Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Explain how a skeletal muscle twitch and a tetanus are generated.
Describe how muscle contractile force can be varied.
Describe the mechanical properties of muscle that are characterized by the length-tension relationship and the force-velocity relationship.
Describe the roles of the three main types of phasic skeletal muscle.
Explain how the mechanical properties of skeletal muscle, cardiac muscle, and smooth muscle differ.
Describe the diversity of smooth muscle types.
Describe how the kinetic properties of some smooth muscles contribute to their ability to generate tonic contractions or “tone.”
The sliding filament mechanism of contraction and E-C coupling are described in Chapters 14 and 15 . We now consider the mechanical properties of muscle.
Skeletal muscle is normally activated through its innervation by α motor neurons. An individual α motor neuron may innervate just a few muscle fibers (myocytes) in extraocular muscles or as many as several thousand in large limb and trunk muscles. A single α motor neuron, together with all the myocytes it innervates, is referred to as a motor unit . The motor neurons release ACh at NMJs ( Chapter 12 ). A single motor neuron AP normally releases sufficient ACh at each NMJ to trigger an AP in all the muscle fibers it innervates. Consequently, all the muscle cells in a motor unit contract synchronously when the motor neuron generates an AP. Thus the motor unit is the functional force-generating unit in whole muscle. The primary mechanism by which the central nervous system increases whole muscle force is by recruiting (i.e., simultaneously activating) motor units. The amount of force generated by whole muscle, therefore, is directly related to the number of motor units that are activated.
A single AP in a skeletal myocyte triggers Ca 2+ release from the SR ( Chapter 15 ) to increase [Ca 2+ ] i and, thus, causes a twitch contraction. A comparison of the time course of [Ca 2+ ] i elevation and force development during a twitch is shown in Fig. 16.1 A. Shortly after the AP, [Ca 2+ ] i rapidly rises; the Ca 2+ ions bind to TnC and activate the formation of actin-myosin cross-bridges. The resultant increase in force develops over a slower time course than the rise in [Ca 2+ ] i ( Fig. 16.1 A).
![Fig. 16.1, Time Courses of the Sarcolemmal AP, Average Rise in [Ca 2+ ] i , and Force Development in Skeletal and Cardiac Muscle. (A) A single stimulus applied to skeletal muscle evokes an AP, which causes Ca 2+ to be released from the SR ( Chapter 15 ). [Ca 2+ ] i (C) rises rapidly after the AP and reaches a level that saturates TnC binding sites, thus permitting actin-myosin cross-bridges to form. Because elastic elements in the muscle need to be stretched, force development (F) is much slower than the [Ca 2+ ] i rise and the activation of the contractile apparatus. (B) Time courses of the AP, [Ca 2+ ] i , and force for a cardiac muscle activated by a single stimulus. The force transient lasts much longer than in skeletal muscle because Ca 2+ channels remain open during the long-duration AP, thus allowing prolonged Ca 2+ influx and, consequently, prolonged CICR. Fig. 16.1, Time Courses of the Sarcolemmal AP, Average Rise in [Ca 2+ ] i , and Force Development in Skeletal and Cardiac Muscle. (A) A single stimulus applied to skeletal muscle evokes an AP, which causes Ca 2+ to be released from the SR ( Chapter 15 ). [Ca 2+ ] i (C) rises rapidly after the AP and reaches a level that saturates TnC binding sites, thus permitting actin-myosin cross-bridges to form. Because elastic elements in the muscle need to be stretched, force development (F) is much slower than the [Ca 2+ ] i rise and the activation of the contractile apparatus. (B) Time courses of the AP, [Ca 2+ ] i , and force for a cardiac muscle activated by a single stimulus. The force transient lasts much longer than in skeletal muscle because Ca 2+ channels remain open during the long-duration AP, thus allowing prolonged Ca 2+ influx and, consequently, prolonged CICR.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/Mechanicsofmusclecontraction/0_3s20B9780323596190000255.jpg)
Force generated by skeletal muscle is transmitted to the bones through elastic elements (e.g., tendons). These elastic elements are connected in series with the contractile elements and bones and must be stretched before force can be transmitted. Stretching these series elastic elements causes a delay between the activation of actin-myosin cross-bridges and the development of force. The following analogy helps explain this effect: you hold in your hand a taut, but not stretched, rubber band that is attached to a weight resting on a table. As you raise your hand, the weight is not lifted immediately, because the rubber band must first be stretched. As the rubber band is stretched (i.e., the length is increased), a force is developed in the rubber band, which pulls against the attached weight. The development of this force takes time, and the more elastic the rubber band, the longer the delay in lifting the weight ( Box 16.1 ).
