Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Upon completion of this chapter the student should be able to answer the following questions :
Which structures in the glomerulus are filtration barriers to plasma proteins?
What is the physiological significance of the juxtaglomerular apparatus?
What blood vessels supply the kidneys?
What nerves innervate the kidneys?
What is the location of the kidneys, and what are their gross anatomical features?
What are the different parts of the nephron, and what is their locations within the cortex and medulla?
What are the major components of the glomerulus, and what are the cell types located in each component?
How can the concepts of mass balance be used to measure the glomerular filtration rate (GFR)?
Why can inulin clearance and creatinine clearance be used to measure GFR?
Why is plasma creatinine concentration used clinically to monitor GFR?
What are elements of the glomerular filtration barrier, and how do they determine how much protein enters Bowman’s space?
What Starling forces are involved in formation of the glomerular ultrafiltrate, and how do changes in each force affect GFR?
What is autoregulation of renal blood flow and GFR, and which factors and hormones are responsible for autoregulation?
Which hormones regulate renal blood flow?
Why do hormones influence renal blood flow despite autoregulation?
The kidney presents in the highest degree the phenomenon of sensibility, the power of reacting to various stimuli in a direction, which is appropriate for the survival of the organism; a power of adaptation which almost gives one the idea that its component parts must be endowed with intelligence. E. STARLING—1909
Certainly, mental integrity is a sine qua non of the free and independent life. But let the composition of our internal environment suffer change, let our kidneys fail for even a short time to fulfill their tasks, and our mental integrity, or personality is destroyed. HOMER W. SMITH—1939
As both Starling and Smith recognized, the kidneys are more appropriately considered regulatory rather than excretory organs. The kidneys regulate (1) body fluid osmolality and volumes, (2) electrolyte balance, and (3) acid-base balance. In addition the kidneys excrete metabolic products and foreign substances and produce and secrete hormones.
Control of body fluid osmolality is important for maintenance of normal cell volume in all tissues of the body. Control of body fluid volume is necessary for normal function of the cardiovascular system. The kidneys are also essential in regulating the amount of several important inorganic ions in the body, including Na + , K + , Cl − , bicarbonate (HCO 3 − ), hydrogen (H + ), Ca ++ , and inorganic phosphate (P i ). Excretion of these electrolytes must be equal to daily intake to maintain appropriate total body balance. If intake of an electrolyte exceeds its excretion, the amount of this electrolyte in the body increases and the individual is in positive balance for that electrolyte. Conversely, if excretion of an electrolyte exceeds its intake, its amount in the body decreases and the individual is in negative balance for that electrolyte. For many electrolytes the kidneys are the sole or principal route for excretion from the body.
Another important function of the kidneys is regulation of acid-base balance. Many metabolic functions of the body are exquisitely sensitive to pH. Thus the pH of body fluids must be maintained within narrow limits. Normal pH is maintained by buffers within body fluids and by the coordinated action of the lungs, liver, and kidneys.
The kidneys excrete a number of the end products of metabolism. These waste products include urea (from amino acids), uric acid (from nucleic acids), creatinine (from muscle creatine), end products of hemoglobin metabolism, and metabolites of hormones. The kidneys eliminate these substances from the body at a rate that matches their production. Thus the kidneys regulate hormone concentrations within body fluids. The kidneys also represent an important route for elimination of foreign substances such as drugs, toxins (e.g., pesticides), and other chemicals from the body.
Finally, the kidneys are important endocrine organs that produce and secrete renin, calcitriol, and erythropoietin. Renin is not a hormone but an enzyme that activates the renin-angiotensin-aldosterone system, which helps regulate blood pressure and Na + and K + balance. Calcitriol, a metabolite of vitamin D 3 , is necessary for normal absorption of Ca ++ by the gastrointestinal tract and for its deposition in bone (see Chapter 36 ). In patients with renal disease the kidneys’ ability to produce calcitriol is impaired and levels of this hormone are reduced. As a result, Ca ++ absorption by the intestine is decreased, which over time contributes to abnormalities in the bone formation and remodeling seen in patients with chronic renal disease. Another consequence of many kidney diseases is a reduction in erythropoietin production and secretion. Erythropoietin stimulates red blood cell formation by bone marrow. Decreased erythrocyte production contributes to the anemia that occurs in chronic kidney disease (CKD), a progressive loss in kidney function over a period of months or years.
