Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Upon completion of this chapter the student should be able to answer the following questions :
Why do changes in water balance alter [Na + ] of extracellular fluid (ECF)?
How is the secretion of arginine vasopressin (AVP) controlled by changes in the body fluid osmolality, blood volume, and systemic blood pressure?
What cellular events are associated with the AVP action on the collecting duct and how do they increase the water permeability of this nephron segment?
What is the role of Henle’s loop in the production of both dilute and concentrated urine?
What is the composition of the medullary interstitial fluid, and how does it contribute to urinary concentration?
What are the roles of vasa recta in the process of diluting and concentrating urine?
How is the diluting and concentrating ability of the kidneys quantitated?
Why do changes in Na + balance alter the volume of extracellular fluid?
What is the effective circulating volume, how is it influenced by changes in Na + balance, and how does it influence renal Na + excretion?
What mechanisms regulate the effective circulating volume?
What are the major signals for altering renal Na + excretion?
How do changes in extracellular fluid volume alter Na + transport in the different nephron segments and how these changes regulate renal Na + excretion?
What mechanisms contribute to edema formation and what roles do the kidneys play in this process?
Body fluid osmolality represents one of the most highly regulated parameters of human physiology. The kidney controls the osmolality and volume of body fluid by regulating excretion of water and NaCl, respectively. This chapter discusses the renal mechanisms of water and NaCl excretion. The composition and volumes of the various body fluid compartments are reviewed in Chapter 2 .
As described in Chapter 2 , water constitutes approximately 60% of the healthy adult human body. Water is distributed between two major compartments in the body—the intracellular fluid (ICF) and extracellular fluid (ECF)—that exist in osmotic equilibrium because aquaporins (e.g., AQP1) make the cellular membranes permeable to water. The major source of body water is oral intake of liquids and solid foods containing a liquid component. Water is also generated during metabolism of ingested foods (e.g., carbohydrates). Intravenous fluids are an important route of water supply during disease states.
The kidneys are responsible for regulating water balance and under most conditions are the major route for eliminating water from the body ( Table 35.1 ). Water is also lost through the gastrointestinal tract. Fecal water loss is normally small (≈100 mL/day) but can increase dramatically with diarrhea (e.g., 20 L/day with cholera). Vomiting can also cause gastrointestinal water losses. The production of sweat is an active process of water and electrolyte elimination. The water loss through kidneys, gastrointestinal tract, and sweat glands is termed sensible water loss because the person is aware of its occurrence. Other routes of water elimination from the body are evaporation from cells of the skin and respiratory passages; collectively, the loss is termed insensible water loss because the individual is unaware of its occurrence. Sweating and insensible water loss can increase dramatically in a hot environment, during exercise, or in the presence of fever ( Table 35.2 ).
| Gain | (mL/day) |
| Fluid a | 1200 |
| Food | 1000 |
| Metabolically produced from food | 300 |
| Total | 2500 |
| Loss (mL/day) | |
| Insensible | 700 |
| Sweat | 100 |
| Feces | 100 |
| Urine | 1600 |
| Total | 2500 |
a Fluid intake may vary widely for social and cultural reasons.
| Normal Temperature | Hot Weather a | Prolonged Heavy Exercise a | |
|---|---|---|---|
| Water Loss | |||
| Insensible loss | |||
| Skin | 350 | 350 | 350 |
| Lungs | 350 | 250 | 650 |
| Sweat | 100 | 1400 | 5000 |
| Feces | 100 | 100 | 100 |
| Urine a | 1600 | 1200 | 500 |
| T otal loss | 2500 | 3300 | 6600 |
a In hot weather and during prolonged heavy exercise, water balance is maintained by increased water ingestion. Decreased excretion of water by the kidneys alone is insufficient to maintain water balance.
Although water loss from sweating, defecation, and evaporation from the lungs and skin can vary depending on the environmental conditions or during disease states, loss of water by these routes cannot be regulated. In contrast, renal excretion of water is tightly regulated to maintain water balance. Maintenance of water balance requires that water intake and loss are precisely matched. If intake exceeds loss, positive water balance exists. Conversely, when intake is lower than loss, negative water balance exists (see Chapter 2 for review of steady-state balance).
During states of decreased water intake or excessive water losses, the kidneys conserve water by producing low-volume, concentrated urine that is hyperosmotic with respect to plasma. Conversely, when water intake is high, a large volume of hypoosmotic urine is produced. In a healthy individual, urine osmolality (U osm ) can vary from approximately 50 to 1200 mOsm/kg H 2 O, and the corresponding urine volume can vary from approximately 18 L/day to 0.5 L/day. Importantly the kidneys can regulate excretion of water separately from excretion of total solute (e.g., Na + , K + , urea, etc.) ( Fig. 35.1 ). The ability to regulate water excretion separate from excretion of solutes is essential for survival because it allows water balance to be achieved without upsetting other homeostatic functions of the kidneys.

It is important to recognize that disorders of water balance are manifested by alterations in body fluid osmolality, which are usually measured by changes in plasma osmolality (P osm ). Because the major determinant of plasma osmolality is Na + (with its anions Cl − and HCO 3 − ), these disorders also result in alterations in plasma or serum [Na + ] ( Fig. 35.2 ). One of the most common fluid and electrolyte disorders seen in clinical practice is an alteration in serum [Na + ]. When an abnormal serum [Na + ] is found in an individual, it is tempting to suspect a problem in Na + balance. However, the problem most often relates to water balance, not Na + balance. As described later, changes in Na + balance result in alterations in the ECF volume (ECFV), not its osmolality.
