Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neonatal encephalopathy is a clinical syndrome that can result from a variety of underlying causes.
Hypoxic-ischemic encephalopathy (HIE) is a major cause of neonatal brain injury and mortality worldwide.
Magnetic resonance imaging and magnetic resonance spectroscopy are useful imaging tools to identify brain injury and help predict long-term neurodevelopmental outcomes in neonates with HIE.
Cerebral arterial or venous thrombosis may be the cause of unexplained seizures.
Infections acquired in utero can present with neonatal encephalopathy.
Metabolic disorders, genetic conditions, and brain malformations can present as neonatal encephalopathy.
Neonatal encephalopathy describes a clinical syndrome unique to the term and near-term newborn in the immediate perinatal period. The clinical findings may include seizures and are characterized by an impairment of one or more components of the central nervous system (CNS) including mental status, tone, primitive reflexes, autonomic functions including respiration, as well as abnormal feeding. The causes of neonatal encephalopathy are diverse and include infections, stroke, genetic conditions, metabolic disorders, and brain malformations ( Table 55.1 ). The incidence of neonatal encephalopathy in high-income countries ranges from 2 to 6:1000 term live births with a combined average of 3.9:1000; the exact incidence, however, is difficult to determine due to the heterogeneous nature of the presenting clinical picture and might be much higher than currently described in the literature. Factors associated with neonatal encephalopathy are plentiful and can be divided into maternal, utero-placental, and fetal in origin ( Table 55.2 ).
|
|
|
|
|
| Maternal |
|
| Uteroplacental |
|
| Intrapartum Sentinel Event |
|
| Fetal |
|
Recent placental pathology studies have given insight into pathophysiologic changes associated with neonatal encephalopathy. Commonly observed findings in placentas from newborns with neonatal encephalopathy can be acute or chronic, and include infection, inflammatory changes, and vasculopathies, particularly those altering fetal vascular perfusion. It is understood that findings consistent with fetal vascular malperfusion take at least 48 hours to evolve and therefore can be an indicator of the acuity of neonatal encephalopathy. Changes consistent with global fetal vascular malperfusion, including avascular villi and fetal thrombotic vasculopathy (FTV), occurred in 20–24% of cases diagnosed with neonatal encephalopathy, 3 to 4× higher than in control newborns. The severity of clinical encephalopathy can be linked to the severity of fetal vascular placental changes. In some series, thrombosed placental vessels and fetal vascular malperfusion have been demonstrated in up to 50% of cases with perinatal arterial-ischemic stroke. The impact of placental inflammatory changes is less clear. Those affecting the fetal side of the placenta, histologically known as funisitis, are six times higher in neonates with encephalopathy than in healthy term newborns (31.4% vs. 4.4–5.4%); however, alterations affecting the maternal side, also referred to as chorioamnionitis, are only twice as common. Based on these observations, abnormal fetal vascular perfusion and inflammatory changes can be associated with neonatal encephalopathy.
Birth asphyxia is one of the leading causes of neonatal mortality worldwide, accounting for an estimated 20–30% of neonatal deaths. Hypoxic-ischemic encephalopathy (HIE) accounts for an estimated 25–45% of neonatal encephalopathy and is the most common form of acute brain injury in newborns. The incidence of HIE is approximately 1–3:1000 term/near-term live births in high-resource countries and as high as 31:1000 live births in low-resource settings.
Motor disabilities and long-term neurodevelopmental impairment (NDI) including cognitive, neuropsychological, epilepsy, educational, and behavioral problems are common in surviving neonates with moderate or severe HIE. Despite advances in therapeutic intervention, 31–55% of neonates with moderate or severe HIE still experience significant sequelae and either don’t survive the neonatal period (17–38%) or suffer from significant neurodevelopmental impairment (14–35%), including intellectual disabilities, cerebral palsy (13–32%), and seizures. Brain injury secondary to hypoxia-ischemia during labor can be seen in up 24% of term-born children with cerebral palsy. The emotional impact and costs associated with medical and rehabilitative care of infants with HIE are substantial. The Centers for Disease Control and Prevention estimated the lifetime costs for all people with cerebral palsy born in 2000 to be US $14.7 billion, which equals approximately US $1.2 million per person with cerebral palsy.
HIE results from a significant intrapartum hypoxic-ischemic sentinel event (acute or prolonged). Factors contributing to such events are plentiful and can be divided into maternal, utero-placental, and fetal in origin (see Table 55.2 ). Maternal and utero-placental causes are often sudden and severe and are estimated to occur in one-third of HIE cases.
