.
At 30 weeks PMA, bursts of delta waves have slightly lower amplitude and are more frequent over the occipital area. They can show a superimposed fast activity with the morphology of delta brushes, which, at younger PMAs, are called spindle-like fast rhythms and can be recorded at as soon as 27 weeks. Bursts of theta rhythm, either diffuse or predominant over the temporal areas, can be recorded from 24 weeks, become more abundant at 26 to 27 weeks, and appear more localized over the temporal and occipital areas. At 30 weeks, these theta bursts are observed mainly during quiet sleep and over the temporal areas. Delta waves, mainly synchronous, with superimposed theta, are predominant in the occipital-temporal areas, especially during active sleep, whereas theta activity predominates over the temporal areas, mainly in quiet sleep. Between 32 and 34 weeks, delta brushes are most abundant, and temporal theta activity completely declines ( Fig. 130.8 ). Physiologic duration of IBI corresponds to 15 seconds or less at 32 weeks and 10 seconds or less at 34 weeks CA. At 36 weeks, it is possible to differentiate between AS 1 and AS 2, whereas quiet sleep appears discontinuous or, according to some authors, semi-discontinuous, with periods of low amplitude activity lasting less than 10 seconds.
Important developmental features of neonatal EEG are summarized in Tables 130.1 and 130.2 and are described in the section below.
| Parameter | Postmenstrual Age | |||||||
|---|---|---|---|---|---|---|---|---|
| 24–25 wk | 26–27 wk | 28–29 wk | 30–31 wk | 32–34 wk | 35–36 wk | 37–38 wk | 39–41 wk | |
| Organization and Behavioral States | Activity and rest Association EEG-behavior: inconsistent |
AW ASQS Inconsistent |
AW ASQS Inconsistent |
AW ASQS Rare QW Concordance between EEG and behavioral criteria |
AW ASQS Rare QW |
AW QW QS AS (from 36 wk CA: AS 1 AS 2) |
AW QW QS AS 1, AS 2 |
AW QW QS AS 1, AS 2 |
| Background Activity | Very discontinuous | Very discontinuous Brief semicontinuous periods |
Discontinuous Very variable IBI (≤30 s), longer periods of continuity |
AS: continuous/semidiscontinuous QS: discontinuous (bursts ≥3 s; IBI ≤20 s) |
AW: continuous (frequent artefacts) QW: continuous AS: continuous QW: discontinuous (IBI ≤15 s at 32 and ≤10 s at 34 wk CA) |
AW, QW: activité moyenne (36 wk CA) AS 1: high amplitude continuous AS 2: continuous, lower amplitude, > rapid activity QS: discontinuous/semidiscontinuous IBI <10 s |
AW, QW, AS 2: activité moyenne AS 1: high amplitude mixed frequency QS: semidiscontinuous; TA |
AW, QW, AS 2: activité moyenne AS 1: mixed frequency tracing QS: TA and/or slow continuous tracing |
| Lability | Present | Present | Present | Present | Present | Present | Present | Present |
| Reactivity | Not visible on the EEG Painful stimuli → limb withdrawal |
Barely visible with tactile stimulations | Inconstant, variable | AS: decrease in amplitude QS: transiently continuous activity |
AS: diffuse decrease in EEG activity QS: transient appearance of continuous slow activity |
AS: diffuse transient decrease in background activity QS: continuous high amplitude slow waves |
AS: general transient decrease QS: lengthening of slow wave bursts or IBI |
Auditory, tactile, and painful stimuli → attenuation of background activity |
| Parameter | Postmenstrual Age | |||||||
|---|---|---|---|---|---|---|---|---|
| 24–25 wk CA | 26–27 wk CA | 28–29 wk CA | 30–31 wk CA | 32–34 wk CA | 35–36 wk CA | 37–38 wk CA | 39–41 wk CA | |
| PMA-Dependent Graphoelements | Mono/diphasic delta, >smooth Sharp theta bursts |
Diphasic delta (smooth/theta-alpha superimposed) Sharp theta waves > in bursts (+) |
High amplitude mono/diphasic delta Theta waves in isolated bursts (++) Delta brushes (+) |
Delta waves with superimposed theta ↑ Theta activity Diffuse delta brushes 31 > 30 wk CA (++) |
Temporal theta disappears in AS at 32 and in QS at 33–34 wk CA Delta brushes (+++) Immature frontal transients at 34 wk (>smooth, incomplete, asymmetric) |
