Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
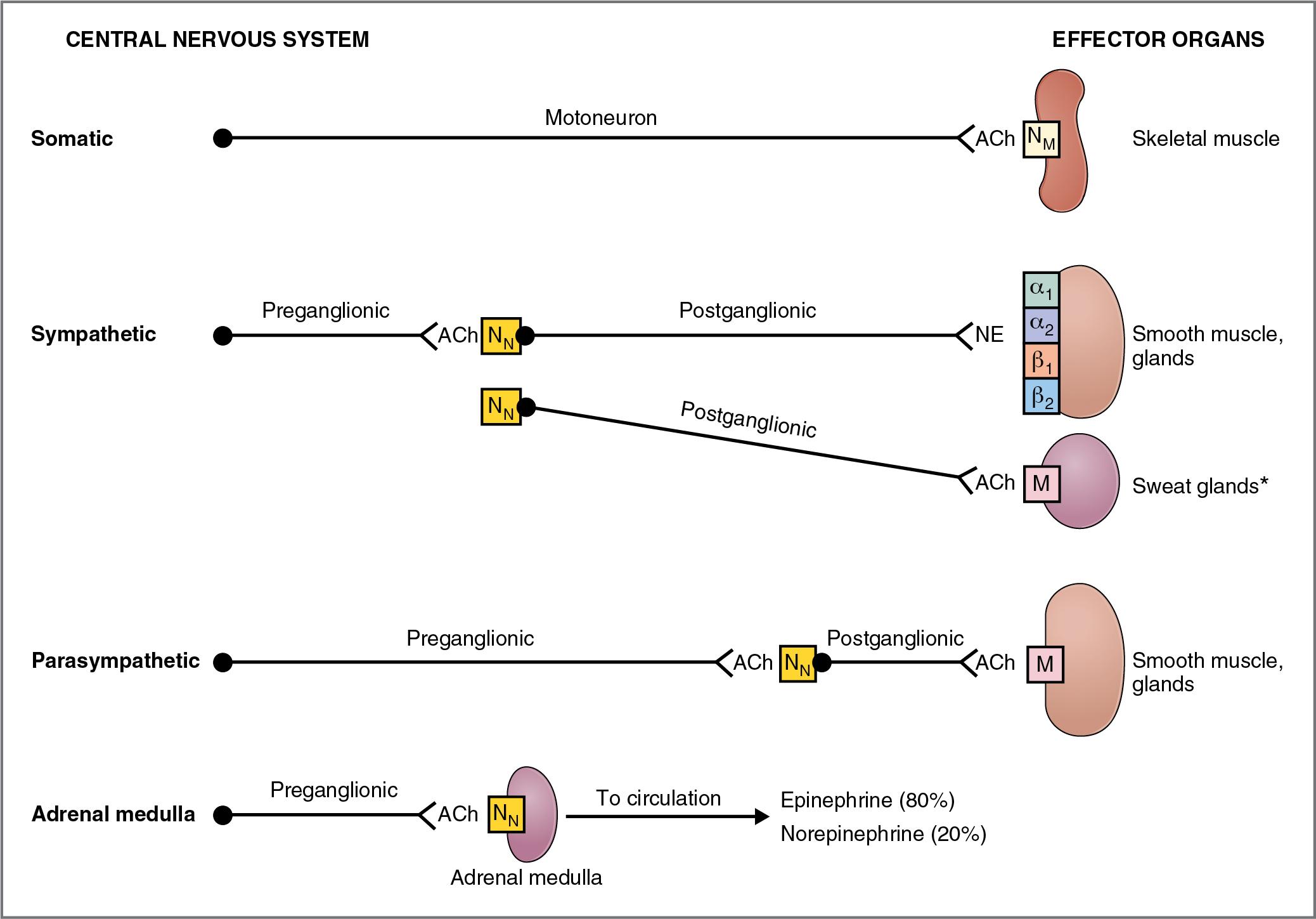
The motor (efferent) nervous system has two components: the somatic and the autonomic. These two systems differ in a number of ways but are chiefly distinguished by the types of effector organs they innervate and the types of functions they control.