A variety of conditions impair kidney function. Reduced renal function can be transient or permanent and may progress over time. Patients in whom the glomerular filtration rate (GFR) is less than 10% of normal are said to have kidney failure, also called end-stage kidney disease (ESKD) and must receive renal replacement therapy (RRT) in the form of either dialysis, hemofiltration or kidney transplantation to survive.
To understand the mechanisms that contribute to renal disease, it is first necessary to understand the normal physiology of renal function. Thus in the following chapters in this section of the book, various aspects of renal function are considered.
Kidney disease is a major health problem worldwide. In the United States alone:
Kidney disease affects over 37 million people—that is 1 out of 7 or 15% of the adult population.
Chronic kidney disease (CKD) is the most under-recognized public health crisis. Approximately 90% of people who have CKD are not even aware of it and 50% of people with very low kidney function, and who are not on dialysis, do not know they have CKD.
CKD is the eight leading cause of death accounting for more than 100,000 deaths annually.
The health care cost for CKD in Medicare patients alone exceeds $70 billion per year, and in those patients with end-stage kidney disease (ESKD) it exceeds an additional $50 billion.
Over 786,000 people are living with ESKD with 71% on dialysis and 29% with a kidney transplant.
Diabetes and hypertension are the leading causes of ESKD.
More than 23,000 kidney transplants are performed each year. Unfortunately, in excess of 90,000 patients are awaiting kidney transplants.
Individuals with ESKD must undergo RRT, which includes peritoneal dialysis, hemodialysis, hemofiltration, and kidney transplantation. Both peritoneal dialysis and hemodialysis, as their names suggest, rely on the ability to remove small dialyzable molecules from the blood—including metabolic waste products normally removed by intact kidneys—via diffusion across a selectively permeable membrane into a solution lacking these substances, thereby mitigating both their accumulation and associated adverse health effects. In addition, dialysis helps reestablish both fluid and electrolyte balance via removal of excess fluid, correction of acid-base changes, and normalization of plasma electrolyte concentrations). In peritoneal dialysis, the peritoneal membrane lining the abdominal cavity acts as a dialyzing membrane. Several liters of a defined dialysis solution are typically introduced into the abdominal cavity, and small molecules in blood diffuse across the peritoneal membrane into the solution, which can then be iteratively removed, discarded, and replaced. In hemodialysis, a patient’s blood is pumped through an extracorporeal artificial kidney in which blood is separated from a defined dialysis solution by an artificial semipermeable membrane that allows small molecules to diffuse from the blood down their concentration gradient into the dialysis solution, thereby removing small molecules associated with adverse health effects if allowed to accumulate in patients without functioning kidneys. Hemofiltration is a form of RRT based on convection, a process during which solutes (metabolic waste products and other small molecules normally cleared by functioning kidneys) and solvent (water) move according to the pressure gradient. In hemofiltration, patient’s blood is pumped through an extracorporeal artificial kidney in which solutes are transported across an artificial semipermeable membrane along with movement of solvent (ultrafiltration) that occurs in response to positive transmembrane pressure gradient. Ultrapure replacement fluid is then reinfused into the patient to keep the volume and electrolyte homeostasis. Hemofiltration is used only as a form of continuous RRT in acutely ill, hospitalized patients. Hemodialysis and peritoneal dialysis can also be used in hospitalized patients or as a form of chronic RRT. Patients who are candidates for RRT are often treated with dialysis until an appropriate donor kidney can be procured. Although anemia has historically been a significant problem in ESKD patients owing to severely reduced endogenous erythropoietin production, this problem can now be easily corrected in patients undergoing chronic dialysis via administration of erythropoiesis-stimulating agents (e.g., recombinant human erythropoietin).
Structure and function are closely linked in the kidneys. Consequently an appreciation of the gross anatomical and histological features of the kidneys is a prerequisite for understanding their functions.
The kidneys are paired organs that lie on the posterior wall of the abdomen behind the peritoneum on either side of the vertebral column. In an adult human, each kidney weighs between 115 and 170 g and is approximately 11 cm long, 6 cm wide, and 3 cm thick.