Factors that increase the risk of a sentinel event include maternal medical conditions associated with impaired placental perfusion such as chronic hypertension, diabetes, autoimmune diseases, preeclampsia, and alterations originating from within the placenta leading to inflammatory changes, such as chorioamnionitis, villitis, vasculitis, and funisitis. Intrapartum clinical inflammatory factors, such as maternal fever, chorioamnionitis, and prolonged rupture of membranes, may also increase the risk and severity of HIE.
Regardless of the cause, significant hypoxia-ischemia can lead to cardiac and vascular compromise, with subsequent diminished cerebral perfusion and oxidative metabolism, energy failure, cell death, and depending on duration and extent, may result in hypoxic-ischemic brain injury.
The pathophysiologic changes after an acute hypoxic-ischemic event follow characteristic phases defined as primary injury, latent, secondary, and tertiary phases ( Fig. 55.1 ). The primary injury phase is characterized by decreased cerebral oxygen and glucose delivery as the immediate result of compromised cerebral perfusion. Glycolysis in an oxygen-poor environment leads to depletion of adenosine triphosphate (ATP), and accumulation of lactic acid. Cellular mechanisms dependent on ATP, such as Na + /K + ATPase pumps, can no longer maintain their functions. This results in depolarization of neuronal cell membranes with a subsequent increase in intracellular sodium and calcium concentration, which generates osmotic and electrochemical gradients across the cell membrane and leads to cytotoxic edema. Induction of destructive enzymes (e.g., phospholipases, proteases, and endonucleases) also occurs. Activated phospholipases (e.g., phospholipase A2) hydrolyze cellular membrane phospholipids and release free fatty acids such as arachidonic acid, which can further increase glutamate release, perpetuate membrane peroxidation, and activate N-methyl-D-aspartate (NMDA) receptors, which results in a further increase in intracellular calcium. Abnormally high intracellular calcium concentrations have toxic effects on intracellular organelles, particularly the mitochondria, and stimulate the activation of nitric oxide synthase resulting in increased production of reactive oxygen species (ROS), including nitric oxide (NO). NO damages the mitochondrial membrane leading to devastating mitochondrial dysfunction. The consequences for cellular health and metabolism are significant and include the induction of necrotic and apoptotic pathways as well as stimulation of the inflammatory cascade.
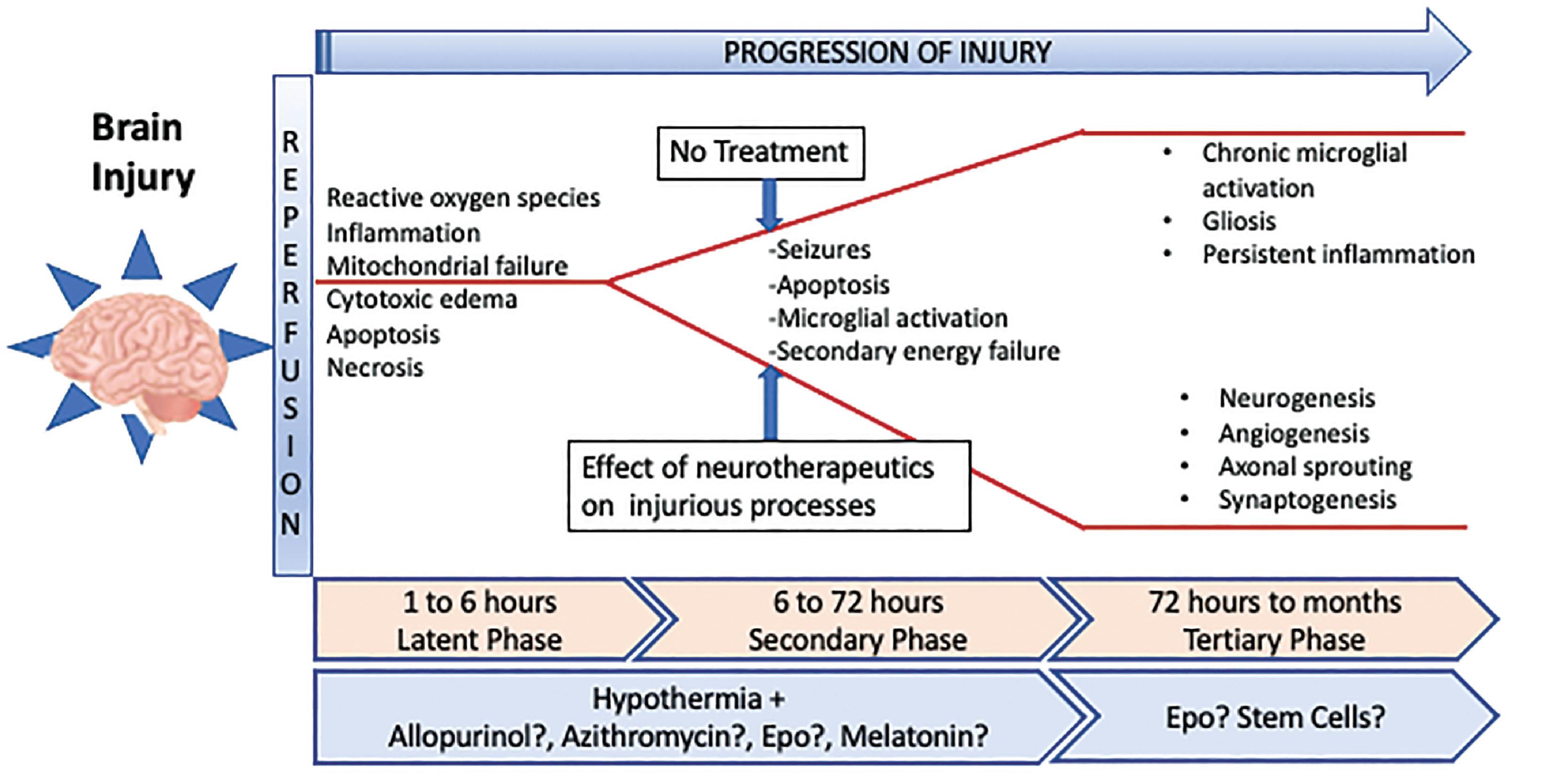
The next phase of injury, referred to as the latent phase, is characterized by reperfusion with active suppression of cerebral metabolism. A partial rebound of neuronal oxidative energy metabolism occurs by meeting a decreased demand, leading to improvement of cytotoxic edema, and increased cerebral tissue oxygenation. While the neurotoxic cascade is suppressed during this stage, increased perfusion and oxygenation continue to contribute to additional ROS production. This phase occurs between 1 and 6 hours after insult and is the optimal window in which to initiate therapeutic hypothermia.