The somatic nervous system is a voluntary motor system under conscious control. Each of its pathways consists of a single motoneuron and the skeletal muscle fibers it innervates. The cell body of the motoneuron is located in the central nervous system (CNS) in either the brain stem or spinal cord, and its axon synapses directly on skeletal muscle, the effector organ. The neurotransmitter acetylcholine is released from presynaptic terminals of the motoneurons and activates nicotinic receptors located on the motor end plates of the skeletal muscle. An action potential in the motoneuron causes an action potential in the muscle fiber, which causes the muscle to contract. (For a complete discussion of the somatic nervous system, see Chapter 1 .)
The autonomic nervous system is an involuntary system that controls and modulates the functions primarily of visceral organs. Each pathway in the autonomic nervous system consists of two neurons: a preganglionic neuron and a postganglionic neuron. The cell body of each preganglionic neuron resides in the CNS. The axons of these preganglionic neurons synapse on the cell bodies of postganglionic neurons in one of several autonomic ganglia located outside the CNS. The axons of the postganglionic neurons then travel to the periphery, where they synapse on visceral effector organs such as the heart, bronchioles, vascular smooth muscle, gastrointestinal tract, bladder, and genitalia. All preganglionic neurons of the autonomic nervous system release acetylcholine. Postganglionic neurons release either acetylcholine or norepinephrine or, in some cases, neuropeptides.
The autonomic nervous system has two major divisions: the sympathetic and the parasympathetic, which often complement each other in the regulation of organ system function. A third division of the autonomic nervous system, the enteric nervous system, is located in plexuses in the wall of the gastrointestinal tract. (The enteric nervous system is discussed in Chapter 8 .)
The organization of the autonomic nervous system is described in Figure 2.1 and in its companion, Table 2.1 . The sympathetic and parasympathetic divisions are included and, for comparison, so is the somatic nervous system.
| Characteristics | Sympathetic Division | Parasympathetic Division | Somatic Nervous System a |
|---|---|---|---|
| Origin of preganglionic neurons | Spinal cord segments T1–L3 (thoracolumbar) | Nuclei of CN III, VII, IX, and X; spinal cord segments S2–S4 (craniosacral) | — |
| Location of autonomic ganglia | Paravertebral and prevertebral | In or near effector organs | — |
| Length of preganglionic axons | Short | Long | — |
| Length of postganglionic axons | Long | Short | — |
| Effector organs | Smooth muscle; cardiac muscle; glands | Smooth muscle; cardiac muscle; glands | Skeletal muscle |
| Neuroeffector junctions | Diffuse, branching; receptors not concentrated in one region | Diffuse, branching; receptors not concentrated in one region | Discrete, organized; ACh receptors localized on motor end plate |
| Neurotransmitter and receptor type in ganglion | ACh/nicotinic receptor | ACh/nicotinic receptor | — |
| Neurotransmitter in effector organs | Norepinephrine (except sweat glands) | ACh | Ach |
| Receptor types in effector organs | α 1 , α 2 , β 1 , β 2 | Muscarinic | Nicotinic |
The terms sympathetic and parasympathetic are strictly anatomic terms and refer to the anatomic origin of the preganglionic neurons in the CNS (see Table 2.1 ). Preganglionic neurons in the sympathetic division originate in the thoracolumbar spinal cord. Preganglionic neurons in the parasympathetic division originate in the brain stem and sacral spinal cord.
The terms adrenergic and cholinergic are used to describe neurons of either division, according to which neurotransmitter they synthesize and release. Adrenergic neurons release norepinephrine; receptors for norepinephrine on the effector organs are called adrenoreceptors. Adrenoreceptors may be activated by norepinephrine, which is released from adrenergic neurons, or by epinephrine, which is secreted into the circulation by the adrenal medulla. Cholinergic neurons release acetylcholine (ACh); receptors for ACh are called cholinoreceptors. (A third term is nonadrenergic, noncholinergic, which describes some postganglionic parasympathetic neurons of the gastrointestinal tract that release peptides [e.g., substance P] or other substances [e.g., nitric oxide (NO)] as their neurotransmitter rather than ACh.)