![Fig. 35.2, Response to changes in water balance. Illustrated are the effects of adding or removing 1 L of water from the ECF of a 70-kg individual. Positive Water Balance: (1) Addition of 1 L of water increases the ECFV and reduces its osmolality. The [Na + ] is also decreased (hyponatremia). (2) The normal renal response is to excrete 1 L of water as hypoosmotic urine. (3) As a result of the renal excretion of water, the ECFV, osmolality, and [Na + ] are returned to normal. Negative Water Balance: (4) The loss of 1 L of water from the ECF decreases its volume and increases its osmolality. The [Na + ] is also increased (hypernatremia). (5) The renal response is to conserve water by excreting a small volume of hyperosmotic urine. (6) With ingestion of water, stimulated by thirst, and conservation of water by the kidneys, the ECF volume, osmolality, and [Na + ] are returned to normal. Size of the boxes indicates relative ECF volume. Fig. 35.2, Response to changes in water balance. Illustrated are the effects of adding or removing 1 L of water from the ECF of a 70-kg individual. Positive Water Balance: (1) Addition of 1 L of water increases the ECFV and reduces its osmolality. The [Na + ] is also decreased (hyponatremia). (2) The normal renal response is to excrete 1 L of water as hypoosmotic urine. (3) As a result of the renal excretion of water, the ECFV, osmolality, and [Na + ] are returned to normal. Negative Water Balance: (4) The loss of 1 L of water from the ECF decreases its volume and increases its osmolality. The [Na + ] is also increased (hypernatremia). (5) The renal response is to conserve water by excreting a small volume of hyperosmotic urine. (6) With ingestion of water, stimulated by thirst, and conservation of water by the kidneys, the ECF volume, osmolality, and [Na + ] are returned to normal. Size of the boxes indicates relative ECF volume.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/ControlofBodyFluidOsmolalityandVolume/1_3s20B9780323847902000357.jpg)
In the clinical setting, hypoosmolality (a reduction in plasma osmolality) shifts water into cells, and this process results in cell swelling (see Chapter 2 ). Symptoms associated with hypoosmolality are related primarily to swelling of brain cells. For example, a rapid fall in P osm can alter neurological function and thereby cause nausea, malaise, headache, confusion, lethargy, seizures, and coma. When P osm is increased (i.e., hyperosmolality ), water is lost from cells. Symptoms of an increase in P osm are also primarily neurological and include lethargy, weakness, seizures, coma, and even death.
Symptoms associated with changes in body fluid osmolality vary depending on how quickly osmolality changes. The rapid osmolality changes (i.e., over hours) are less well tolerated than changes that occur more gradually (i.e., over days to weeks). Indeed, individuals who have developed alterations in their body fluid osmolality over an extended period of time may be entirely asymptomatic. This reflects the compensatory mechanisms in neurons to minimize changes in cell volume over time. For example, cells eliminate intracellular osmoles in response to hypoosmolality while they generate new intracellular osmoles in response to hyperosmolality (see Chapter 2 ).
The following sections discuss the mechanisms by which the kidneys excrete either hypoosmotic (dilute) or hyperosmotic (concentrated) urine. Control of arginine vasopressin secretion and its essential role in regulating water excretion by the kidneys are also explained (see also Chapter 41 ).
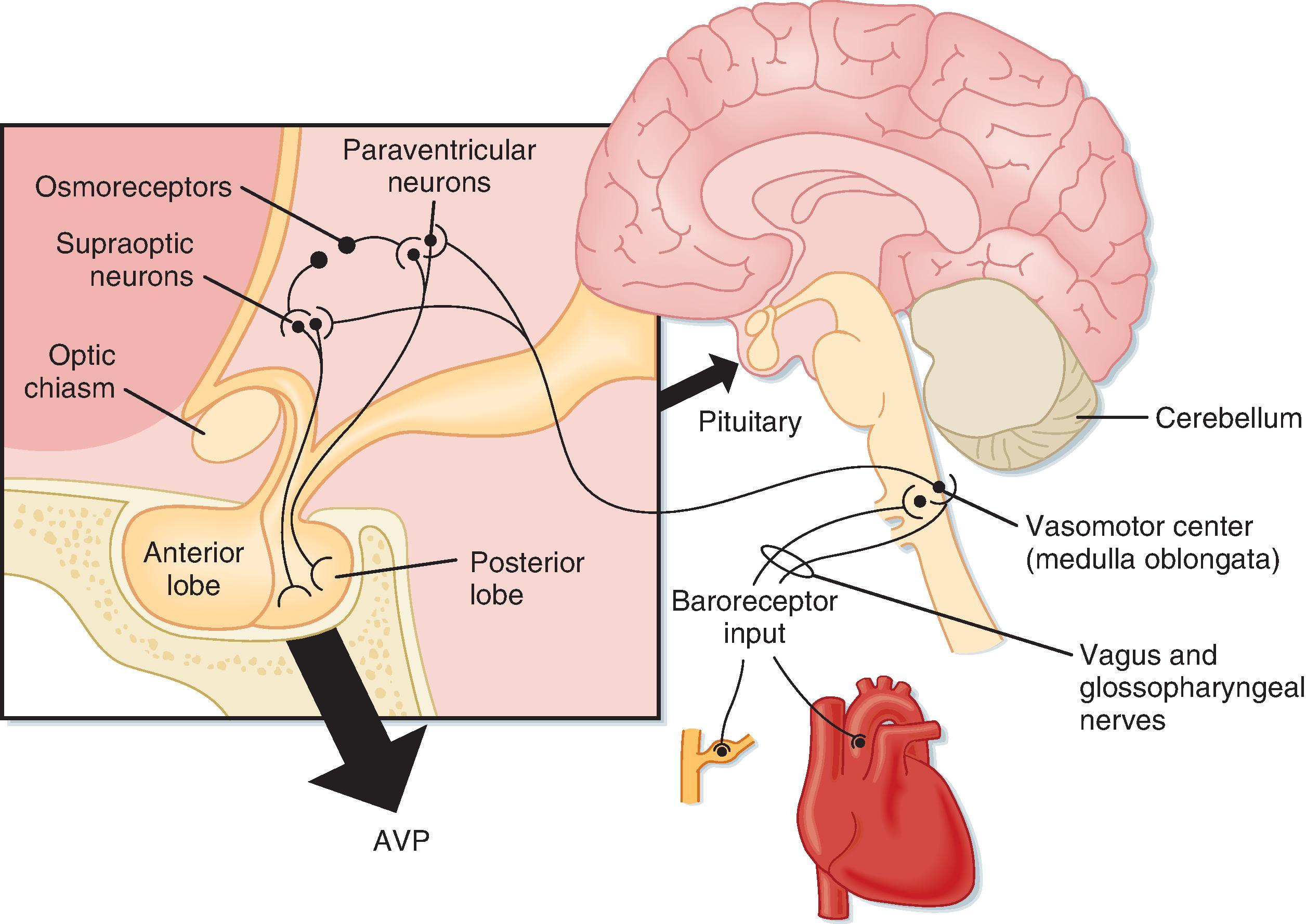
Arginine vasopressin (AVP) , a nonapeptide, is synthesized in the hypothalamic supraoptic (SON) and paraventricular (PVN) nuclei. a
a The SON and PVN synthesize either AVP or oxytocin. AVP-secreting cells predominate in the SON, whereas oxytocin-secreting neurons are primarily found in the PVN. The synthesized hormone is packaged in granules that are transported down the axon of the cell and stored in nerve terminals located in the neurohypophysis (posterior pituitary). The anatomy of the hypothalamus and pituitary gland is shown in Fig. 35.3 (see also Chapter 41 ).