The following, secondary phase, is characterized by secondary energy failure, ongoing inflammation, oxidative injury, and excitotoxicity. This phase may be observed as early as 6 hours after the initial insult and typically lasts for multiple days. While some repletion of energy sources takes place during the latent phase, rapid depletion of these resources occurs again during the secondary phase leading to cytotoxic edema, mitochondrial failure, and accumulation of excitotoxins and cytokines, ultimately resulting in delayed cell death. Ongoing injury during secondary energy failure is thought to be directly related to the extent of primary energy failure, partially mediated by neuroglial activation after early neuronal cell death. This leads to the release of proinflammatory cytokines and ROS, which attracts peripheral immune cells that can enter the parenchyma via the disrupted blood-brain barrier and facilitate cell death. The degree of secondary energy failure and degree of cellular energy deficit determines the extent and pathway of neuronal death, for example, necrosis, apoptosis, and autophagy, all of which occur on a continuum.
The tertiary phase is characterized by reparative and restorative processes. This phase can last weeks to years. The immune response shifts towards the healing of the neuroinflammatory process, with microglia exerting their phagocytic capabilities alongside penetrating peripheral macrophages. Phagocytosis of dead cells triggers the release of anti inflammatory cytokines and the production of neuronal growth factors, an essential component of the remodeling process that includes neurogenesis, angiogenesis, and axonal sprouting.
Newborns who have undergone hypoxic-ischemic compromise often present with encephalopathy, although other organ systems may be affected. Obtaining a careful pregnancy and labor history to identify any acute sentinel events complicating labor and/or delivery such as decreased fetal movement, an abnormal fetal heart rate pattern (bradycardia or category III tracing), or meconium passage prior to delivery may provide important information regarding the timing of the insult.
Following delivery, newborns with HIE commonly present with respiratory failure requiring positive pressure ventilation for a prolonged period of time (≥10 minutes), often needing endotracheal intubation with assisted ventilation. Hypoxia also leads to cardiac and vascular compromise, and newborns with HIE may therefore present with persistent bradycardia or even asystole requiring cardiopulmonary resuscitation including epinephrine administration. The cardio-respiratory compromise is reflected in persistent low Apgar scores (≤5) by 10 minutes of age. Laboratory biomarkers of hypoxia-ischemia include significant fetal acidemia (pH ≤ 7.0 and/or a base deficit of ≥12) and an elevated serum lactate diagnosed from either arterial or venous umbilical cord blood samples, or an arterial or venous sample obtained from the newborn within the first postnatal hour.
