Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The term hemostasis means prevention of blood loss. Whenever a vessel is severed or ruptured, hemostasis is achieved by several mechanisms: (1) vascular constriction; (2) formation of a platelet plug; (3) formation of a blood clot as a result of blood coagulation; and (4) eventual growth of fibrous tissue into the blood clot to close the hole in the vessel permanently.
Immediately after a blood vessel has been cut or ruptured, the trauma to the vessel wall causes smooth muscle in the wall to contract; this instantaneously reduces the flow of blood from the ruptured vessel. The contraction results from the following: (1) local myogenic spasm; (2) local autacoid factors from the traumatized tissues, vascular endothelium, and blood platelets; and (3) nervous reflexes. The nervous reflexes are initiated by pain nerve impulses or other sensory impulses that originate from the traumatized vessel or nearby tissues. However, even more vasoconstriction probably results from local myogenic contraction of the blood vessels initiated by direct damage to the vascular wall. And, for the smaller vessels, the platelets are responsible for much of the vasoconstriction by releasing a vasoconstrictor substance, thromboxane A 2 .
The more severely a vessel is traumatized, the greater the degree of vascular spasm. The spasm can last for many minutes or even hours, during which time the processes of platelet plugging and blood coagulation can take place.
If the cut in the blood vessel is very small—many very small vascular holes develop throughout the body each day—the cut is often sealed by a platelet plug rather than by a blood clot. To understand this process, it is important that we first discuss the nature of platelets themselves.
Platelets (also called thrombocytes ) are minute discs 1 to 4 micrometers in diameter. They are formed in the bone marrow from megakaryocytes , which are extremely large hematopoietic cells in the marrow; the megakaryocytes fragment into the minute platelets in the bone marrow or soon after entering the blood, especially as they squeeze through capillaries. The normal concentration of platelets in the blood is between 150,000 and 450,000/μl.
Platelets have many functional characteristics of whole cells, even though they do not have nuclei and cannot reproduce. In their cytoplasm are the following: (1) actin and myosin molecules , which are contractile proteins similar to those found in muscle cells, and still another contractile protein, thrombosthenin , that can cause the platelets to contract; (2) residuals of both the endoplasmic reticulum and Golgi apparatus that synthesize various enzymes and especially store large quantities of calcium ions; (3) mitochondria and enzyme systems that are capable of forming adenosine triphosphate (ATP) and adenosine diphosphate (ADP); (4) enzyme systems that synthesize prostaglandins , which are local hormones that cause many vascular and other local tissue reactions; (5) an important protein called fibrin-stabilizing factor , which we discuss later in relation to blood coagulation; and (6) a growth factor that causes vascular endothelial cells, vascular smooth muscle cells, and fibroblasts to multiply and grow, thus causing cellular growth that eventually helps repair damaged vascular walls.
On the platelet cell membrane surface is a coat of glycoproteins that repulses adherence to normal endothelium and yet causes adherence to injured areas of the vessel wall, especially to injured endothelial cells and even more so to any exposed collagen from deep within the vessel wall. In addition, the platelet membrane contains large amounts of phospholipids that activate multiple stages in the blood-clotting process, as discussed later.
Thus, the platelet is an active structure. It has a half-life in the blood of only 8 to 12 days, so over several weeks its functional processes run out; it is then eliminated from the circulation mainly by the tissue macrophage system. More than half of the platelets are removed by macrophages in the spleen, where the blood passes through a latticework of tight trabeculae.