Skeletal muscle can be modeled as a system consisting of three mechanical components ( Fig. 16.2 ). A contractile element is in series with an elastic element, represented by a spring. A parallel elastic element is in parallel with the contractile element and the series elastic element. The series elastic element resides primarily in the tendons. The sarcolemma, intracellular components of the cytoarchitecture, and extracellular connective tissue are part of the parallel elastic element. The series elastic element explains the delay that occurs between the activation of the contractile elements and the development of force: the series elastic element must be stretched before force can be transmitted to a load. Furthermore, if a muscle is rapidly stretched immediately after stimulation and before it begins to develop tension, it will develop more force than if it had not been stretched. The rapid stretch extends the series elastic element, so that the shortening of the contractile elements will pull on the external load sooner. When resting muscle is stretched to relatively long sarcomere lengths, the resting tension increases. This maintained resting tension is evidence of a parallel elastic element because without it the nonelastic contractile elements would passively stretch and eliminate steady-state tension.

Sufficient Ca 2+ is released from the SR by a single AP to saturate all the TnC binding sites. These sites are saturated for only a brief time because [Ca 2+ ] i reaches a peak and then begins to fall rapidly as the Ca 2+ is pumped back into the SR. Therefore the muscle does not develop its maximum possible force during a twitch because Ca 2+ dissociates from TnC (as [Ca 2+ ] i declines) before the series elastic elements are completely stretched. If [Ca 2+ ] i remains elevated for a longer period, however, muscle force will continue to increase. This occurs physiologically if a second AP is evoked before [Ca 2+ ] i has returned to the resting level. Note that contractile force can also be enhanced by increasing the amount of Ca 2+ stored in the SR. This is an important mechanism for altering contractile force in the heart (see later), but not in skeletal muscle.
The maximum force generated by skeletal muscle in response to two consecutive stimuli depends on the interval between the stimuli ( Fig. 16.3 ). If the two stimuli are separated by more than 100 msec, both stimuli will produce twitch contractions ( Fig. 16.3 A). If the second stimulus is given before the first twitch relaxes completely, the force from the second twitch will be added to the force from the first ( Fig. 16.3 B) and the resultant peak force will be larger than the force generated by a single stimulus. This occurs because the second stimulus rereleases Ca 2+ from the SR before all the Ca 2+ released by the first stimulus dissociates from TnC. Thus TnC remains Ca 2+ -bound for a longer period. This gives the contractile elements more time to stretch the series elastic elements and, thus, generate more force. If the interval between the two stimuli is short enough, the second twitch will become completely fused with the first, thus generating a single force transient with a smooth rising phase ( Fig. 16.3 C). If a train of stimuli is applied at a frequency of 20 to 40 per second, a partially fused contraction will result ( Fig. 16.3 D). This is called a semifused tetanus; a tetanus a
Tetanus toxin, from the bacterium Clostridium tetani, causes tetanic convulsions by blocking inhibitory synaptic receptors in the spinal cord. The resultant disinhibition greatly increases the frequency at which α motor neurons fire APs.
is a large contraction caused by a high-frequency train of APs in skeletal muscle. The tetanus becomes completely fused at higher rates of stimulation (80 to 100 stimuli per second; Fig. 16.3 E). Thus force generation in skeletal muscle is regulated through two mechanisms: (1) control of the frequency at which the α motor neuron generates APs and (2) control of the number of α motor neurons (and consequently the number of motor units) activated.

The length-tension curve and the force-velocity relationship characterize the mechanical properties of muscle . Note that “tension” and “force” have the same meaning in muscle physiology and are used interchangeably. The experimental apparatus diagrammed in Fig. 16.4 is used to determine these relationships, either for a single muscle fiber or for whole muscle. In this experimental setup, two mechanical variables are measured as a function of time: contractile force and muscle length. The initial length of the muscle and the attached load can both be varied.