At birth, neonates with HIE are commonly depressed and manifest clinical symptoms consistent with neurologic injury. The clinical picture evolves significantly over the first hours after birth. When assessing a depressed neonate for therapeutic intervention, the worst exam between one and six hours of life is the most informative regarding the severity of HIE and eligibility for therapeutic hypothermia. It is, however, important to note that the exam continues to evolve over the first week of life and becomes more predictive for long-term outcomes towards the end of the first week. The Modified Sarnat Exam ( Table 55.3A ) and the Thompson Encephalopathy Score ( Table 55.3B ) are the standardized neurologic assessments used to assess the severity of HIE and eligibility for therapeutic hypothermia. The Modified Sarnat Exam, or alternatively the Thompson Encephalopathy Score, can then be followed sequentially over the first week of life to monitor changes in neurologic status.
| Category | Normal | Mild | Moderate | Severe |
|---|---|---|---|---|
| Level of Consciousness | Normal | Hyperalert or irritable | Lethargic or poorly responsive | Minimal or no responsiveness |
| Spontaneous Activity | Normal | Slightly decreased | Decreased | Absent |
| Posture | Normal | Mild distal flexion | Distal flexion, complete extension | Decerebrate |
| Muscle Tone | Normal | Hypertonic | Hypotonic | Flaccid |
|
|
|
|
|
|
|
|
|
|
| Category | Score 0 | Score 1 | Score 2 | Score 3 |
|---|---|---|---|---|
| Muscle tone | Normal | Hypertonicity | Hypotonicity | Flaccid |
| Level of consciousness | Normal | Hyperalert, stare | Lethargic | Comatose |
| Seizures | None | Infrequent <3/day | Frequent >2/day | N/A |
| Posture | Normal | Fisting, cycling | Strong distal flexion | Decerebrate |
| Moro reflex | Normal | Partial | Absent | N/A |
| Grasp reflex | Normal | Poor | Absent | N/A |
| Suck reflex | Normal | Poor | Absent ± bites | N/A |
| Respirations | Normal | Hyperventilation | Brief apnea | Apnea, ventilated |
| Fontanel | Normal | Full, not tense | Tense | N/A |
Neonates with mild encephalopathy appear hyperalert and irritable with wide-open eyes, often with a “stunned look” or a blank stare, and dilated pupils. An increased muscle tone and a heightened Moro reflex reactive to tactile and sensory stimuli are observed. Neonates with moderate encephalopathy appear lethargic with low tone, a weak suck, constricted pupils, and a decreased Moro reflex. Neonates with severe encephalopathy appear stuporous, flaccid and have decerebrate posture, absent reflexes (suck, gag, and Moro), and poorly reactive pupils. Respiratory disturbances are also common with moderate and severe HIE, with periodic breathing and apnea present in most affected neonates.
HIE is the most common cause of seizures in the neonatal period. Seizures are often subclinical or subtle in presentation, manifesting as apneic events, vital sign instability, or less commonly, with stereotypic movements such as abnormal eye movements (e.g., tonic, horizontal eye deviation with or without jerking, eyelid blinking), oral-buccal-lingual movements (e.g., sucking or lip smacking), or limb movements (e.g., finger flicking, swimming motions or bicycle pedaling). Seizures occur in 56–88% of patients with HIE, are subclinical in 43–67%, and progress to status epilepticus in 23–67%.
Other organ system involvement is commonly seen in neonates with HIE including cardiac dysfunction, respiratory failure, acute kidney injury, abnormal glucose homeostasis, syndrome of inappropriate antidiuretic hormone secretion (SIADH), liver dysfunction, and disseminated intravascular coagulopathy (DIC).