To summarize, whether located in the sympathetic division or in the parasympathetic division, all preganglionic neurons release ACh and therefore are called cholinergic. Postganglionic neurons may be either adrenergic (they release norepinephrine) or cholinergic (they release ACh). Most postganglionic parasympathetic neurons are cholinergic; postganglionic sympathetic neurons may be either adrenergic or cholinergic.
The junctions between postganglionic autonomic neurons and their effectors (target tissues), the neuroeffector junctions, are analogous to the neuromuscular junctions of the somatic nervous system. There are, however, several structural and functional differences with the neuromuscular junction. (1) The neuromuscular junction (discussed in Chapter 1 ) has a discrete arrangement, whereby the “effector,” a skeletal muscle fiber, is innervated by a single motoneuron. In contrast, in the autonomic nervous system, the postganglionic neurons that innervate target tissues form diffuse, branching networks. Beads, or varicosities, line these branches and are the sites of neurotransmitter synthesis, storage, and release. The varicosities are therefore analogous to the presynaptic nerve terminals of the neuromuscular junction. (2) There is overlap in the branching networks from different postganglionic neurons, such that target tissues may be innervated by many postganglionic neurons. (3) In the autonomic nervous system, postsynaptic receptors are widely distributed on the target tissues, and there is no specialized region of receptors analogous to the motor end plate of skeletal muscle.
The overall function of the sympathetic nervous system is to mobilize the body for activity. In the extreme, if a person is exposed to a stressful situation, the sympathetic nervous system is activated with a response known as “fight or flight,” which includes increased arterial pressure, increased blood flow to active muscles, increased metabolic rate, increased blood glucose concentration, and increased mental activity and alertness. Although this response, per se, is rarely employed, the sympathetic nervous system operates continuously to modulate the functions of many organ systems such as heart, blood vessels, gastrointestinal tract, bronchi, and sweat glands.
Figure 2.2 depicts the organization of the sympathetic nervous system in relation to the spinal cord, the sympathetic ganglia, and the effector organs in the periphery. The preganglionic sympathetic neurons originate in nuclei of the thoracolumbar spinal cord, leave the spinal cord via the ventral motor roots and white rami, and project either to the paravertebral ganglia of the sympathetic chain or to a series of prevertebral ganglia. Thus one category of preganglionic neuron synapses are on postganglionic neurons