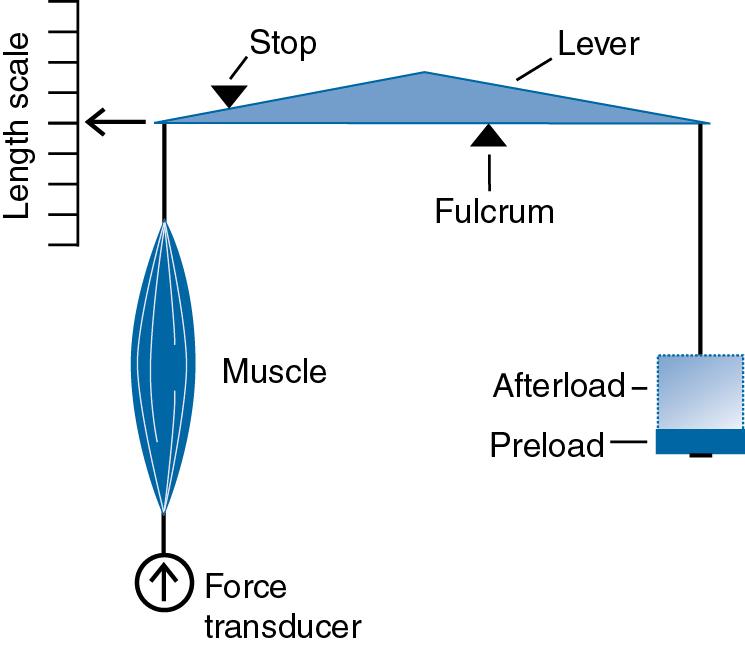
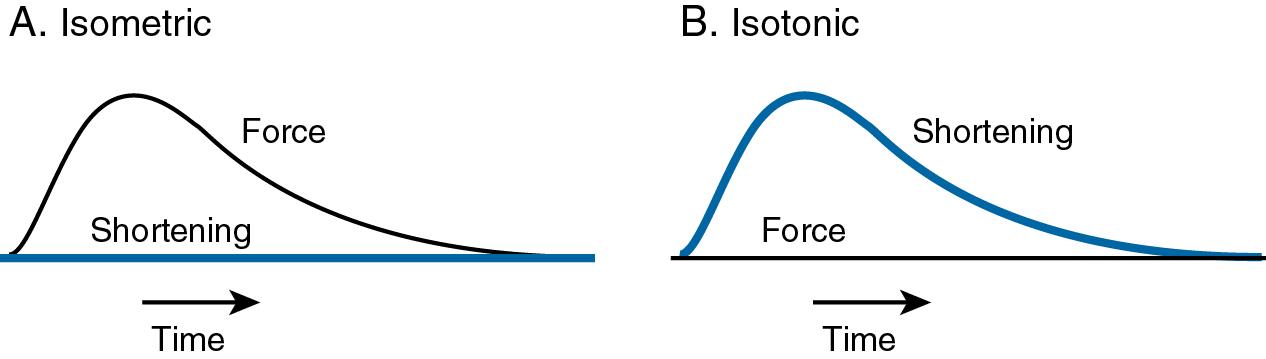
When muscle is activated and filaments begin to slide, two types of mechanical events can occur. The muscle can generate force as it pulls on the tendons, or the muscle can shorten. If the force generated by the muscle is too small to lift the attached load, the muscle will not shorten ( Fig. 16.5 A). In this isometric contraction , the muscle generates force at a constant length . The magnitude of the force varies with the length of the muscle in a manner that is characterized by the length-tension curve ( Fig. 16.6 ). If the muscle is activated with no weight attached, the contraction will result in shortening with no force generation ( Fig. 16.5 B). This is an isotonic contraction —the muscle shortens while generating either no force or a constant force . Macroscopic movement and force generation are the results of summing numerous molecular power strokes that are generated by interactions between individual actin and myosin molecules in the form of cross-bridges. The force and movement generated by a single actin-myosin cross-bridge have been measured ( Box 16.2 ).