AVP acts though vasopressin (V) receptors. Several nephron segments express the type-2 receptor (V 2 ) that mediates the kidneys’ ability to regulate the urine volume and osmolality. When the plasma AVP level is low, a large volume of urine is excreted (diuretic effect) , and the urine osmolality is lower than that of plasma (i.e., dilute). When the plasma AVP level is high, a small volume of urine is excreted (antidiuretic effect) , and the urine osmolality is greater than that of plasma (i.e., concentrated). Hence, AVP is also known as the antidiuretic hormone (ADH) .
The gene for AVP is located on chromosome 20. It contains approximately 2000 base pairs with three exons and two introns. The gene codes for a preprohormone that consists of a signal polypeptide, the AVP molecule, neurophysin, and a glycopeptide (copeptin). As the cell processes the preprohormone the signal peptide is cleaved off in the rough endoplasmic reticulum. Once packaged in neurosecretory granules, the preprohormone is further cleaved into AVP, neurophysin, and copeptin molecules. The neurosecretory granules are then transported down the axon to the posterior pituitary and stored in the nerve endings until released. When the neurons are stimulated to secrete AVP, the action potential opens Ca ++ channels in the nerve terminal, which raises the intracellular [Ca ++ ] and causes exocytosis of the neurosecretory granules. All three peptides are secreted in this process. Neurophysin and copeptin do not have an identified physiological function.
Secretion of AVP by the posterior pituitary can be influenced by several factors. Under physiological conditions AVP secretion is controlled by two major mechanisms: osmotic (changes in plasma osmolality) and hemodynamic (changes in blood pressure or volume). Other factors that alter AVP secretion include nausea, acute hypoglycemia, angiotensin II (stimulate), and atrial natriuretic peptide (inhibits). A number of medications and recreational drugs affect AVP secretion. For example, nicotine stimulates secretion, whereas ethanol and anti-emetics inhibit it.
Changes in the body fluid osmolality play the most important role in regulating AVP secretion. Specialized neurons termed osmoreceptors , located in the organum vasculosum of the lamina terminalis (OVLT) of the hypothalamus, modulate osmolality changes within a normal range between 275 and 295 mOsm/kg H 2 O. The osmoreceptor cells sense changes in body fluid osmolality in response to small changes in the concentration of effective solutes, such as Na + and its anions, and are insensitive to ineffective solutes, such as urea and glucose (see Chapter 1 ).
Effective solutes are those that penetrate cells slowly or not at all, thereby creating an osmotic gradient resulting in efflux of water across the cell membrane. When the effective plasma osmolality (tonicity) increases, osmoreceptor cell shrinkage activates membrane nonselective cationic channels that generate inward current depolarizing the cells. In turn, the osmotically evoked action potential in the OVLT neurons synaptically propagates the electrical activity to downstream effector neurons in the SON and PVN leading to AVP release. Conversely, when the effective plasma osmolality decreases, AVP synthesis and secretion are inhibited. Because AVP is rapidly degraded in the plasma, circulating levels can be reduced to zero within minutes. Recent data demonstrate that cell membrane stretch rather than cell volume determines osmoreceptor activity. The transient receptor potential vanilloid (TRPV) family of cation channels, including TRPV1, TRPV2, and TRPV4, mediate osmotic stimuli in mammals. The channels are activated by cell membrane stretch and mediate inactivation of the osmoreceptors in hypoosmolal states. The mediators of stretch-inactivated cationic channels that respond to cell shrinkage in hyperosmolal states are currently unknown.
The coordinated action of the stimulatory and inhibitor components of the osmoreceptors creates a threshold, or set point. Fig. 35.4A illustrates the effect of changes in plasma osmolality on circulating AVP levels. The slope of the relationship is quite steep and accounts for the sensitivity of the system. The set point is the plasma osmolality value at which AVP secretion begins to increase. Below the set point, virtually no AVP is released. The absolute level of the effective plasma osmolality at which minimally and maximally effective levels of plasma AVP occur, varies appreciably from person to person, due to genetic influences on the set and sensitivity of the system. However, the average set point for AVP release corresponds to a plasma osmolality of 280 mOsmol/kg H 2 O and levels only 2% to 4% higher normally result in maximum antidiuretic effect. The set point is relatively stable in healthy individuals but can be decreased by pregnancy, menstrual cycle, estrogen, or a significant drop in blood pressure or blood loss. The mechanism responsible for the set point shift during pregnancy is likely due to increased levels of certain hormones (e.g., relaxin and chorionic gonadotropin).