Platelet repair of vascular openings is based on several important functions of the platelet. When platelets come in contact with a damaged vascular surface, especially with collagen fibers in the vascular wall, the platelets rapidly change their own characteristics drastically ( Figure 37-1 ). They begin to swell, they assume irregular forms with numerous irradiating pseudopods protruding from their surfaces, their contractile proteins contract forcefully and cause the release of granules that contain multiple active factors, and they become sticky so that they adhere to collagen in the tissues and to a protein called von Willebrand factor (vWF), which leaks into the traumatized tissue from the plasma. The platelet surface glycoproteins bind to vWF in the exposed matrix below the damaged endothelium. The platelets then secrete increased quantities of ADP and platelet-activating factor (PAF), and their enzymes form thromboxane A 2 . Thromboxane is a vasoconstrictor and, along with ADP and PAF, acts on nearby platelets to activate them as well; the stickiness of these additional activated platelets causes them to adhere to the original activated platelets.
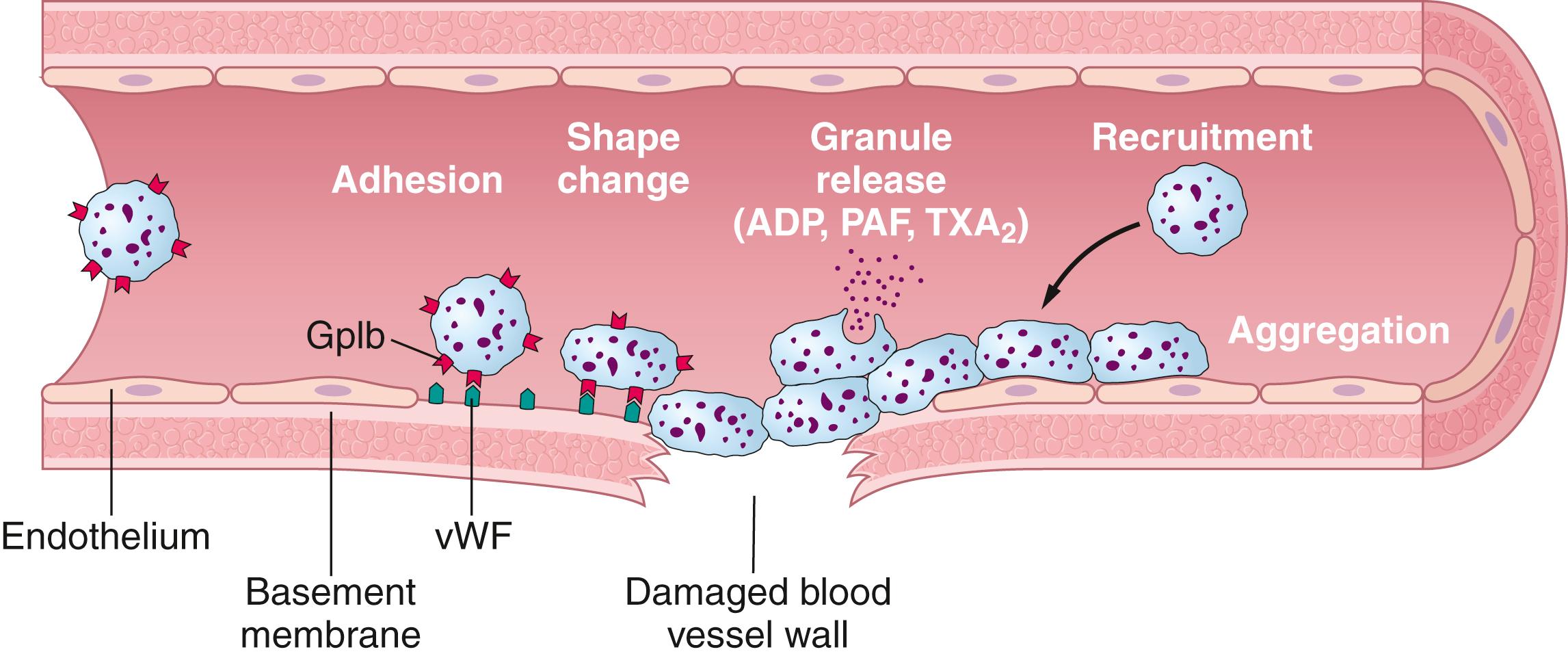
Therefore, at the site of a puncture in a blood vessel wall, the damaged vascular wall activates successively increasing numbers of platelets that attract more and more additional platelets, thus forming a platelet plug . This plug is loose at first but is usually successful in blocking blood loss if the vascular opening is small. Then, during the subsequent process of blood coagulation, fibrin threads form. These threads attach tightly to the platelets, thus constructing an unyielding plug.