in paravertebral ganglia (e.g., superior cervical ganglion) of the sympathetic chain. These synapses may occur in ganglia at the same segmental level of the chain, or the preganglionic fibers may turn in the cranial or caudal direction and innervate ganglia at higher or lower levels in the chain, thereby permitting synapses in multiple ganglia (consistent with the diffuseness of sympathetic functions). The other category of preganglionic neuron passes through the sympathetic chain without synapsing and continues on to synapse in prevertebral ganglia (celiac, superior mesenteric, and inferior mesenteric) that supply visceral organs, glands, and the enteric nervous system of the gastrointestinal tract. In the ganglia, the preganglionic neurons synapse on postganglionic neurons, which travel to the periphery and innervate the effector organs.
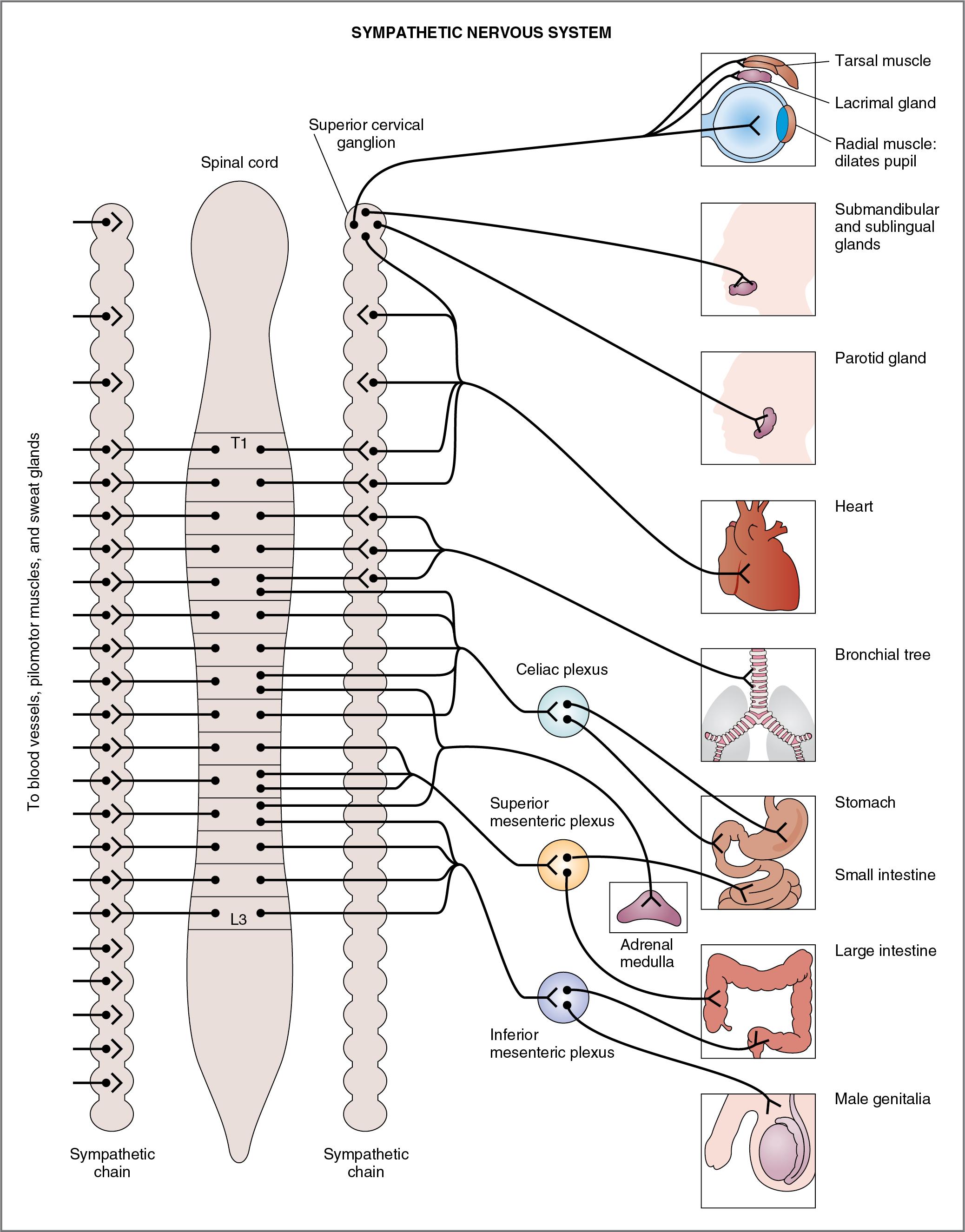
The features of the sympathetic nervous system discussed in the following sections are listed in Table 2.1 and are illustrated in Figure 2.2 .
The preganglionic neurons of the sympathetic division arise from nuclei in the thoracic and lumbar spinal cord segments, specifically from the first thoracic segment to the third lumbar segment (T1–L3). Thus the sympathetic division is referred to as thoracolumbar.
Generally, the origin of preganglionic neurons in the spinal cord is anatomically consistent with the projection to the periphery. Thus the sympathetic pathways to organs in the thorax (e.g., heart) have preganglionic neurons originating in the upper thoracic spinal cord. Sympathetic pathways to organs in the pelvis (e.g., colon, genitals) have preganglionic neurons that originate in the lumbar spinal cord. Blood vessels, thermoregulatory sweat glands, and pilomotor muscles of the skin have preganglionic neurons that synapse on multiple postganglionic neurons up and down the sympathetic chain, reflecting their broad distribution throughout the body.
The ganglia of the sympathetic nervous system are located near the spinal cord, either in the paravertebral ganglia (known as the sympathetic chain) or in the prevertebral ganglia. Again, the anatomy is logical. The superior cervical ganglion projects to organs in the head such as the eyes and the salivary glands. The celiac ganglion projects to the stomach and the small intestine. The superior mesenteric ganglion projects to the small and large intestine, and the inferior mesenteric ganglion projects to the lower large intestine, anus, bladder, and genitalia.
The adrenal medulla is simply a specialized sympathetic ganglion whose preganglionic neurons originate in the thoracic spinal cord (T5–T9), pass through the sympathetic chain and the celiac ganglion without synapsing, and travel in the greater splanchnic nerve to the adrenal gland.