Laboratory tests and ancillary studies are pertinent in assessing and treating newborns with suspected HIE, particularly since multisystem organ involvement is common. Metabolic derangements such as hypoglycemia, hypocalcemia, hyponatremia, hypomagnesemia, hypoxemia, decreased Pco 2 and metabolic acidosis are frequently seen in the first 24 hours after birth. Prompt corrective intervention may avoid further compromise. Serum lactate is often significantly elevated following hypoxia or ischemia, reflecting the anaerobic metabolism of glucose for energy in the setting of decreased tissue oxygenation. A complete blood count may demonstrate leukopenia or leukocytosis, which may suggest infection, anemia (e.g., in cases of known or suspected abruption), or thrombocytopenia, which may also occur with DIC. Coagulation studies to assess for DIC and to guide clinical management should include prothrombin time (PT), activated partial thromboplastin time (aPTT), international normalized ratio (INR), and fibrinogen level. Liver function may be abnormal and should be assessed by monitoring serum alanine transferase (ALT), aspartate transferase (AST), particularly since many medications given to critically ill newborns are metabolized in the liver. ALT and AST typically don’t reach their peak level until 24 to 48 hours after the insult, hence daily monitoring of liver function is crucial for appropriate medication adjustment. Initial newborn creatinine levels may reflect maternal values, but a rising creatinine, especially in the setting of poor urine output, is consistent with acute kidney injury (AKI) and provides pivotal information for managing renally cleared pharmacologic agents in the critically ill newborn. Myocardial perfusion is frequently compromised in neonates with HIE and can lead to decreased ventricular function and reduced cardiac output in as many as 75–100% of patients with HIE, Electrocardiogram and cardiac enzymes (serum creatine kinase, creatine kinase-MB isoenzyme, and troponin I) are good markers to assess cardiac dysfunction after perinatal asphyxia, and abnormalities are seen in up to 90% of neonates with HIE; those changes are more significant as the severity of HIE increases. However, echocardiography remains the preferred method for assessment of cardiac compromise in this population, allowing for serial examinations and individually targeted treatment. Furthermore, neonates with HIE often have decreased heart rate variability on continuous electrocardiogram, which is associated with the severity of injury and outcome.
Given the high incidence of seizures and frequent lack of clear clinical expression in this high-risk population, the American Clinical Neurophysiology Society recommends that all neonates with HIE undergoing therapeutic hypothermia are monitored with electroencephalogram (EEG) throughout cooling and rewarming, preferentially as continuous video-EEG (cEEG). Alternatively, in centers where cEEG is unavailable, amplitude-integrated EEG (aEEG) may be used.
Prompt recognition and treatment of HIE-related seizures are indicated, as increased seizure burden is associated with increased brain injury on magnetic resonance imaging (MRI) and worse long-term outcomes independent of the degree of HIE. Moreover, acute symptomatic seizures become more difficult to treat the longer they last, which may lead to increased exposure to antiepileptic medications and associated side effects such as respiratory depression and hemodynamic compromise, in addition to worsening brain injury. Seizures in neonates with severe HIE tend to be longer in duration and more frequent, resulting in a higher seizure burden compared to neonates with moderate HIE. Therapeutic hypothermia does not affect the incidence of seizures (54% vs. 60%), but does significantly decrease the seizure burden, particularly in neonates with moderate HIE.
Near-infrared spectroscopy (NIRS) allows noninvasive, continuous bedside monitoring of cerebral hemodynamics and oxygenation by measuring the absorption spectra in the near-infrared region of light (700 to 1000 nm) of oxygenated and deoxygenated hemoglobin (Hgb) in the cortical tissue. The calculated percentage ratio of oxygenated Hgb to total Hgb (oxygenated plus deoxygenated Hgb) is commonly referred to as cerebral oxygenation. Regional cerebral blood flow measured by MRI showed a strong correlation with mixed venous saturation values measured by NIRS in neonates with HIE undergoing therapeutic hypothermia. Further observations in neonates with HIE showed that cerebral oxygenation decreases in the first 4 to 6 hours of life and then slowly increases within the first 24 hours. The initial decrease is less pronounced in more severely affected newborns, likely due to a lack of metabolic activity within the severely injured brain. Correlations with injury on MRI imaging and long-term outcome have shown that neonates who continue to have increased cerebral tissue oxygenation after 24 hours have more significant injury on MRI and less favorable neurodevelopmental outcome at 2 years.
Cranial ultrasonography (CUS) can be used as a first-line imaging tool in newborns with HIE since it can be performed at the bedside in critically unstable neonates. However, extra-axial structures are poorly visualized, and the sensitivity and specificity of CUS findings in HIE are low and correlate poorly with long-term outcomes. White matter echogenicity detected by early CUS suggests antenatal events rather than acute injury at the time of birth. The accuracy of CUS increases towards the middle and end of the first week of life. Significant cerebral edema may be demonstrated as increased focal or diffuse brain parenchyma echogenicity, slit-like ventricles, and obliteration of the extracerebral cerebrospinal fluid (CSF) spaces and the interhemispheric fissure. Cerebral edema can be seen secondary to HIE, metabolic encephalopathies, seizures, or infections, among other causes. While signs of HIE may be difficult to detect with CUS, this imaging modality may provide preliminary information about brain malformations or other potential causes of neonatal encephalopathy that may clinically resemble HIE. Although CUS is a valuable tool to assess neonates with suspected brain injury, MRI is the study of choice in the diagnostic assessment of neonates with HIE.