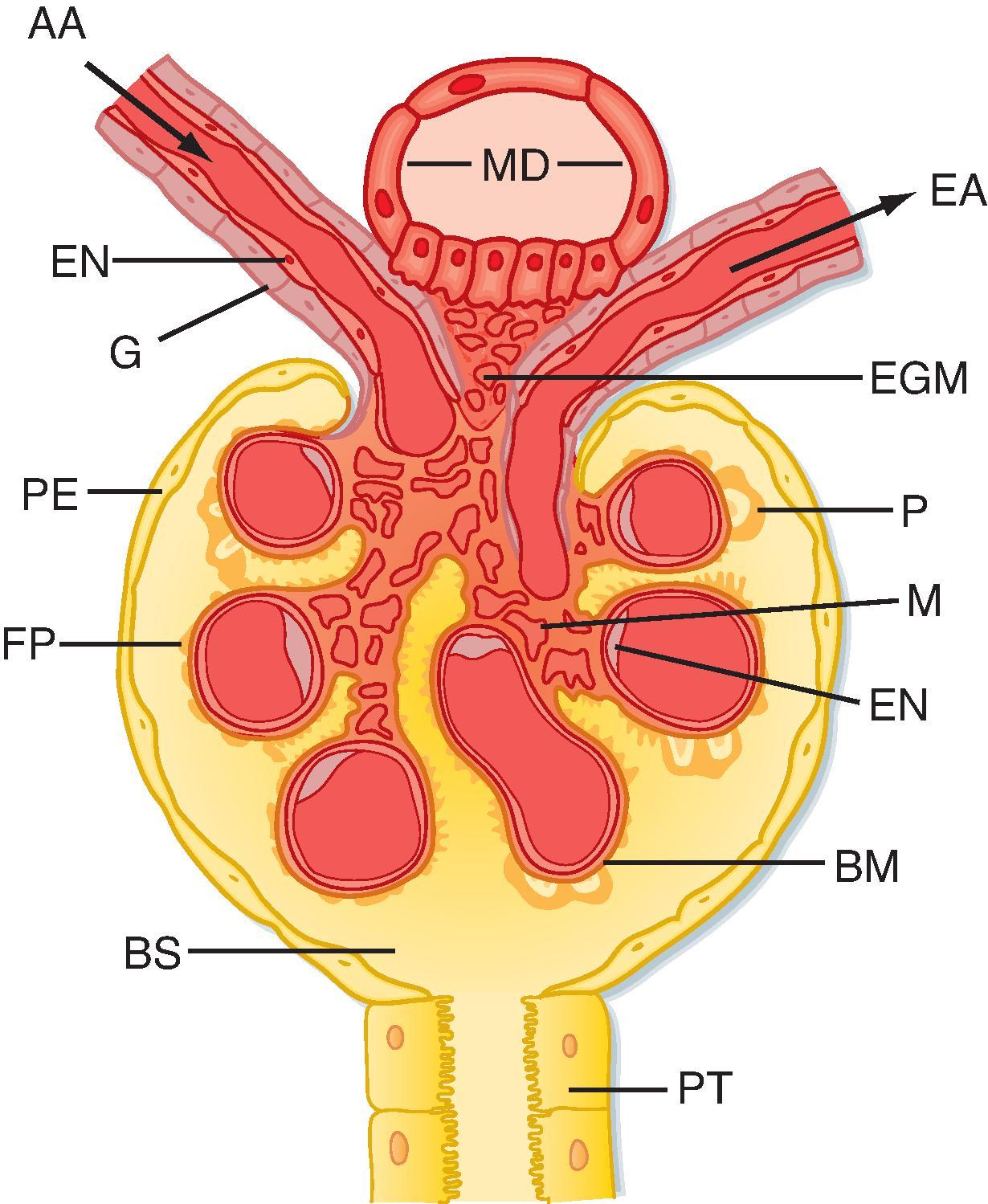
The gross anatomical features of the human kidney are illustrated in Fig. 33.1 . The medial side of each kidney contains an indentation through which pass the renal artery and vein, nerves, and pelvis. If a kidney were cut in half, two regions would be evident: an outer region called the cortex and an inner region called the medulla. The cortex and medulla are composed of nephrons (the functional units of the kidney), blood vessels, lymphatics, and nerves. The medulla in the human kidney is divided into conical masses called renal pyramids . The base of each pyramid originates at the corticomedullary border, and the apex terminates in a papilla , which lies within a minor calyx . Minor calyces collect urine from each papilla. The numerous minor calyces expand into two or three open-ended pouches, the major calyces . The major calyces in turn feed into the pelvis . The pelvis represents the upper expanded region of the ureter , which carries urine from the pelvis to the urinary bladder. The walls of the calyces, pelvis, and ureters contain smooth muscle that contracts to propel the urine toward the urinary bladder .