The platelet-plugging mechanism is extremely important for closing minute ruptures in very small blood vessels that occur many thousands of times daily. Indeed, multiple small holes through the endothelial cells themselves are often closed by platelets actually fusing with the endothelial cells to form additional endothelial cell membranes. Literally thousands of small hemorrhagic areas develop each day under the skin ( petechiae , which appear as purple or red dots on the skin) and throughout the internal tissues of a person who has few blood platelets. This phenomenon does not occur in persons with normal numbers of platelets.
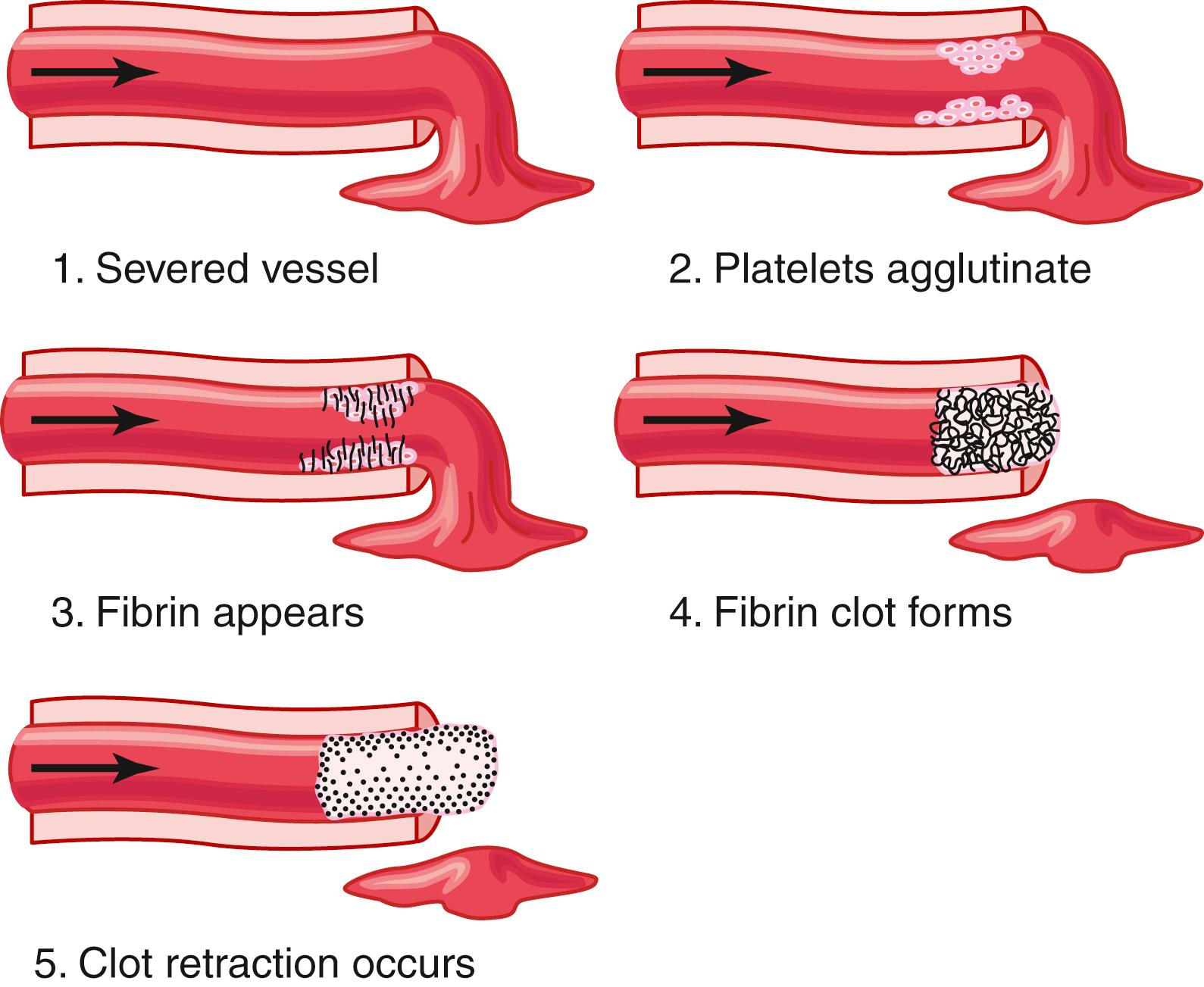
The third mechanism for hemostasis is formation of the blood clot. The clot begins to develop in 15 to 20 seconds if the trauma to the vascular wall is severe and in 1 to 2 minutes if the trauma is minor. Activator substances from the traumatized vascular wall, from platelets, and from blood proteins adhering to the traumatized vascular wall initiate the clotting process. The physical events of this process are shown in Figure 37-2 ; Table 37-1 lists the most important clotting factors.
| Clotting Factor | Synonym(s) |
|---|---|
| Fibrinogen | Factor I |
| Prothrombin | Factor II |
| Tissue factor | Factor III; tissue thromboplastin |
| Calcium | Factor IV |
| Factor V | Proaccelerin; labile factor; Ac-globulin (Ac-G) |
| Factor VII | Serum prothrombin conversion accelerator (SPCA); proconvertin; stable factor |
| Factor VIII | Antihemophilic factor (AHF); antihemophilic globulin (AHG); antihemophilic factor A |
| Factor IX | Plasma thromboplastin component (PTC); Christmas factor; antihemophilic factor B |
| Factor X | Stuart factor; Stuart-Prower factor |
| Factor XI | Plasma thromboplastin antecedent (PTA); antihemophilic factor C |
| Factor XII | Hageman factor |
| Factor XIII | Fibrin-stabilizing factor |
| Prekallikrein | Fletcher factor |
| High-molecular-weight kininogen | Fitzgerald factor; high-molecular-weight kininogen (HMWK) |
| Platelets |