A decrease in blood volume or pressure also stimulates AVP secretion. The receptors responsible for this response are located in both the low-pressure (left atrium and large pulmonary vessels) and the high-pressure (aortic arch and carotid sinus) sides of the circulatory system. Because the low-pressure receptors are located in the high-compliance side of the circulatory system (i.e., venous), and because the majority of blood is on the venous side, these low-pressure receptors can be viewed as responding to the overall vascular volume. The high-pressure receptors respond to arterial pressure. Both groups of receptors are sensitive to stretch of the wall of the structure in which they are located (e.g., cardiac atrial and aortic arch) and are termed baroreceptors . Signals from these receptors are carried in afferent fibers of the vagus and glossopharyngeal nerves to the brainstem (solitary tract nucleus of the medulla oblongata), which is part of the center that regulates heart rate and blood pressure (see also Chapter 18 ). Signals are then relayed from the brainstem to the AVP secretory cells of the supraoptic and paraventricular hypothalamic nuclei. The sensitivity of the baroreceptor system is less than that of the central osmoreceptors, and a 5% to 10% decrease in blood volume or pressure is required before AVP secretion is stimulated. This is illustrated in Fig. 35.4B . A number of substances have been shown to alter the secretion of AVP through their effects on blood pressure. These include bradykinin and histamine, which lower pressure and thus stimulate AVP secretion, and norepinephrine, which increases blood pressure and inhibits AVP secretion.
Alterations in blood volume and pressure also affect the response to changes in body fluid osmolality (see Fig. 35.4C ). With a decrease in blood volume or pressure, the set point is shifted to lower osmolality values and the slope of the relationship is steeper. In terms of survival of the individual this means that faced with circulatory collapse, the kidneys will continue to conserve water, even though by doing so they reduce the osmolality of the body fluids. With an increase in blood volume or pressure, the opposite occurs. The set point is shifted to higher osmolality values and the slope is decreased.
Inadequate release of AVP from the posterior pituitary results in excretion of a large volume of dilute urine (polyuria) . To compensate for this loss of water the individual must ingest a large volume of water (polydipsia) to maintain constant body fluid osmolality. If the individual is deprived of water, body fluid will become hyperosmotic. This condition is called central (pituitary) diabetes insipidus (CDI) . It can be inherited, although this is rare. CDI occurs more commonly after head trauma and with brain neoplasms or infections. Individuals with CDI have a urine-concentrating defect that can be corrected by administration of exogenous AVP. The inherited (autosomal dominant) form of CDI is due to mutations in different regions of the AVP gene (i.e., AVP, copeptin, and neurophysin). The human placenta produces a cysteine aminopeptidase that degrades AVP. In some women the levels of this vasopressinase result in diabetes insipidus. The associated polyuria can be treated by administration of the synthetic AVP analog desmopressin (DDAVP) .
The syndrome of inappropriate AVP (ADH) secretion (SIADH) is a relatively common clinical problem characterized by plasma AVP levels that are elevated above what would be expected on the basis of body fluid osmolality, blood volume, or blood pressure—hence the term inappropriate AVP (ADH) secretion . The AVP action in the kidney collecting duct causes recruitment of water channels (see below), thus augmenting the effect of AVP to stimulate renal water retention. Individuals with SIADH retain water, and their body fluids become progressively hypoosmotic. In addition, their urine is more hyperosmotic than expected based on the low body-fluid osmolality. SIADH can be caused by drugs, central nervous system infection or tumors, pulmonary diseases, or lung carcinoma. These conditions either stimulate AVP secretion by altering neural input to the AVP secretory cells or secrete AVP (small cell carcinoma). Drug-related SIADH is increasingly common and can be associated with many classes of over the counter and prescription medications, including proton pump inhibitors, nonsteroidal anti-inflammatory, antidepressants, antiseizure, antipsychotic, and antitumor drugs. AVP receptor antagonists bind to V 1A and V 2 receptors and induce water diuresis ( aquaresis ) to treat SIADH and other conditions resulting from AVP-dependent water retention by the kidneys (e.g., congestive heart failure and hepatic cirrhosis).
The primary action of AVP on the kidneys is to enhance absorption of water from the tubular fluid by increasing the water permeability of the latter portion of the distal tubule and collecting duct. In addition, and importantly, AVP increases the permeability of the medullary portion of the collecting duct to urea. Finally, AVP stimulates NaCl reabsorption by the thick ascending limb of Henle’s loop, distal tubule, and collecting duct.
In the absence of AVP, the apical membrane of principal cells (see Chapter 34 ), located in the latter portion of the distal tubule and along the collecting duct, is relatively impermeable to water. This reflects the fact that in the absence of AVP the apical membrane of these cells contains few water channels (aquaporins), since they are stored inside cells. Thus, in the absence of AVP, little water is reabsorbed by these nephron segments. Binding of AVP to the V 2 receptor located in the basolateral membrane of principal cells results in the recruitment of aquaporin (AQP2) water channels to the apical membrane, allowing water to enter the cell from the tubular lumen. This water then exits the cell across the basolateral membrane, which is always freely permeable to water owing to the presence of AQP3 and AQP4 water channels. Thus in the presence of AVP, water is reabsorbed from the renal tubules.
The gene for the V 2 receptor is located on the X chromosome and codes for a 371–amino acid protein that belongs to the family of receptors with seven membrane spanning domains coupled to heterotrimeric G proteins. As shown in Fig. 35.5 , binding of AVP to its receptor on the basolateral membrane activates adenyl cyclase. The increase in intracellular cyclic adenosine monophosphate (cAMP) then activates protein kinase A (PKA), which results in the phosphorylation of AQP2 water channels and increased transcription of the AQP2 gene via activation of a cAMP-response element (CRE). Intracellular vesicles containing phosphorylated AQP2 move toward the apical membrane along microtubules driven by the molecular motor dynein. Once near the apical membrane, proteins called SNAREs interact with the AQP2 containing vesicles and facilitate their fusion with the plasma membrane. Insertion of AQP2 to the membrane allows water to enter the cell driven by the osmotic gradient (lumen osmolality < cell osmolality). The water then exits the cell across the basolateral membrane through AQP3 and AQP4 water channels, which are constitutively present in the basolateral membrane. When the V 2 receptor is not occupied by AVP, the AQP2 water channels are removed from the apical membrane by clathrin-mediated endocytosis, thus rendering the apical membrane impermeable to water. The endocytosed AQP2 molecules may be either stored in intracellular vesicles, ready for reinsertion into the apical membrane when AVP levels in the plasma increase, or degraded.