In encephalopathic newborns, MRI done at 4 to 5 days after birth, and following rewarming from therapeutic hypothermia, can be used to assess for injury patterns consistent with HIE and rule out other causes, including neurogenetic, neurovascular, or inflammatory diseases. A combination of conventional imaging sequences (T1- and T2-weighted images), diffusion-weighted imaging (DWI), diffusion tensor imaging, plus magnetic resonance spectroscopy (MRS) can provide information on brain microstructure, connectivity, and metabolism and can assist with determining the timing and severity of brain injury in newborns with HIE. MRI performed in the newborn period has a high predictive value for subsequent neurologic impairment at 18 months of age. However, the timing is important, and MRI should ideally be performed on postnatal days 4 to 6. DWI, which measures the random Brownian motion of water molecules within a voxel of tissue, can identify cytotoxic edema and ischemic brain neonatal tissue days before T1 and T2 sequences. Pseudo normalization of the brain MRI occurs after the first week and may underestimate the final extent of basal ganglia and thalamic lesions. MRS is a tool to assess brain metabolism and is usually focused on a region of interest most commonly the basal ganglia. Elevated lactate peaks on MRS have been associated with poor long-term outcome, while N-acetyl aspartate (NAA), a marker of neuronal viability, is commonly decreased when neuronal loss is observed. The ratio of lactate to NAA can be helpful in estimating the severity of injury and predict long-term outcome.
Characteristic patterns of brain injury seen in HIE give valuable information about onset, extent, and evolution of brain injury, and can be predictive of outcome. The neuropathology and pathogenesis of neonatal brain injury associated with HIE have been thoroughly described by Volpe, who summarizes three major patterns of injury based on historic fetal monkey and human studies. The three injury patterns include (1) diffuse, (2) cerebral cortical-deep nuclear, and (3) deep nuclear-brain stem.
A deep nuclear pattern of injury is observed in the case of abrupt onset (within 10 to 46 minutes) of complete asphyxia. An example of such a sentinel event is a cord prolapse. The cerebral cortical-deep nuclear pattern can be observed after partial and prolonged asphyxia. Sentinel events corresponding to injury in the cerebral cortex and deep gray matter (basal ganglia and thalamus) evolve over longer periods, often hours. If this insult is prolonged and/or profound, additional effect on the brain stem can be observed and this injury pattern is referred to as deep nuclear-brainstem pattern .
The parasagittal cerebral injury pattern, also referred to as a watershed predominant pattern , is found after subacute partial and prolonged intrapartum hypoxia and involves the parasagittal white matter, in severe cases also the cortical gray matter.
Computed tomography (CT) lacks the sensitivity to evaluate the characteristics and extent of brain injury in term encephalopathic infants and is therefore rarely used to assess newborns with HIE. An advantage of CT over CUS is that it can detect hemorrhages in areas where CUS is difficult to apply and can visualize the extra-axial regions better. An advantage of CT over MRI used to be the speed at which it can be done. Newer limited-sequence (rapid) MRI protocols are now of similar duration and cost. MRI can detect hemosiderin depositions via susceptibility-weighted images (SWI) and ischemic stroke on DWI more accurately than CT while sparing the developing brain from radiation exposure.
Neonates with HIE are frequently critically ill and demonstrate signs of multiorgan involvement. For that reason, the initial management steps should focus on promoting adequate oxygenation, ventilation, circulation, and correction of metabolic derangements. Myocardial ischemia can result in a low cardiac output state. Cardiovascular instability and hypotension are commonly observed in affected patients. Continuous blood pressure monitoring facilitates prompt recognition of systemic hypotension and timely treatment to avoid additional compromise. Treatment options include inotropic agents as well as support with hydrocortisone. Hydrocortisone may be beneficial given the common compromise of the adrenal glands in HIE patients.