Within 3 to 6 minutes after rupture of a vessel, the entire opening or broken end of the vessel is filled with clot if the vessel opening is not too large. After 20 to 60 minutes, the clot retracts, which closes the vessel still further. Platelets also play an important role in this clot retraction, as discussed later.
Once a blood clot has formed, it can follow one of two courses: (1) it can become invaded by fibroblasts , which subsequently form connective tissue all through the clot; or (2) it can dissolve. The usual course for a clot that forms in a small hole of a vessel wall is invasion by fibroblasts, beginning within a few hours after the clot is formed, which is promoted at least partially by growth factor secreted by platelets. This process continues to complete organization of the clot into fibrous tissue within about 1 to 2 weeks.
Conversely, when excess blood has leaked into the tissues, and tissue clots have formed where they are not needed, special substances in the clot usually become activated. These substances function as enzymes to dissolve the clot, as discussed later in the chapter.
More than 50 important substances that cause or affect blood coagulation have been found in the blood and in the tissues—some that promote coagulation, called procoagulants , and others that inhibit coagulation, called anticoagulants . Whether blood will coagulate depends on the balance between these two groups of substances. In the blood stream, the anticoagulants normally predominate, so the blood does not coagulate while it is circulating in the blood vessels. However, when a vessel is ruptured, procoagulants from the area of tissue damage become activated and override the anticoagulants, and then a clot does develop.
Clotting takes place in three essential steps:
In response to rupture of the vessel or damage to the blood itself, a complex cascade of chemical reactions occurs in the blood involving more than 12 blood coagulation factors. The net result is the formation of a complex of activated substances collectively called prothrombin activator.