AVP also regulates long-term expression of AQP2 and AQP3. When large volumes of water are ingested over an extended period of time (e.g., psychogenic polydipsia), the abundance of AQP2 and AQP3 in principal cells is reduced. As a consequence, when water ingestion is restricted, these individuals cannot maximally concentrate urine. Conversely, in states of restricted water ingestion, AQP2 and AQP3 protein expression in principal cells increases, thereby facilitating excretion of maximally concentrated urine.
It is also clear that expression of AQP2 (and in some instances also AQP3) varies in pathological conditions associated with disturbances in urine concentration and dilution. AQP2 expression is reduced in a number of conditions associated with impaired urine-concentrating ability (e.g., hypercalcemia, hypokalemia). By contrast, in conditions associated with water retention (e.g., congestive heart failure, hepatic cirrhosis, pregnancy) AQP2 expression is increased.
AVP also increases the permeability of the terminal portion of the inner medullary collecting duct to urea leading to increased reabsorption of urea and increased osmolality of the medullary interstitial fluid necessary for maximal urine concentration. The cells of the collecting duct express two types of urea transporters (UT), UT-A1 localized to the apical membrane and UT-A3 localized to the basolateral membrane. AVP, acting through the cAMP/PKA cascade, increases expression of UT-A1 and UT-A3. Increasing the osmolality of the interstitial fluid of the renal medulla also increases the permeability of the inner medullary collecting duct to urea. This effect is mediated by the phospholipase C/protein kinase C (PKC) pathway, which increases UT-A1 and UT-A3 expression.
AVP also stimulates reabsorption of NaCl by the thick ascending limb of Henle’s loop and by the distal tubule and cortical segment of the collecting duct. This increase in Na + reabsorption is associated with increased abundance of three Na + transporters: the Na + /K + /2Cl − symporter (thick ascending limb of Henle’s loop), the Na + /Cl − symporter (distal tubule), and the Na + channel ENaC (the latter portion of the distal tubule and collecting duct). Stimulation of thick ascending limb NaCl transport may help maintain the hyperosmotic medullary interstitium necessary for absorption of water from the medullary portion of the collecting duct (see below).
When the collecting ducts do not respond normally to AVP, urine cannot be maximally concentrated leading to polyuria and polydipsia. This clinical entity is termed nephrogenic diabetes insipidus (NDI) to distinguish it from central diabetes insipidus. NDI can result from a number of systemic disorders and rarely can be inherited. Acquired NDI is caused by decreased expression of AQP2 in the collecting duct. Decreased expression of AQP2 impairs the urine-concentrating ability during hypokalemia, lithium ingestion (35% of individuals who take lithium for bipolar disorder develop some degree of NDI), urinary tract obstruction, low-protein diet, and hypercalcemia. Mutations in the AVP V 2 receptor AVPR2 gene or the AQP2 gene lead to inherited NDI. Approximately 90% of the hereditary forms result from mutations in the AVPR2 gene and the remaining 10% result from AQP2 gene mutations. Since the AVPR2 gene is located on the X chromosome, its mutations lead to X-linked NDI. The AQP2 gene is located on chromosome 12, and its mutations can lead to autosomal recessive and very rarely autosomal dominant NDI. The AQP2 channel functions at the cell membrane as homotetramers. Mutations leading to recessive NDI affect the region of AQP2 gene associated with the formation of the homotetramer water channel pore. Heterozygous carriers produce both normal and defective AQP2 monomers. Since the defective AQP2 monomers are retained in the endoplasmic reticulum, the water channel forms only from normal monomers and the carriers remain asymptomatic. By contrast, mutations leading to the dominant NDI affect the region of AQP2 gene associated with post-translational modifications, such as AQP2 phosphorylation and not the water channel pore.
Activating (gain-of-function) mutations in the AVPR2 gene lead to nephrogenic syndrome of inappropriate antidiuresis (NSIAD) . In this X-linked disorder, V 2 receptors are constitutively activated. These individuals have laboratory findings similar to those seen in SIADH, including reduced plasma osmolality, hyponatremia (reduced plasma [Na + ]), and urine more concentrated than would be expected from the reduced body fluid osmolality. However, unlike SIADH where circulating levels of AVP are elevated and thus responsible for water retention by the kidneys, these individuals have undetectable levels of AVP in their plasma.
The perception of thirst is affected by changes in plasma osmolality, blood volume, or blood pressure. Increased plasma osmolality and reduced blood volume or pressure increase thirst. Of these stimuli, hyperosmolality is more potent and its increase by only 2% to 3% produces a strong desire to drink, whereas loss of blood volume or decrease in blood pressure in the range of 10% to 15% is required to produce the same response in thirst.
As already discussed, there is a genetically determined threshold for AVP secretion (i.e., a body fluid osmolality above which AVP secretion increases). Similarly there is a genetically determined threshold for triggering the sensation of thirst. However, the thirst threshold is higher than the threshold for AVP secretion. On average the threshold for AVP secretion is approximately 280 mOsm/kg H 2 O, whereas the thirst threshold is approximately 295 mOsm/kg H 2 O. Because of this difference, thirst is stimulated at a body fluid osmolality when AVP secretion is almost maximal.
The center involved in regulating water intake (the thirst center) is located in the same region of the hypothalamus involved with regulating AVP secretion. However, it is not certain whether the same cells serve both functions. Indeed, the thirst response, like the regulation of AVP secretion, only occurs in response to effective solute (e.g., NaCl). Even less is known about the pathways involved in the thirst response to decreased blood volume or pressure, but it is believed that the pathways are the same as those involved in the volume- and pressure-related regulation of AVP secretion. Angiotensin II, acting on cells of the thirst center, also evokes the sensation of thirst. Because angiotensin II levels are increased when blood volume and pressure are reduced, this effect of angiotensin II contributes to the homeostatic response that restores and maintains body fluids at their normal volume.