Because the sympathetic ganglia are located near the spinal cord, the preganglionic nerve axons are short and the postganglionic nerve axons are long (so that they can reach the peripheral effector organs).
Preganglionic neurons of the sympathetic division are always cholinergic. They release ACh, which interacts with nicotinic (N N ) receptors on the cell bodies of postganglionic neurons. Postganglionic neurons of the sympathetic division are adrenergic in all of the effector organs, except in the thermoregulatory sweat glands (where they are cholinergic). The effector organs that are innervated by sympathetic adrenergic neurons have one or more of the following types of adrenoreceptors: alpha 1 , alpha 2 , beta 1 , or beta 2 (α 1 , α 2 , β 1 , or β 2 ). The thermoregulatory sweat glands innervated by sympathetic cholinergic neurons have muscarinic cholinoreceptors.
As described previously, sympathetic postganglionic adrenergic nerves release their neurotransmitters from varicosities onto their target tissues (e.g., vascular smooth muscle). The sympathetic adrenergic varicosities contain both the classic neurotransmitter (norepinephrine) and nonclassic neurotransmitters (adenosine triphosphate [ATP] and neuropeptide Y). The classic neurotransmitter, norepinephrine, is synthesized from tyrosine in the varicosities (see Fig. 1.18 ) and stored in small dense-core vesicles, ready for release; these small dense-core vesicles also contain dopamine β-hydroxylase, which catalyzes the conversion of dopamine to norepinephrine (the final step in the synthetic pathway), and ATP. ATP is said to be “colocalized” with norepinephrine. A separate group of large dense-core vesicles contain neuropeptide Y.
When sympathetic postganglionic adrenergic neurons are stimulated, norepinephrine and ATP are released from the small dense-core vesicles. Both norepinephrine and ATP serve as neurotransmitters at the neuroeffector junction, binding to and activating their respective receptors on the target tissue (e.g., vascular smooth muscle). Actually, ATP acts first, binding to purinergic receptors on the target tissue and causing a physiologic effect (e.g., contraction of the vascular smooth muscle). The action of norepinephrine follows ATP; norepinephrine binds to its receptors on the target tissue (e.g., α 1 -adrenergic receptors on vascular smooth muscle) and causes a second, more prolonged contraction. Finally, with more intense or higher-frequency stimulation, the large dense-core vesicles release neuropeptide Y, which binds to its receptor on the target tissue, causing a third, slower phase of contraction.
The adrenal medulla is a specialized ganglion in the sympathetic division of the autonomic nervous system. The cell bodies of its preganglionic neurons are located in the thoracic spinal cord. The axons of these preganglionic neurons travel in the greater splanchnic nerve to the adrenal medulla, where they synapse on chromaffin cells and release ACh, which activates nicotinic receptors. When activated, the chromaffin cells of the adrenal medulla secrete catecholamines (epinephrine and norepinephrine) into the general circulation. In contrast with sympathetic postganglionic neurons, which release only norepinephrine, the adrenal medulla secretes mainly epinephrine (80%) and a small amount of norepinephrine (20%). The reason for this difference is the presence of phenylethanolamine- N -methyltransferase (PNMT) in the adrenal medulla but not in sympathetic postganglionic adrenergic neurons (see Fig. 1.18 ). PNMT catalyzes the conversion of norepinephrine to epinephrine, a step that, interestingly, requires cortisol from the nearby adrenal cortex; cortisol is supplied to the adrenal medulla in venous effluent from the adrenal cortex.
A tumor of the adrenal medulla, or pheochromocytoma, may be located on or near the adrenal medulla or at a distant (ectopic) location in the body ( Box 2.1 ). Unlike the normal adrenal medulla, which secretes mainly epinephrine, a pheochromocytoma secretes mainly norepinephrine, which is explained by the fact that the tumor is located too far from the adrenal cortex to receive the cortisol that is required by PNMT.
A 48-year-old woman visits her physician complaining of what she calls “panic attacks.” She reports that she has experienced a racing heart and that she can feel (and even see) her heart pounding in her chest. She also complains of throbbing headaches, cold hands and cold feet, feeling hot, visual disturbances, and nausea and vomiting. In the physician’s office, her blood pressure is severely elevated (230/125). She is admitted to the hospital for evaluation of her hypertension.