Blood flow to the two kidneys is equivalent to about 25% (1.25 L/minute) of the cardiac output in resting individuals. However, the kidneys constitute less than 0.5% of total body weight. As illustrated in Fig. 33.2 (left), the renal artery branches progressively to form the interlobar artery , the arcuate artery , the interlobular artery, and the afferent arteriole , which leads into the glomerular capillaries . The glomerular capillaries come together to form the efferent arteriole , which leads into a second capillary network, the peritubular capillaries , which supply blood to the nephron. The vessels of the venous system run parallel to the arterial vessels and progressively form the interlobular vein, arcuate vein, interlobar vein , and renal vein , which courses beside the ureter.

The functional unit of the kidneys is the nephron. Each human kidney contains approximately 1.2 million nephrons, which are essentially hollow tubes composed of a single epithelial cell layer. The nephron consists of a renal corpuscle, proximal tubule, loop of Henle, distal tubule, and collecting duct system a
a The organization of the nephron is actually more complicated than presented here. However, for simplicity and clarity of presentation in subsequent chapters, the nephron is divided into five segments. The collecting duct system is not actually part of the nephron. However, again for simplicity, we consider the collecting duct system part of the nephron.
( Fig. 33.3 ; also see Fig. 33.2 ). The renal corpuscle b
b Although the renal corpuscle is composed of glomerular capillaries and Bowman’s capsule, the term glomerulus is commonly used to described the renal corpuscle.
consists of glomerular capillaries enclosed within Bowman’s capsule . The proximal tubule exits this structure and initially forms several coils, followed by a straight piece that descends toward the medulla. The next segment is the loop of Henle, which is composed of the straight part of the proximal tubule, the descending thin limb (which ends in a hairpin turn), the ascending thin limb (only in nephrons with long loops of Henle), and the thick ascending limb. Near the end of the thick ascending limb, the nephron passes between the afferent and efferent arterioles of the same nephron. This short segment of the thick ascending limb abutting the glomerulus is called the macula densa (see Figs. 33.2 and 33.3 ). The distal tubule begins a short distance beyond the macula densa and extends to the point in the cortex where two or more nephrons join to form a cortical collecting duct. The cortical collecting duct enters the medulla and becomes the outer medullary collecting duct and then the inner medullary collecting duct .

Each nephron segment is made up of cells that are uniquely suited to perform specific transport functions (see Fig. 33.3 ). Proximal tubule cells have an extensively amplified apical membrane (the ultrafiltrate or urine side of the cell) called the brush border , which is present only in the proximal tubule. The basolateral membrane (the interstitial or blood side of the cell) is highly invaginated. These invaginations contain many mitochondria. In contrast, the descending and ascending thin limbs of the loop of Henle have poorly developed apical and basolateral surfaces and few mitochondria. The cells of the thick ascending limb and the distal tubule have abundant mitochondria and extensive infoldings of the basolateral membrane.
The collecting duct is composed of two cell types: principal cells and intercalated cells. Principal cells have a moderately invaginated basolateral membrane and contain few mitochondria (see Fig. 33.3 ). Principal cells play an important role in reabsorption of NaCl (see Chapters 34 and 35 ) and secretion of K + (see Chapter 36 ). Intercalated cells , which play an important role in regulating acid-base balance, have a high density of mitochondria (see Fig. 33.3 ). One population of intercalated cells secretes H + (i.e., reabsorbs HCO 3 − ), and a second population secretes HCO 3 − and can also reabsorb NaCl (see Chapter 37 ). The final segment of the nephron, the inner medullary collecting duct, is composed of inner medullary collecting duct cells, which have poorly developed apical and basolateral surfaces and few mitochondria.
All cells in the nephron except intercalated cells have in their apical plasma membrane a single nonmotile primary cilium that protrudes into the tubule fluid ( Fig. 33.4 ). Primary cilia are mechanosensors (i.e., they sense changes in the rate of flow of tubule fluid) and chemosensors (i.e., they sense or respond to compounds in the surrounding fluid), and they initiate Ca ++ -dependent signaling pathways, including those that control kidney cell function, proliferation, differentiation, and apoptosis (i.e., programmed cell death).
Polycystin 1 (encoded by the PKD1 gene) and polycystin 2 (encoded by the PKD2 gene) are expressed in the membrane of primary cilia and mediate entry of Ca ++ into cells. PKD1 and PKD2 are thought to play an important role in flow-dependent K + secretion by principal cells of the collecting duct. As described in more detail in Chapter 36 , increased flow of tubule fluid in the collecting duct is a strong stimulus for secretion of K + . Increased flow bends the primary cilium in principal cells, which activates the PKD1/PKD2 Ca ++ -conducting channel complex and allows Ca ++ to enter the cell and increase intracellular [Ca ++ ]. The increase in [Ca ++ ] activates K + channels in the apical plasma membrane, which enhances secretion of K + from the cell into the tubule fluid.