The sensation of thirst is satisfied by the act of drinking, even before sufficient water is absorbed from the gastrointestinal tract to correct the plasma osmolality. It is interesting to note that cold water is more effective in reducing the thirst sensation. Oropharyngeal and upper gastrointestinal receptors appear to be involved in this response. However, relief of the thirst sensation via these receptors is short lived, and thirst is only completely satisfied when the plasma osmolality or blood volume or pressure is corrected.
It should be apparent that the AVP and thirst systems work in concert to maintain water homeostasis. An increase in plasma osmolality evokes drinking and, via AVP action in the kidneys, conservation of water. Conversely, when plasma osmolality is decreased, thirst is suppressed and, in the absence of AVP, renal water excretion is enhanced. When the fluid intake is dictated by cultural and social determinants rather than thirst, maintaining normal body fluid osmolality relies solely on the ability of the kidneys to excrete water. How the kidneys accomplishes this is discussed in detail in the following sections of this chapter.
With adequate access to water, the thirst mechanism can prevent development of hyperosmolality. This mechanism is responsible for the polydipsia seen in response to the polyuria of both CDI and NDI. Most individuals ingest water/beverages even in the absence of the thirst sensation. Normally the kidneys are able to excrete this excess water because they can excrete up to 18 L/day of urine. However, in some instances, the volume of water ingested exceeds the kidneys’ capacity to excrete water, especially over short periods of time. When this occurs, body fluids become hypoosmotic.
An example of how water intake can exceed the capacity of the kidneys to excrete water is long-distance running. A study of participants in the Boston Marathon found that 13% of the runners developed hyponatremia during the race. b
b Almond CS, et al. Hyponatremia among runners in the Boston Marathon. N Engl J Med ; 2005;352:1150-1556.
This reflected the practice of some runners to ingest water or other hypotonic drinks during the race to remain “well hydrated.” In addition, water is produced from the metabolism of glycogen and triglycerides used as fuels by the exercising muscle. Hyponatremia developed because, over the course of the race, the runners achieved a positive water balance resulting from higher ingestion and generation of water compared to its excretion by the kidneys and loss with sweat. In some racers the hyponatremia was severe enough to elicit the neurological symptoms.
The maximum amount of water that can be excreted by the kidneys depends on the amount of solute excreted, which in turn depends on food intake. For example, with maximally dilute urine (U osm = 50 mOsm/kg H 2 O), the maximum urine output of 18 L/day will be achieved only if the solute excretion rate is 900 mmol/day:
If solute excretion is reduced, as commonly occurs in the elderly with reduced food intake, the maximum urine output will decrease. For example, if solute excretion is only 400 mmol/day, a maximum urine output (at U osm = 50 mOsm/kg H 2 O) of only 8 L/day can be achieved. Thus individuals with reduced food intake have a reduced capacity to excrete water.
As already noted, water excretion is regulated separately from solute excretion. For this to occur, the kidneys must be able to excrete urine that is either hypoosmotic or hyperosmotic with respect to body fluid. This ability to excrete urine of varying osmolality in turn requires that solute be separated from water at some point along the nephron. As discussed in Chapter 34 , reabsorption of solute in the proximal tubule results in reabsorption of a proportional amount of water. Hence solute and water are not separated in this portion of the nephron. Moreover, this proportionality between proximal tubule water and solute reabsorption occurs regardless of whether the kidneys excrete dilute or concentrated urine. Thus, the proximal tubule reabsorbs a large portion of the filtered amount of solute and water, but it does not produce dilute or concentrated tubular fluid. The loop of Henle, in particular the thick ascending limb, is the major site where solute and water are separated. Thus excretion of both dilute and concentrated urine requires normal function of the Henle’s loop.
Excretion of hypoosmotic urine is relatively easy to understand. The nephron must simply reabsorb solute from the tubular fluid and not allow water reabsorption to also occur. As just noted, and as described in greater detail later, reabsorption of solute without concomitant water reabsorption occurs in the ascending limb of Henle’s loop. Under appropriate conditions (i.e., in the absence of AVP) the distal tubule and collecting duct also dilute the tubular fluid by reabsorbing solute but not water.
Excretion of hyperosmotic urine (or urinary concentration) is more complex and in essence involves removing water from the tubular fluid without solute. Because water movement is passive, driven by an osmotic gradient, the kidney must generate a hyperosmotic compartment into which water is reabsorbed, without solute, osmotically from the tubular fluid. The hyperosmotic compartment that serves this function is the interstitium of the renal medulla. Henle’s loop is critical for generating the hyperosmotic medullary interstitium. Once established, this hyperosmotic compartment drives water reabsorption from the collecting duct and thereby concentrates urine.
Fig. 35.6 summarizes tubular fluid osmolality at several points along the nephron, in both the absence and presence of AVP. Note that tubular fluid entering the loop of Henle from the proximal tubule is isosmotic with respect to plasma and is so regardless of the absence or presence of AVP. Also, tubular fluid leaving the thick ascending limb is hypoosmotic with respect to plasma, in both the absence and presence of AVP. The osmolality of tubular fluid along the collecting duct is hypoosmotic with respect to plasma in the absence of AVP and becomes progressively hyperosmotic (i.e., from the cortex to inner medulla) in the presence of AVP.

Establishment and maintenance of the hyperosmotic medullary interstitium has been a subject of study since the 1940s and the model remains incomplete. While it is generally accepted that the outer medulla contributes to the osmotic gradient by means of an active process termed countercurrent multiplication , the source of the gradient in the inner medulla is still incompletely understood. With the caveat that the current model needs refinement, it is presented here because it embodies some fundamental concepts that underlie the process.