The prothrombin activator catalyzes the conversion of prothrombin into thrombin.
The thrombin acts as an enzyme to convert fibrinogen into fibrin fibers that enmesh platelets, blood cells, and plasma to form the clot.
We will first discuss the mechanism whereby the blood clot is formed, beginning with conversion of prothrombin to thrombin, and then come back to the initiating stages in the clotting process whereby prothrombin activator is formed.
Prothrombin activator is formed as a result of rupture of a blood vessel or as a result of damage to special substances in the blood.
Prothrombin activator, in the presence of sufficient amounts of ionic calcium (Ca 2+ ), causes conversion of prothrombin to thrombin ( Figure 37-3 and 37-4 ).
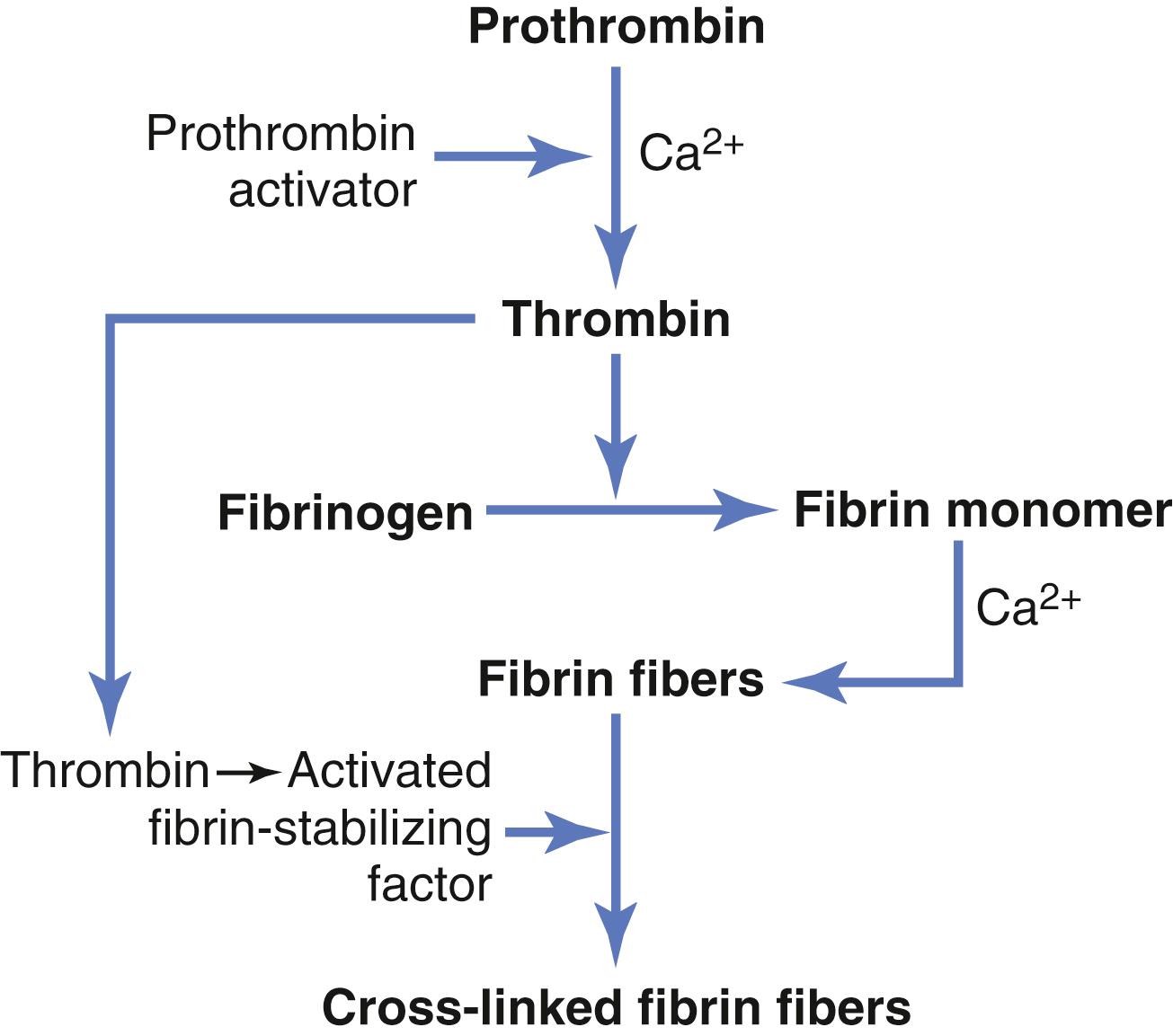
Thrombin causes polymerization of fibrinogen molecules into fibrin fibers within another 10 to 15 seconds.