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited kidney disease, occurring in about 1 in 1000 people. More than 12.5 million people worldwide have ADPKD, which is caused primarily by mutations in PKD1 (85%–90% of cases) or PKD2 (≈15% of cases). The major phenotype of ADPKD is enlargement of the kidneys due to the presence of hundreds or thousands of fluid-filled renal cysts that can grow to the size of 20 cm in diameter. Cysts are also seen in the liver and other organs in this condition. About 50% of patients with ADPKD progress to ESKD by the age of 60. Although it is not clear how mutations in PKD1 and PKD2 cause ADPKD, renal cyst formation may result from defects in Ca ++ uptake that alter Ca ++ -dependent signaling pathways, including those controlling kidney cell proliferation, differentiation, and apoptosis.
Nephrons may be subdivided into superficial and juxtamedullary types (see Fig. 33.2 ), with approximately 10 superficial nephrons for each juxtamedullary nephron. The glomerulus of each superficial nephron is located in the outer region of the cortex. The corresponding loops of Henle are short, and associated efferent arterioles branch into peritubular capillaries that surround its associated nephron segments as well as adjacent nephrons. This capillary network conveys oxygen and important nutrients to the nephron segments in the cortex, delivers substances to individual nephron segments for secretion (i.e., movement of a substance from blood into tubular fluid), and serves as a pathway for return of reabsorbed water and solutes to the circulatory system. A few species, including humans, also possess very short superficial nephrons whose loops of Henle never enter the medulla.
The glomerulus of each juxtamedullary nephron is located in the region of the cortex adjacent to the medulla (see Fig. 33.2 , right ). When compared with superficial nephrons, juxtamedullary nephrons differ anatomically in two important ways: the loop of Henle is longer and extends deeper into the medulla, and the efferent arteriole forms not only a network of peritubular capillaries but also a series of accompanying vascular loops called the vasa recta.
As shown in Fig. 33.2 (left), the vasa recta descend into the medulla, where they form capillary networks that surround the collecting ducts and ascending limbs of the loop of Henle. The blood returns to the cortex via the ascending vasa recta. Although less than 0.7% of the renal blood flow (RBF) enters the vasa recta, these vessels serve important functions in the renal medulla that include (1) conveying oxygen and important metabolic substrates to support nephron function, (2) delivering substances to the nephron for secretion, (3) serving as a pathway for return of reabsorbed water and solutes to the circulatory system, and (4) concentrating and diluting urine (urine concentration and dilution are discussed in more detail in Chapter 35 ).
The first step in urine formation begins with passive movement of a plasma ultrafiltrate from the glomerular capillaries (i.e., glomerulus) into Bowman’s space . The term ultrafiltration refers to this passive movement of fluid—similar in composition to plasma, except for the fact that the ultrafiltrate protein concentration is much lower than that in the plasma—from the glomerular capillaries into Bowman’s space. To appreciate this process, one must understand the anatomy of the glomerulus, which consists of a network of capillaries supplied by the afferent arteriole and drained by the efferent arteriole ( Figs. 33.5 and 33.6 ). During embryological development, the glomerular capillaries press into the closed end of the proximal tubule, forming Bowman’s capsule. As the epithelial cells thin on the outside circumference of Bowman’s capsule, they form the parietal epithelium (see Fig. 33.5 ). The epithelial cells in contact with the capillaries thicken and develop into podocytes , which form the visceral layer of Bowman’s capsule ( Figs. 33.7 – 33.9 ). The visceral cells face outward at the vascular pole (i.e., where afferent and efferent arterioles enter and exit Bowman’s capsule) to form the parietal layer of Bowman’s capsule. The space between the visceral layer and the parietal layer is Bowman’s space, which at the urinary pole (i.e., where the proximal tubule joins Bowman’s capsule) of the glomerulus becomes the lumen of the proximal tubule.