Countercurrent multiplication involves reabsorption of solute (principally NaCl) without water from the ascending limb of Henle’s loop into the surrounding medullary interstitium. This decreases the osmolality in the tubular fluid and raises the osmolality of the interstitium at this point. The increased osmolality of the interstitium then causes water to be reabsorbed from the descending limb of Henle’s loop, thus increasing the tubular fluid osmolality in this segment. Thus at any point along the loop of Henle the fluid in the ascending limb has an osmolality less than fluid in the adjacent descending limb. This osmotic difference is termed the single effect . Because of the countercurrent flow of tubular fluid in the descending limb (fluid flowing into the medulla) and ascending limb (fluid flow out of the medulla), this single effect could be multiplied, resulting in an osmotic gradient within the medullary interstitium, where the tip of the papilla has an osmolality of 1200 mOsm/kg H 2 O compared to 300 mOsm/kg H 2 O at the corticomedullary junction.
Fig. 35.7 schematically depicts the processes for diluting and concentrating urine. Three key concepts underlie these processes:
Urine is concentrated by AVP-dependent reabsorption of water from the collecting duct.
Reabsorption of NaCl from the ascending limb of Henle’s loop dilutes the tubular fluid and at the same time generates a high [NaCl] in the medullary interstitium (up to 600 mmol/L at the tip of the papilla), which then drives water reabsorption from the collecting duct.
Urea accumulates in the medullary interstitium (up to 600 mmol/L), which allows the kidneys to excrete urine with the same high urea concentration. This allows large amounts of urea to be excreted with relatively little water.

First, how the kidneys excrete dilute urine (water diuresis or aquaresis) when AVP levels are low or zero is considered. The following numbers refer to those encircled in Fig. 35.7A :
Fluid entering the descending thin limb of the loop of Henle from the proximal tubule is isosmotic with respect to plasma. This reflects the essentially isosmotic nature of solute and water reabsorption in the proximal tubule (see Chapter 34 ). (N ote : Water is reabsorbed from the segments of the proximal tubule via AQP1.)
Water is reabsorbed from the thin descending limb of Henle’s loop. Most of this water is reabsorbed in the outer medulla, thereby limiting the amount of water added to the deepest part of the inner medullary interstitial space and thus preserving the hyperosmolality of this region of the medulla. (N ote : Water is reabsorbed via AQP1.)
In the inner medulla the terminal portion of the descending thin limb and all of the thin ascending limb is impermeable to water. (N ote : AQP1 is not expressed.) These same nephron segments express the Cl − channel CLC-K1, which mediates Cl − reabsorption, with Na + following via the paracellular pathway. This passive reabsorption of NaCl without concomitant water reabsorption begins the process of diluting the tubular fluid.
The thick ascending limb of the loop of Henle is also impermeable to water and actively reabsorbs NaCl from the tubular fluid and thereby dilutes it further (see Chapter 34 ). Dilution occurs to such a degree that this segment is often referred to as the diluting segment of the kidney. Fluid leaving the thick ascending limb is hypoosmotic with respect to plasma (see Fig. 35.6 ).
The distal tubule and cortical portion of the collecting duct actively reabsorb NaCl. In the absence of AVP these segments are not permeable to water (i.e., AQP2 is not present in the apical membrane of the cells). Thus, when AVP is absent or present at low levels (i.e., decreased plasma osmolality), the osmolality of tubule fluid in these segments is reduced further because NaCl is reabsorbed without water. Under this condition, fluid leaving the cortical portion of the collecting duct is hypoosmotic with respect to plasma (see Fig. 35.6 ).
The medullary collecting duct actively reabsorbs NaCl. Even in the absence of AVP, this segment is slightly permeable to water and some water is reabsorbed.
The urine has an osmolality as low as approximately 50 mOsm/kg H 2 O and contains low concentrations of NaCl. The volume of urine excreted can be as much as 18 L/day, or approximately 10% of the glomerular filtration rate (GFR).
Next, how the kidneys excrete concentrated urine (antidiuresis) when plasma osmolality and plasma AVP levels are high is considered. The following numbers refer to those encircled in Fig. 35.7B :
1–4. These steps are similar to those for production of dilute urine. An important point in understanding how a concentrated urine is produced is to recognize that while reabsorption of NaCl by the ascending thin and thick limbs of the loop of Henle dilutes the tubular fluid, the reabsorbed NaCl accumulates in the medullary interstitium and raises the osmolality of this compartment. Accumulation of NaCl in the medullary interstitium is crucial for production of urine hyperosmotic to plasma because it provides the osmotic driving force for water reabsorption by the medullary collecting duct. As already noted, AVP stimulates NaCl reabsorption by the thick ascending limb of Henle’s loop. This is thought to maintain the medullary interstitial gradient at a time when water is being added to this compartment from the medullary collecting duct, which would tend to dissipate the gradient.
Because of NaCl reabsorption by the ascending limb of the loop of Henle, the fluid reaching the collecting duct is hypoosmotic with respect to the surrounding interstitial fluid. Thus an osmotic gradient is established across the collecting duct. In the presence of AVP, which increases the water permeability of the latter portion of the distal tubule and the collecting duct by causing insertion of AQP2 into the luminal membrane of the cells, water diffuses out of the tubule lumen and the tubule fluid osmolality increases. This diffusion of water out of the lumen of the collecting duct begins the process of urine concentration. The maximum osmolality the fluid in the distal tubule and cortical portion of the collecting duct can attain is approximately 290 mOsm/kg H 2 O (i.e., the same as plasma), which is the osmolality of the interstitial fluid and plasma within the cortex of the kidney.
As the tubular fluid descends deeper into the medulla, water continues to be reabsorbed from the collecting duct, increasing the tubular fluid osmolality to 1200 mOsm/kg H 2 O at the tip of the papilla.
The urine produced when AVP levels are elevated has an osmolality of 1200 mOsm/kg H 2 O and contains high concentrations of urea and other nonreabsorbed solutes. Urine volume under this condition can be as low as 0.5 L/day.