In an “optical trap” experiment, myosin molecules are bound to a small silica particle, which is then fixed to a glass coverslip. A single actin filament is attached at each end to a plastic bead. The bead is held in place, or trapped, at the focal point of a laser. The operator can move the bead (and thus the attached actin) by moving the laser focal point, and can change the force with which the bead (and actin) is held in place by varying the laser intensity. The actin filament is moved close to the myosin-coated particle to permit single actin-myosin interactions. When the actin filament is permitted to move freely during a single cross-bridge cycle, the displacement generated by that single interaction is approximately 10 nm (nm = 10 −9 m). By adjusting the intensity of the trapping laser so that it is just sufficient to prevent actin filament movement, the isometric force generated by a single cross-bridge can be estimated to be approximately 1 to 3 × 10 −12 Newtons. (The Newton is a unit of force and is equal to 1 kg × m/sec 2 . Applying a 1-Newton force to a 1-kg mass imparts an acceleration of 1 m/sec 2 .)
The force generated by skeletal muscle depends on the length to which a muscle is stretched. This fundamental property of muscle can be studied by setting the initial length of a single myocyte and measuring the maximum isometric tetanic force generated at that length. Some force is required to stretch relaxed muscle; this is termed passive tension . The relationship between passive tension and muscle length is shown in Fig. 16.6 . When the muscle is stimulated to contract, total tension increases. The difference between total tension generated by contracting muscle and passive tension in relaxed muscle is active tension ( Fig. 16.6 ). The active length-tension relationship shows an optimal length ( L 0 ) at which tension is maximal. In skeletal muscle L 0 is 2.0 to 2.2 μm per sarcomere ( Fig. 16.6 , points B and C).
The active length-tension relationship can be understood in terms of the sliding filament mechanism. At L 0 , the maximum number of cross-bridges can form between thick and thin filaments. As the muscle is stretched from 2.2 to 3.65 μm per sarcomere, reduced overlap between thick and thin filaments decreases the number of cross-bridges that can form; therefore active force decreases ( Fig. 16.6 , points A to B). At sarcomere lengths less than 2.0 μm, force declines for at least two reasons ( Fig. 16.6 , points C to E). Thin filaments from opposite ends of the sarcomere can overlap, which allows cross-bridges to form in the wrong orientation, and filament geometry may be disrupted as thick filaments hit the Z line and actin filaments are forced to move away from the thick filaments.
Because the shape of the active length-tension relationship is precisely predicted by the sliding filament mechanism, we conclude that the active force generated by a muscle is proportional to the number of attached cross-bridges .
The force-velocity relationship for a given muscle can also be determined by use of the apparatus shown in Fig. 16.4 . Force and length are measured during contractions with different weights attached. When the muscle generates sufficient force to lift the weight, a partly isometric, partly isotonic contraction occurs ( Fig. 16.7 ). After activation, the muscle begins to develop force isometrically until the force is sufficient to lift the attached weight (“load”). At this point, force development stops and the muscle begins to shorten isotonically. With a small load, the velocity of shortening (i.e., the rate of change of muscle length with time) is relatively fast ( Fig. 16.7 ). With a larger load, more time is spent developing force, thus leaving less time for isotonic shortening. As a result, the velocity of shortening is slower. The overall force-velocity relationship, which is constructed by measuring a series of contractions against different loads, is shown in Fig. 16.8 . The maximum velocity of shortening ( V 0 ) occurs with no load.