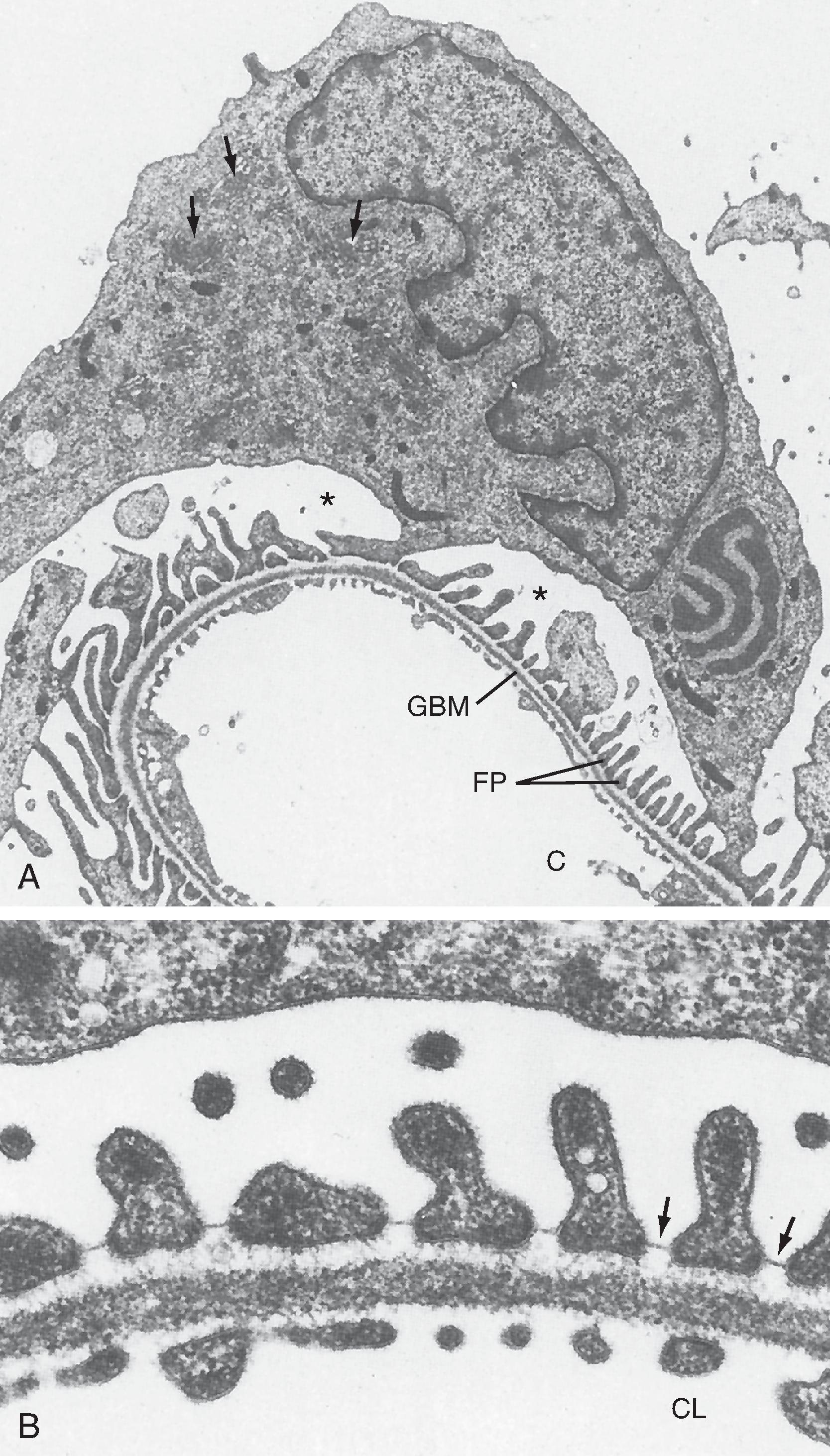

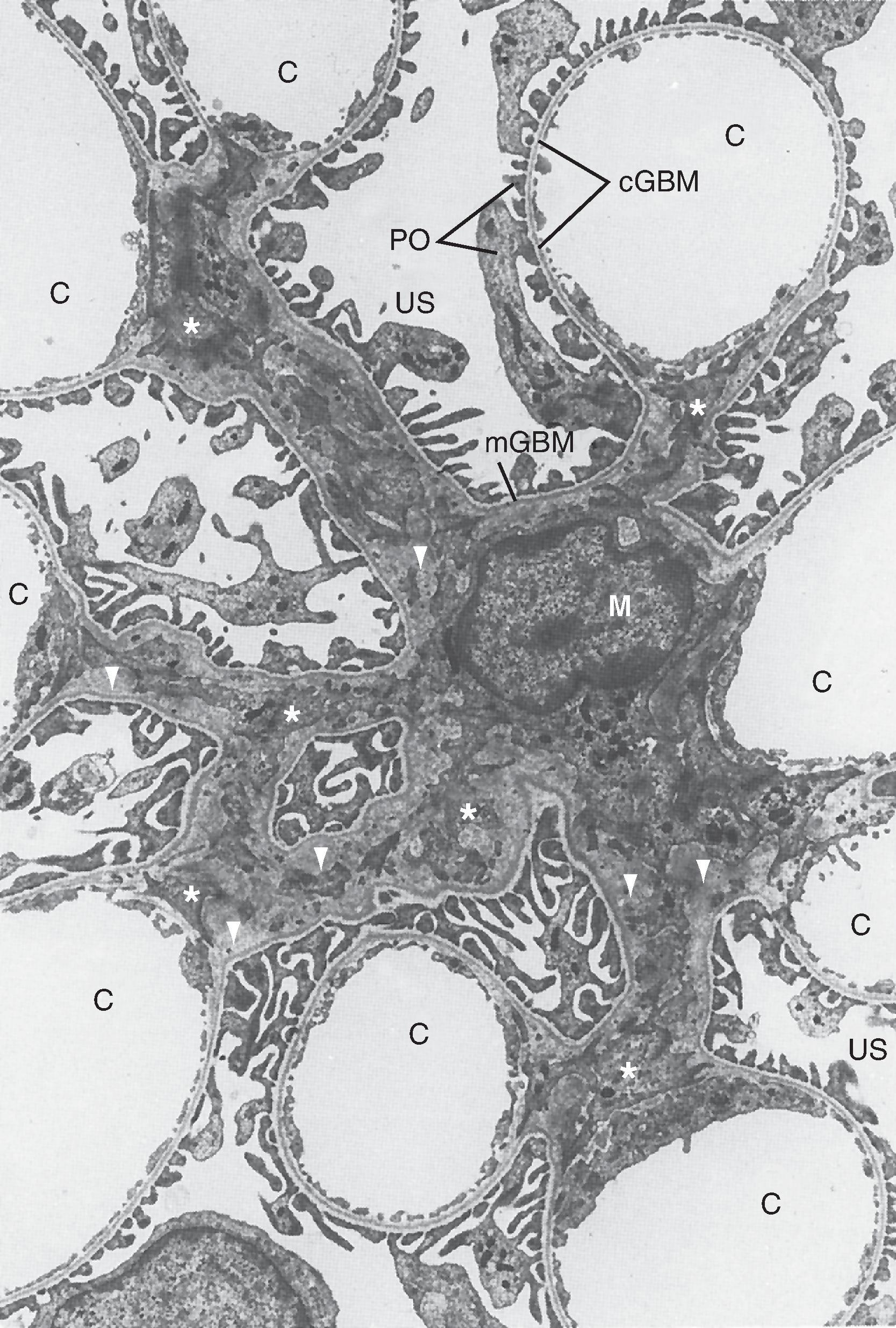
The endothelial cells of glomerular capillaries are covered by a basement membrane surrounded by podocytes . The capillary endothelium, basement membrane, and foot processes of podocytes form the so-called filtration barrier (see Figs. 33.5 and 33.7 –33.9). The endothelium is fenestrated (i.e., contains 700-Å holes, where 1 Å = 10 −10 m) and freely permeable to water, small solutes (e.g., Na + , urea, glucose), and most proteins but is not permeable to red blood cells, white blood cells, or platelets. Because endothelial cells express negatively charged glycoproteins on their surface, they minimize the filtration into Bowman’s space of albumin, the most abundant plasma protein, and most other plasma proteins. In addition to their role as a barrier to filtration, the endothelial cells synthesize a number of vasoactive substances (e.g., nitric oxide [NO], a vasodilator, and endothelin 1 [ET-1], a vasoconstrictor) that are important in controlling renal plasma flow (RPF) . The basement membrane, which is a porous matrix of negatively charged proteins (type IV collagen, laminin, the proteoglycans agrin and perlecan, and fibronectin), is an important filtration barrier to plasma proteins. The basement membrane is thought to function primarily as a charge-selective filter in which the ability of proteins to cross the filter is based on charge.