A 24-hour urine sample reveals elevated levels of metanephrine, normetanephrine, and 3-methoxy-4-hydroxymandelic acid (VMA). After the physician rules out other causes for hypertension, he concludes that she has a tumor of the adrenal medulla, called a pheochromocytoma. A computerized tomographic scan of the abdomen reveals a 3.5-cm mass on her right adrenal medulla. The patient is administered an α 1 antagonist, and surgery is performed. The woman recovers fully; her blood pressure returns to normal, and her other symptoms disappear.
The woman has a classic pheochromocytoma, a tumor of the chromaffin cells of the adrenal medulla. The tumor secretes excessive amounts of norepinephrine and epinephrine, which produce all of the woman’s symptoms and result in elevated levels of catecholamine metabolites in her urine. In contrast to normal adrenal medulla, which secretes mainly epinephrine, pheochromocytomas secrete mainly norepinephrine.
The patient’s symptoms can be interpreted by understanding the physiologic effects of catecholamines. Any tissue where adrenoreceptors are present will be activated by the increased levels of epinephrine and norepinephrine, which reach the tissues via the circulation. The woman’s most prominent symptoms are cardiovascular: pounding heart, increased heart rate, increased blood pressure, and cold hands and feet. These symptoms can be understood by considering the functions of adrenoreceptors in the heart and blood vessels. The increased amounts of circulating catecholamines activated β 1 receptors in the heart, increasing the heart rate and increasing contractility (pounding of the heart). Activation of α 1 receptors in vascular smooth muscle of the skin produced vasoconstriction, which presented as cold hands and feet. The patient felt hot, however, because this vasoconstriction in the skin impaired the ability to dissipate heat. Her extremely elevated blood pressure was caused by the combination of increased heart rate, increased contractility, and increased constriction (resistance) of the blood vessels. The patient’s headache was secondary to her elevated blood pressure.
The woman’s other symptoms also can be explained by the activation of adrenoreceptors in other organ systems (i.e., gastrointestinal symptoms of nausea and vomiting and visual disturbances).
The patient’s treatment consisted of locating and excising the tumor, thereby removing the source of excess catecholamines. Alternatively, if the tumor had not been excised, the woman could have been treated pharmacologically with a combination of α 1 antagonists (e.g., phenoxybenzamine or prazosin) and β 1 antagonists (e.g., propranolol) to prevent the actions of the endogenous catecholamines at the receptor level.
The body responds to fear, extreme stress, and intense exercise with a massive, coordinated activation of the sympathetic nervous system including the adrenal medulla. This activation, the fight or flight response, ensures that the body can respond appropriately to a stressful situation (e.g., take a difficult exam, run away from a burning house, fight an attacker). The response includes increases in heart rate, cardiac output, and blood pressure; redistribution of blood flow away from skin, kidneys, and splanchnic regions and toward skeletal muscle; increased ventilation with dilation of the airways; decreased gastrointestinal motility and secretions; decreased blood clotting time; and increased blood glucose concentration with decreased insulin secretion.
The overall function of the parasympathetic nervous system is restorative, to conserve energy. Figure 2.3 depicts the organization of the parasympathetic nervous system in relation to the CNS (brain stem and spinal cord), the parasympathetic ganglia, and the effector organs. Preganglionic neurons of the parasympathetic division have their cell bodies in either the brain stem (midbrain, pons, and medulla) or the sacral spinal cord. Preganglionic axons project to a series of ganglia located near or in the effector organs.