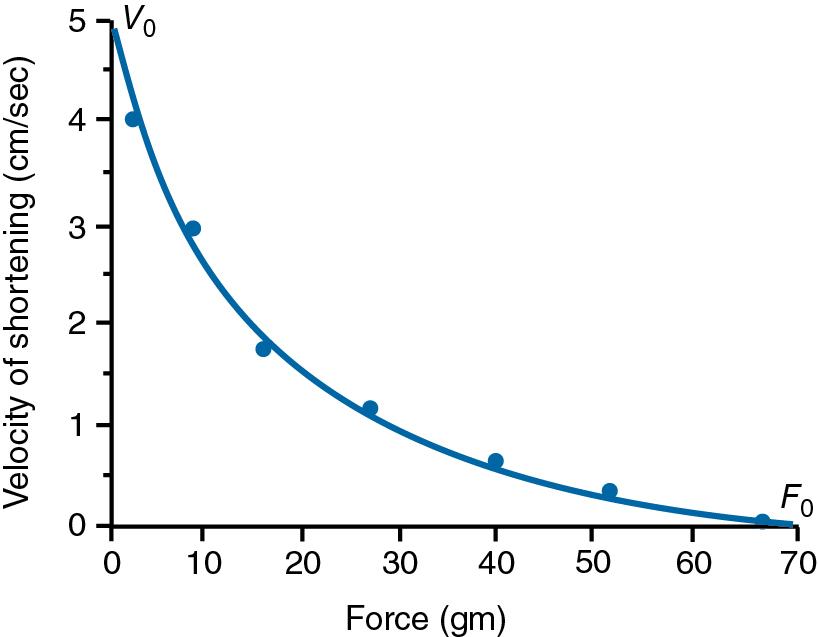
At the molecular level, the rate of cross-bridge cycling determines the rate of muscle shortening. Thus V 0 is a reflection of the maximum rate of cross-bridge cycling. Cross-bridge cycling requires myosin ATPase activity ( Chapter 14 ); therefore V 0 is also directly proportional to the myosin ATPase activity. The inverse relationship between load and shortening velocity can be understood with the help of Fig. 16.9 . Shortening is caused by rotation of the myosin head; the force generated by this rotation is opposed by the load. If the load is relatively small, myosin head rotation will meet little resistance and will occur at a fast rate. In addition, many myosin heads are unattached and available for cycling. Together, these factors lead to fast shortening. In contrast, if the load is large, head rotation will meet stiff opposition and the rotation speed will be slow. Furthermore, many more cross-bridges are attached at any given moment, and fewer are available for recycling. Thus shortening is slow.

The main criteria used to classify motor units are speed of contraction and susceptibility to fatigue. The three types of motor units are fast-twitch, fatigable (type FF); fast-twitch, fatigue-resistant (FR); and slow-twitch, fatigue-resistant (S) . Fast-twitch fibers use anaerobic metabolism for contraction, and slow-twitch fibers use aerobic metabolism. Both FF and FR motor units have twitch contraction times (time to peak twitch force) of 10 to 40 msec, whereas S units take from 60 to more than 100 msec to develop peak twitch force. The contraction time is directly related to the myosin ATPase activity. The myosin isoforms in FF and FR muscle fibers have a higher ATPase activity than the isoform in S fibers. FF motor units generate the largest tetanic forces, S motor units generate the smallest forces, and FR motor units are intermediate. Several factors contribute to the variation in force among motor unit types. These include the innervation ratio (i.e., the number of muscle fibers innervated by the motor neuron) and the force output of individual muscle fibers, which depends, in part, on the cross-sectional area of the individual muscle fibers.
The responses of the different types of motor units to repetitive tetanic stimulation are shown in Fig. 16.10 . The decrease in force that occurs with repetitive trains of tetanic stimuli is called fatigue . This property is quantified by the fatigue index , which is defined as the ratio of the tetanic force after 2 minutes of repetitive stimulation to the force generated by the first tetanus. The fatigue index of FF motor units is typically less than 0.25, whereas it is greater than 0.75 in FR and S motor units. Although fatigue is a well-characterized phenomenon, the underlying cellular mechanisms are poorly understood (but see next section).