The newborn with HIE oftentimes shows signs of respiratory compromise secondary to the ischemic effects on the lungs, impairment of the central respiratory system, and frequent exposure to meconium-stained amniotic fluid. The resulting clinical symptoms include apnea, periodic breathing, impaired gas exchange, and pulmonary hypertension. Hypoxemia is particularly profound in the initial phase and improves with amelioration of the acidosis. Despite respiratory depression, newborns with HIE are frequently hypocarbic because of respiratory compensation of the metabolic acidosis. This can result in further compromise of the cerebral autoregulation and exacerbation of brain injury. Therefore, tight management of respiratory status and blood chemistry is crucial in this critically ill population.
The fluid and electrolyte status may become imbalanced due to cerebral edema and secondary SIADH. Hypoxic injury to the kidneys can also lead to AKI, which presents with oliguria or anuria, fluid retention, and subsequent electrolyte abnormalities, particularly hyponatremia. When profound, hyponatremia can result in seizures, further exacerbate brain injury, and worsen the cerebral edema. Therefore, fluid and electrolyte management are critical to avoid additional compromise, and significant temporary fluid restriction might be necessary.
Glucose homeostasis is often disrupted in neonates with HIE. Hypoglycemia is an emergency and requires prompt correction as it can exacerbate brain injury and induce seizures.
Seizures can manifest clinically, but more often lack a clinical correlate and can be detected by EEG only. Prompt treatment of the acute symptomatic seizures and correction of electrolyte derangements (hypoglycemia, hyponatremia, and hypocalcemia) is pivotal to avoiding further injury.
Liver injury and DIC are frequently observed in neonates with HIE. DIC warrants monitoring and when associated with bleeding, requires immediate treatment. Liver dysfunction impairs the production of clotting factors and additional vitamin K might be necessary to assist with the production of vitamin K-dependent clotting factors until either the liver injury is recovering or DIC resolves. Abnormal renal and hepatic function have a significant impact on metabolism and clearance of medications commonly used in this critically ill population (e.g., phenobarbital, gentamicin, and furosemide).
Hypothermia is currently the only proven neuroprotective therapy for HIE. Hypothermia decreases cellular metabolism by 5–8%/°C, attenuates the inflammatory reaction, stabilizes the blood-brain barrier, and reduces glutamate and oxygen free radicals. In addition, hypothermia has been shown to have anti-epileptic effects. The beneficial effect of therapeutic hypothermia is most pronounced when applied before the onset of the secondary energy failure, which is approximately within 6 hours of birth, preferably as soon as possible after injury. Applying hypothermia within 6 hours of birth to maintain the core body temperature at 33.5 ± 0.5°C for 72 hours followed by slow rewarming at a rate of 0.5°C/h to normothermia, has been shown in numerous randomized controlled trials to improve outcomes in neonates with moderate to severe HIE.
Mild cooling has an effect on all organ systems, and physiologic changes are commonly seen after the initiation of therapeutic hypothermia ( Table 55.4 ). Alteration in pharmacokinetics, particularly enzyme-dependent metabolism (e.g., cytochrome P450—phenobarbital, fentanyl, midazolam, phenytoin, corticosteroids, and vecuronium) and enzyme facilitated conjugation (e.g., hepatic glucuronidation—morphine), is affected by hypothermia. Therefore, particular attention needs to be given to pharmacotherapy during therapeutic hypothermia.
| Effect | Mechanism | |
|---|---|---|
| Metabolic rate | ↓ 5–8% per 1°C below 37.0°C | Decreased—primary energy conserving mechanism |
| Cerebral blood flow | ↓ 5% per 1°C below 37.0°C | Decreased cardiac output and metabolic rate |
| Heart rate | ↓ 10 bpm per 1°C below 37.0°C | Slowing of conduction through the sinoatrial node |
| Cardiac output | ↓ 7% per 1°C below 37.0°C | Decreased metabolic rate |
| Myocardial contractility | ↑ | Increased myofibrillar sensitivity to calcium |
| Peripheral vascular resistance | ↑ | Peripheral vasoconstriction |
| Pco 2 | ↓ 2 mmHg per 1°C below 37.0°C |
|
| Po 2 | ↓ 5 mmHg per 1°C below 37.0°C | |
| pH | ↑ 0.015 per 1°C below 37.0°C | |
| Alteration of platelet function | Mildly thrombophilic | ↑ Platelet aggregation |
| Medication clearance |
|
Altered CYP450 enzymes and glucuronidation |
In animal studies, decreased blood flow to the intestines was described during hypothermia, in human neonates; however, therapeutic hypothermia has not been associated with an increased risk of necrotizing enterocolitis either with hypothermia alone or in conjunction with initiation of enteral feedings during cooling therapy.