Thus, the rate-limiting factor in causing blood coagulation is usually the formation of prothrombin activator and not the subsequent reactions beyond that point because these terminal steps normally occur rapidly to form the clot.
Platelets also play an important role in the conversion of prothrombin to thrombin because much of the prothrombin first attaches to prothrombin receptors on the platelets that are already bound to the damaged tissue.
Prothrombin is a plasma protein, an α 2 -globulin, having a molecular weight of 68,700. It is present in normal plasma in a concentration of about 15 mg/dl. It is an unstable protein that can split easily into smaller compounds, one of which is thrombin , which has a molecular weight of 33,700, almost half that of prothrombin.
Prothrombin is formed continually by the liver, and it is continually being used throughout the body for blood clotting. If the liver fails to produce prothrombin, in a day or so prothrombin concentration in the plasma falls too low to provide normal blood coagulation.
Vitamin K is required by the liver for normal activation of prothrombin, as well as a few other clotting factors. Therefore, lack of vitamin K or the presence of liver disease that prevents normal prothrombin formation can decrease the prothrombin to such a low level that a bleeding tendency results.
Fibrinogen is a high-molecular-weight protein (molecular weight ≈340,000) that occurs in the plasma in quantities of 100 to 700 mg/dl. Fibrinogen is formed in the liver, and liver disease can decrease the concentration of circulating fibrinogen, as it does the concentration of prothrombin, noted earlier.
Because of its large molecular size, little fibrinogen normally leaks from the blood vessels into the interstitial fluids, and because fibrinogen is one of the essential factors in the coagulation process, interstitial fluids ordinarily do not coagulate. Yet, when the permeability of the capillaries becomes pathologically increased, fibrinogen does leak into the tissue fluids in sufficient quantities to allow clotting of these fluids in much the same way that plasma and whole blood can clot.
Thrombin is a protein enzyme with weak proteolytic capabilities. It acts on fibrinogen to remove four low-molecular-weight peptides from each molecule of fibrinogen, forming one molecule of fibrin monomer that has the automatic capability to polymerize with other fibrin monomer molecules to form fibrin fibers. Therefore, many fibrin monomer molecules polymerize within seconds into long fibrin fibers that constitute the reticulum of the blood clot.
In the early stages of polymerization, the fibrin monomer molecules are held together by weak noncovalent hydrogen bonding, and the newly forming fibers are not cross-linked with one another. Therefore, the resultant clot is weak and can be broken apart with ease. However, another process occurs during the next few minutes that greatly strengthens the fibrin reticulum. This process involves a substance called fibrin stabilizing factor that is present in small amounts in normal plasma globulins but is also released from platelets entrapped in the clot. Before fibrin stabilizing factor can have an effect on the fibrin fibers, it must be activated. The same thrombin that causes fibrin formation also activates the fibrin stabilizing factor. This activated substance then operates as an enzyme to form covalent bonds between more and more of the fibrin monomer molecules, as well as multiple cross-linkages between adjacent fibrin fibers, thus adding tremendously to the three-dimensional strength of the fibrin meshwork.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here