Diffuse theta waves (AS 2 > AS 1) Delta brushes (AS 1 > AS 2, QS) Immature frontal transients; more mature at the onset of QS ASD in AS 1 |
Delta waves (> in AS 2) Delta brushes in all behavioral states Clear frontal transients (AS 1; QS onset) ASD in AS 1 |
Delta brushes only in QS Frontal transients and ASD at 37–38 wk CA Short runs of alpha and delta over rolandic areas |
| Spatial and Temporal Organization | T slow waves: isolated/in sequences O and C slow waves: bilaterally synchronous/unilateral Bursts of sharp theta: diffuse/>T |
Delta waves (> in central areas) High amplitude delta bursts (>synchronous) over occipital areas Theta waves (temporal > diffuse) |
↓ Diffuse delta waves, posterior predominance in AS and QS ↓ Synchrony (persisting over occipital areas) ↓↓ Temporal delta bursts Abundant central delta waves Synchronized diffuse bursts of theta rhythms (> T and O) |
Delta waves with theta >O/O-T Delta mainly synchronous, > in AS Theta (>T) > in QS |
Delta brushes: O-T at 32, O at 33–34 wk CA | Delta waves > O in AS, diffuse in QS Constantly synchrony in AS Possible asynchrony at the onset of QS |
Delta brushes > O in AS and QS Interhemispheric synchrony ↑ Theta waves over C areas in AS 1 and AS 2 |
Constant symmetry and synchrony (possible asynchrony at the onset of QS) Slow continuous tracing precedes TA in a single QS period |
In healthy preterm newborns, the posterior regions are dominated by runs of HVS bioccipital monorhythmic positive polarity delta activity, lasting 2 to 60 seconds and often being synchronous on the two hemispheres. While they are present at earlier stages, they show a pick between 30 and 33 weeks, decreasing by 34 weeks of age. , , It is a highly conservative rhythm, usually still recordable even in the context of severe encephalopathies.
This is constituted by sustained trains of semirhythmic delta activity, recorded in the extremely preterm infant, and fading by 34 weeks CA.
Diphasic (small negative deflection followed by a positive deflection), usually synchronous, they first appear at 35 weeks CA, although immature broad, incomplete, and asymmetrical sharp transients may be seen from 33 weeks CA and disappear after 44 weeks CA. They are often present in transitional phases, especially from active to quiet sleep. ,
It is represented by monomorphic/polymorphic delta waves over the frontal areas in short runs of a few seconds, appearing at 36 to 37 weeks CA, typically synchronous and symmetric. They tend to markedly decline during wakefulness in the first week in term newborns but persist in sleep throughout the first month.
Delta waves (50 to 200 μV, 0.3 to 1 Hz) with superimposed fast activity (8 to 30 Hz, 20 to 200 μV) are first observed at 28 weeks CA, have a maximum expression at 32 to 34 weeks CA (after which it decreases), and show progressively reduced amplitude and higher frequencies with maturation as well as an evolution in location from diffuse or localized over the rolandic regions in the very premature, to the temporal and then occipital areas, until they become exclusively occipital at 36 weeks CA. Immature delta brushes (spindle-like fast rhythms) superimposed on slow waves are recorded as soon as 27 weeks CA and are predominantly localized on the occipital and central areas ( Fig. 130.9 ). They disappear first in wakefulness and active sleep, and then in quiet sleep. Their persistence in the term newborn, a persistently focal or hemispheric location, or a unilateral attenuation in the preterm infant are abnormal and might reflect structural lesions. , From a physiologic point of view, delta brushes are thought to play an important role in the development of thalamocortical connections. , In the immature rat, spindle bursts, considered homologous to delta brushes, are abolished after removal of subplate neurons, resulting in reduced thalamocortical connections. Spindle bursts and delta brushes can be spontaneous or triggered by sensory stimuli. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here