In comparing the two conditions just described, it should be apparent that a relatively constant volume of dilute tubular fluid is delivered to the AVP-sensitive portions of the nephron (latter portion of the distal tubule and collecting duct). Plasma AVP levels then determine the amount of water reabsorbed by these segments. When AVP levels are low, a relatively small volume of water is reabsorbed by these segments and a large volume of hypoosmotic urine is excreted (up to 10% of the filtered water). When AVP levels are high, a large volume of water is reabsorbed by these same segments and a small volume of hyperosmotic urine is excreted (<1% of filtered water). During antidiuresis, most of the water is reabsorbed in the distal tubule and cortical and outer medullary portions of the collecting duct. Thus, a relatively small volume of fluid reaches the inner medullary collecting duct where it is then reabsorbed. This distribution of water reabsorption along the length of the collecting duct (i.e., cortex > outer medulla > inner medulla) allows for maintenance of a hyperosmotic interstitial environment in the inner medulla by minimizing the amount of water entering this compartment.
As noted earlier, the interstitial fluid of the renal medulla is critically important in concentrating urine. The osmotic pressure of the interstitial fluid provides the driving force for reabsorbing water from both the descending thin limb of the loop of Henle and the collecting duct. The principal solutes of the medullary interstitial fluid are NaCl and urea, but the concentration of these solutes is not uniform throughout the medulla (i.e., a gradient exists from cortex to papilla). Other solutes also accumulate in the medullary interstitium (e.g., NH 4 + and K + ), but the most abundant solutes are NaCl and urea. For simplicity, this discussion assumes that NaCl and urea are the only solutes.
As depicted in Fig. 35.8 , NaCl and urea accumulate in the renal medulla, and the interstitial fluid at the tip of the papilla of the inner medulla reaches a maximum osmolality of 1200 mOsm/kg H 2 O, with approximately 600 mOsm/kg H 2 O attributable to NaCl (300 mmol/L) and 600 mOsm/kg H 2 O attributable to urea (600 mmol/L). Establishment of the NaCl gradient is essentially complete at the transition between the outer and inner medulla.

The medullary gradient for NaCl results from accumulation of NaCl reabsorbed by the nephron segments in the medulla during countercurrent multiplication. The most important segment in this regard is the ascending limb of the loop of Henle. Urea accumulation within the medullary interstitium is more complex and occurs most effectively when hyperosmotic urine is excreted (i.e., antidiuresis). When dilute urine is produced, especially over extended periods, the osmolality of the medullary interstitium declines (see Fig. 35.7 A ). This reduced osmolality is almost entirely caused by a decrease in the concentration of urea. This decrease reflects washout by the vasa recta (discussed later) and diffusion of urea from the interstitium into the tubular fluid within the medullary portion of the collecting duct, which is permeable to urea even in the absence of AVP. (N ote : The cortical and outer medullary portions of the collecting duct have a low permeability to urea, whereas the inner medullary portion has a relatively high permeability because of the presence of the urea transporters UT-A1 and UT-A3, the expression of which is increased by AVP.) Some of this reabsorbed urea is secreted into the thin descending limbs of Henle’s loops via the urea transporter UT-A2, and some enters the vasa recta via the UT-B transporter. The urea that is secreted into the descending thin limbs of Henle’s loops is then trapped in the nephron until it again reaches the medullary collecting duct, where it can reenter the medullary interstitium. Thus, urea recycles from the interstitium to the nephron and back into the interstitium. This process of urea recycling facilitates accumulation of urea in the medullary interstitium, where it can attain a concentration at the tip of the papilla of 600 mmol/L.
As described, the hyperosmotic medulla is essential for concentrating the tubular fluid within the collecting duct. Because water reabsorption from the collecting duct is driven by the osmotic gradient established in the medullary interstitium, urine can never be more concentrated than that of the interstitial fluid in the papilla. Thus any condition that reduces the medullary interstitial osmolality impairs the ability of the kidneys to maximally concentrate urine. Urea within the medullary interstitium contributes to the total osmolality of the urine. However, because the inner medullary collecting duct is highly permeable to urea, especially in the presence of AVP, urea cannot drive water reabsorption across this nephron segment. Instead, urea in the tubular fluid and medullary interstitium equilibrate, and a small volume of urine with a high concentration of urea is excreted. c
c On a typical diet the kidneys must excrete 450 mmol/day of urea. At a maximal urine [urea] of 600 mmol/L this amount of urea can be excreted in less than 1 L of urine. However, if the maximal urine [urea] is reduced because of a decrease in the medullary interstitial fluid [urea], a larger urine volume would be needed to excrete the 450 mmol/day of urea (e.g., 2.25 L of urine would be required if the maximal urine [urea] was only 200 mM).
It is the medullary interstitial NaCl concentration that is responsible for reabsorbing water from the medullary collecting duct and thereby concentrating the nonurea solutes (e.g., NH 4 + salts, K + salts, creatinine) in the urine.
The vasa recta, the capillary networks that supply blood to the medulla, are highly permeable to solute and water. As with the loop of Henle, the vasa recta form a parallel set of hairpin loops within the medulla (see Chapter 33 ). Not only do the vasa recta bring nutrients and oxygen to the medullary nephron segments, but more importantly they also remove the excess water and solute that is continuously added to the medullary interstitium by these nephron segments. The ability of the vasa recta to maintain the medullary interstitial gradient is flow dependent. A substantial increase in vasa recta blood flow dissipates the medullary gradient (i.e., washout of osmoles from the medullary interstitium). Alternatively, reduced blood flow reduces oxygen delivery to the nephron segments within the medulla. Because transport of salt and other solutes requires oxygen and ATP, reduced medullary blood flow decreases salt and solute transport by nephron segments in the medulla. As a result, the medullary interstitial osmotic gradient cannot be maintained.
In summary, the kidneys maintain an osmotic gradient from the corticomedullary junction to the inner medullary tip. The cortical tissue is isotonic to plasma while the medullary tip is hypertonic. This gradient becomes steeper during antidiuresis and decreases in magnitude during diuresis. Recent studies have been focusing on the detailed understanding of the renal functional architecture, including three-dimensional reconstruction and mathematical modeling to develop a more complete understanding how the permeability properties of nephron segments and their three-dimensional arrangements may contribute to the generation and maintenance of osmotic gradient necessary for urinary concentration. d
d Nawata, CM, and Pannabecker, TL. Mammalian urine concentration: a review of renal medullary architecture and membrane transporters. J Comp Physiol B ; 2018; 188 :899–918.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here