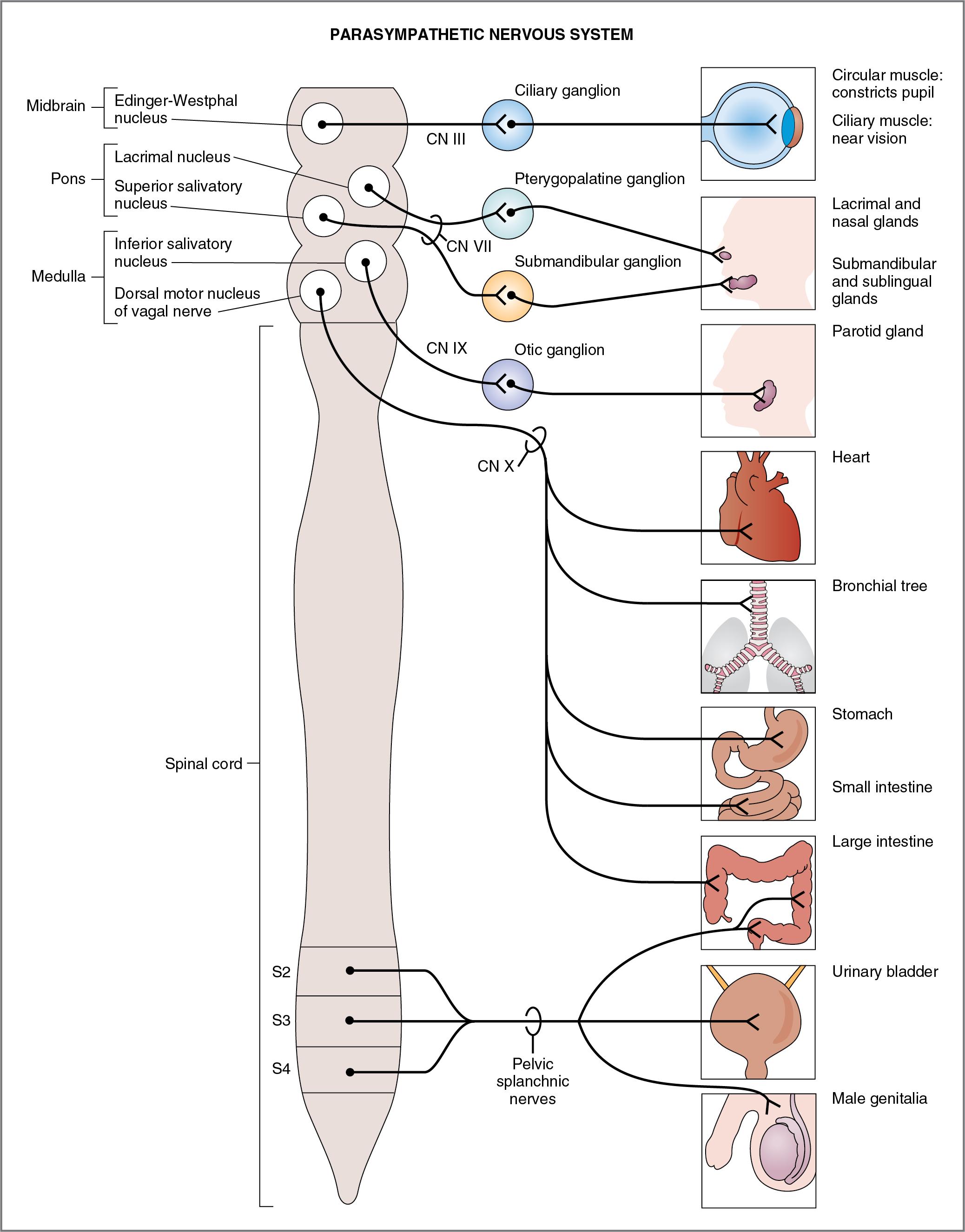
The following features of the parasympathetic nervous system can be noted and compared with the sympathetic nervous system (see Table 2.1 and Fig. 2.3 ).
Preganglionic neurons of the parasympathetic division arise from nuclei of cranial nerves (CNs) III, VII, IX, and X or from sacral spinal cord segments S2–S4; therefore the parasympathetic division is called craniosacral. As in the sympathetic division, the origin of the preganglionic neurons in the CNS is consistent with the projection to effector organs in the periphery. For example, the parasympathetic innervation of eye muscles originates in the Edinger-Westphal nucleus in the midbrain and travels to the periphery in CN III; the parasympathetic innervation of the heart, bronchioles, and gastrointestinal tract originates in nuclei of the medulla and travels to the periphery in CN X (vagus nerve); and the parasympathetic innervation of the genitourinary organs originates in the sacral spinal cord and travels to the periphery in the pelvic nerves.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here