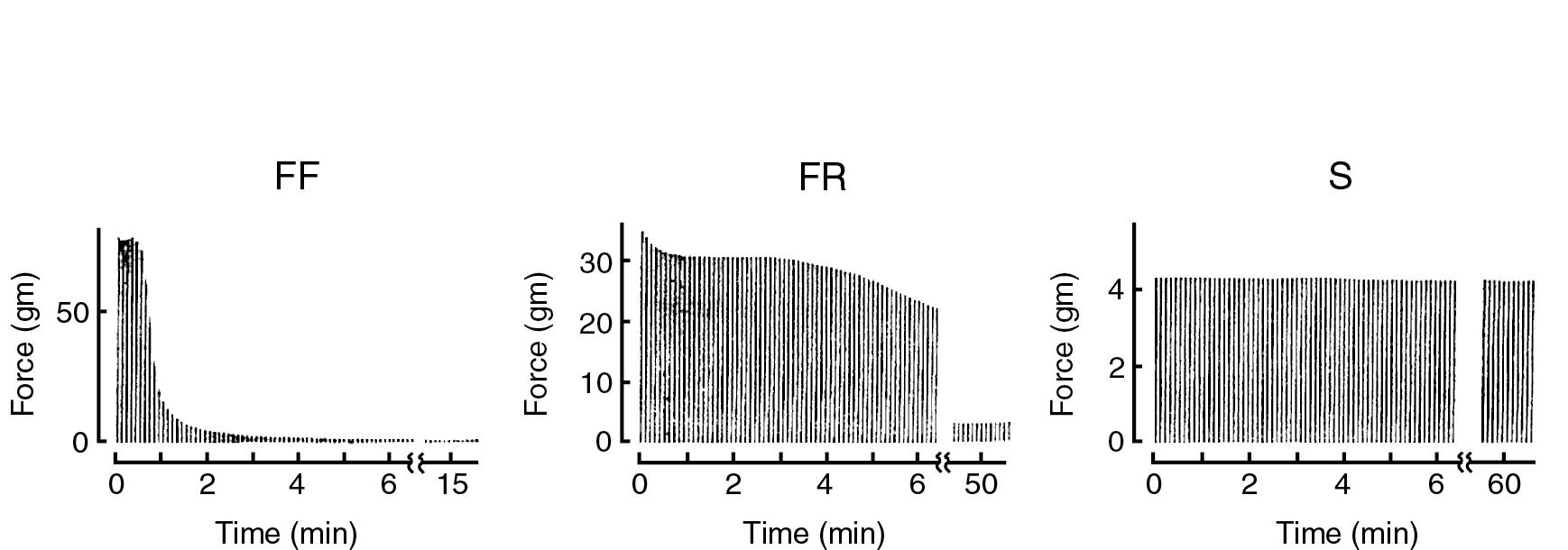
Whole muscle usually contains mixtures of the three types of motor units. For example, the medial gastrocnemius (calf) muscle of the cat contains FF, FR, and S motor units. The recruitment order of these motor units follows the size principle: motor units that generate the smallest force are recruited first, followed by larger motor units as the requirement for additional force increases. This size-ordered recruitment confers certain mechanical advantages on the operation of the whole muscle. Type S units are used to maintain posture; FR and FF units are used, respectively, for sustained and burst movements ( Box 16.3 ).
The medial gastrocnemius (calf) muscle in the cat is a mixture of fast-twitch, fatigable (FF); fast-twitch, fatigue-resistant (FR); and slow (S) motor units. The order in which motor units are recruited depends on the magnitude of the force generated by each motor unit. Consequently, the total muscle force is a nonlinear function of the fraction of motor units activated ( Fig. 16.11 ). First to be recruited are S units, which generate small, slow contractions and are fatigue-resistant; this is advantageous during sustained activity at low force levels, such as maintaining posture. For actions requiring more force, FR units are recruited next. FR motor units contract quickly, generate moderate force, and are relatively resistant to fatigue. These properties are advantageous for sustained activity requiring moderate force, such as running. Together, FR and S motor units constitute 60% of the pool, but generate only approximately 25% of the maximum force. Demand for larger forces is met by activating FF motor units, which generate large forces rapidly, but they fatigue quickly and are used infrequently. They are recruited when a short burst of strenuous activity is required, as in sprinting or jumping.

Tetanic activation of skeletal muscle causes [K + ] to rise in the T-tubules of fast twitch fibers and apparently leads to fatigue when clearance by Na + pumping and diffusion cannot keep pace with K + efflux during the action potentials. Fatigue is augmented in mice whose α2 Na + pumps have been genetically knocked out (equivalent to ouabain blockade), and in isolated muscle by ouabain treatment. Conversely, fatigue is attenuated in mice with skeletal muscle-specific mutation of α2 Na + pumps that confers ouabain resistance (presumably because the normal circulating endogenous ouabain cannot inhibit these pumps; see Chapter 11 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here