Subcutaneous fat necrosis has been described in neonates undergoing therapeutic hypothermia, particularly on the back and shoulders, with an incidence of 2.8% in the Swiss cooling registry. Strict nursing care protocols and awareness can decrease the frequency.
Studies evaluating a different duration (120 hours vs. 72 hours) and depth (32.0°C vs. 33.5°C) of therapeutic hypothermia or later initiation between 6 and 24 hours of life have not shown benefit but did show potential harm.
Since therapeutic hypothermia is most effective when implemented within 6 hours after delivery, timely referral to a center with a therapeutic hypothermia program is crucial. Passive cooling can be done before active cooling with close monitoring of core temperature. However active cooling during transport is preferred as patients reach the target temperature earlier and are less likely to be over or undercooled.
Centers offering therapeutic hypothermia should have access to cEEG or aEEG, a trained pediatric neurologist, MRI with neuroradiology interpretation, and access to ancillary services such as physical therapy, speech therapy, and high-risk infant follow-up programs as recommended by the American Academy of Pediatrics and the state of California.
The use of medication to provide sedation and prevent shivering in cooled newborns with HIE is controversial and needs to be studied in more detail. The current use of morphine during therapeutic hypothermia is not evidence-based and may not be ideal due to its side effect profile (e.g., respiratory depression, urinary retention, constipation) and because it does not specifically prevent shivering. Dexmedetomidine and clonidine both α 2 -adrenergic receptor agonists, are promising alternative sedatives because they specifically prevent shivering without suppressing respirations. Dexmedetomidine also reduces inflammation, without producing abnormal brain histology, and provides neuroprotection in animal models of HIE.
Prior to therapeutic hypothermia, mortality for infants with moderate to severe HIE surpassed 60% and the disability rate has been described to be as high as 100% in neonates with severe HIE. With the introduction of therapeutic hypothermia, the death and disability rates have improved significantly. A Cochrane metaanalysis included 1505 term and late preterm infants with moderate to severe HIE from 11 randomized controlled trials and concluded that therapeutic hypothermia results in fewer deaths (25% compared to 34%, risk ratio [RR] of 0.75; 95% CI 0.65–0.88 and better neurodevelopmental outcomes in survivors. For infants with moderate to severe HIE, outcomes at 18 to 24 months (assessed in seven and eight studies in a Cochrane review ) and at 6 to 7 years (assessed in two major studies, the NICHD and the TOBY-Xe trial ) are summarized in Table 55.5 . The number needed to treat (NNT) to prevent death or moderate to severe neurodevelopmental disability in one newborn is 7 (95% CI 5–10). Based on the proven benefits, therapeutic hypothermia is now standard of care for term and near-term newborns with moderate to severe HIE in high-resource environments.
| Outcome | 18–24 MONTHS | 6–7 YEARS | ||||
|---|---|---|---|---|---|---|
| Hypothermia | Control | RR (95% CI) | Hypothermia | Control | RR (95% CI) | |
|
27% | 37% | 0.73 (0.61–0.89) | 29% | 35% | 0.81 (0.63–1.04) |
|
14% | 23% | 0.60 (0.41–0.88) | NA | NA | NA |
|
53% | 68% | 0.77 (0.64–0.93) | NA | NA | NA |
|
26% | 39% | 0.67 (0.67–0.80) | 29% | 49% | 0.60 (0.44–0.81) |
|
27% | 39% | 0.67 (0.5–0.90) | NA | NA | NA |
|
37% | 51% | 0.75 (0.50–1.12) | NA | NA | NA |
| Cerebral palsy | 23% | 35% | 0.66 (0.54–0.82) | 20% | 33% | 0.59 (0.40–0.87) |
Of course, outcomes vary by severity of presenting HIE. Mild HIE has now been recognized as causing significant brain injury in 18–38% of affected infants on MRI. There remains controversy as to whether these infants should be routinely cooled. Common practice trend leans clearly towards cooling, however, the benefit of therapeutic hypothermia for mild HIE remains under investigation.
In general, neonates who have a quick clinical recovery, a normal EEG background pattern, a normal MRI, and a normal neurological examination at 7 to 10 days of age often have a favorable long-term outcome. An early abnormal neurologic examination has limited long-term predictability, in contrast to an abnormal examination noted at the time of discharge which correlates significantly with adverse outcomes. Excellent prediction of outcomes at 18 to 24 months can be made by combining early laboratory data, the need for anticonvulsant and pressor support with MRI evaluation of cortex, basal ganglia/thalami, white matter, and posterior limb of the internal capsule.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here