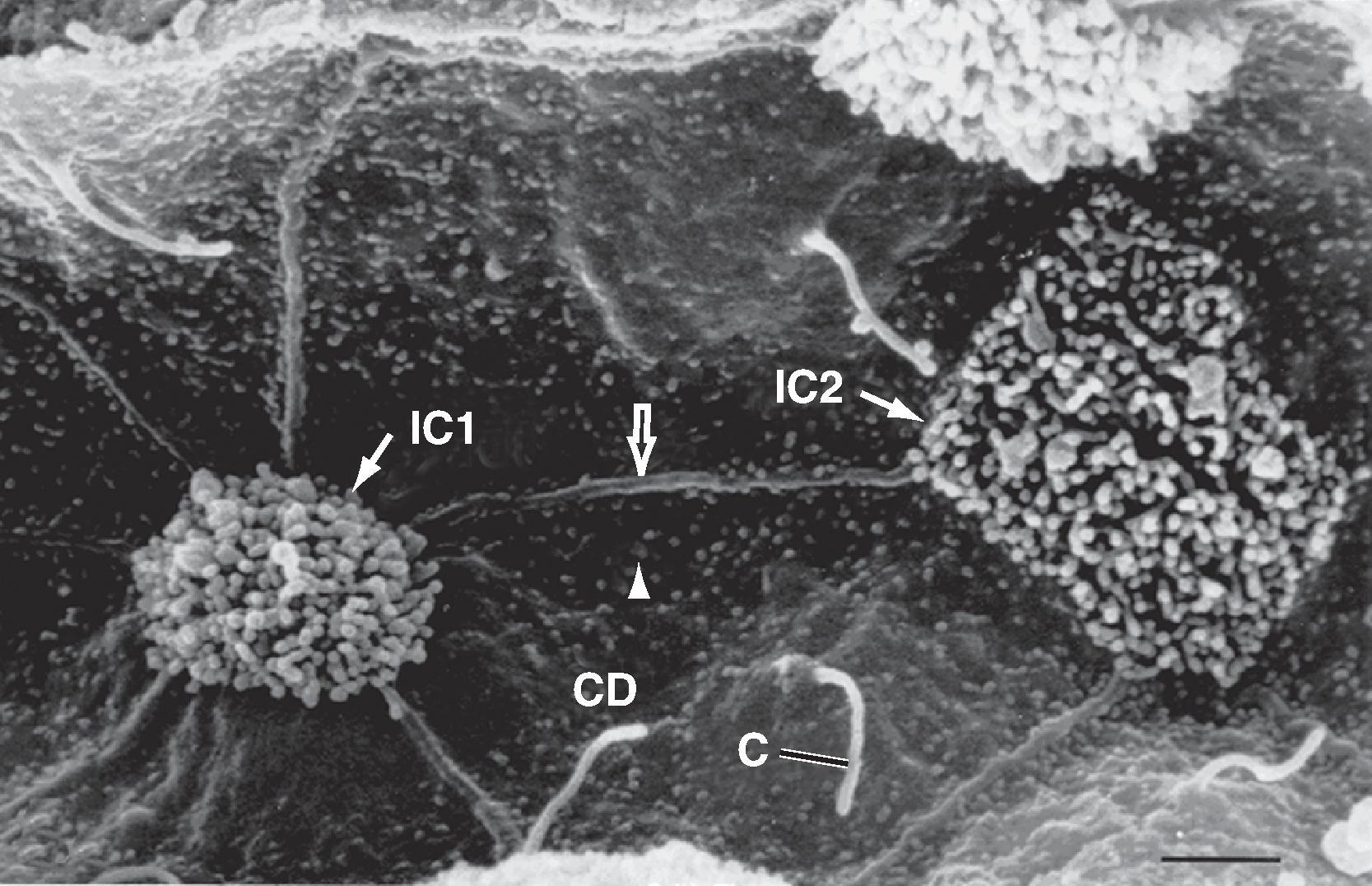
The podocytes, which are endocytic, have long finger-like processes that completely encircle the outer surface of the capillaries (see Figs. 33.7 and 33.8 A). The processes of the podocytes interdigitate to cover the basement membrane and are separated by apparent gaps called filtration slits (see Figs. 33.7 and 33.8 A). Each filtration slit is bridged by a thin diaphragm that contains pores with a dimension of 40 × 140 Å. The filtration slit diaphragm , which appears as a continuous structure when viewed by electron microscopy (see Fig. 33.7 B), is composed of several proteins, including nephrin (NPHS1), NEPH-1 , and podocin (NPHS2) , and intracellular proteins that associate with the slit diaphragm, including α-actinin-4 (ACTN4) and CD2-AP ( Figs. 33.10 and 33.11 ). Filtration slits, which function primarily as a size-selective filter, minimize the filtration of proteins and macromolecules that cross the basement membrane from entering Bowman’s space. Because both the basement membrane and filtration slits contain negatively charged glycoproteins, some proteins are held back (i.e., not filtered into Bowman’s space) on the basis of size and charge. For molecules with an effective molecular radius between 18 and 42 Å, cationic molecules are filtered more readily than anionic molecules.

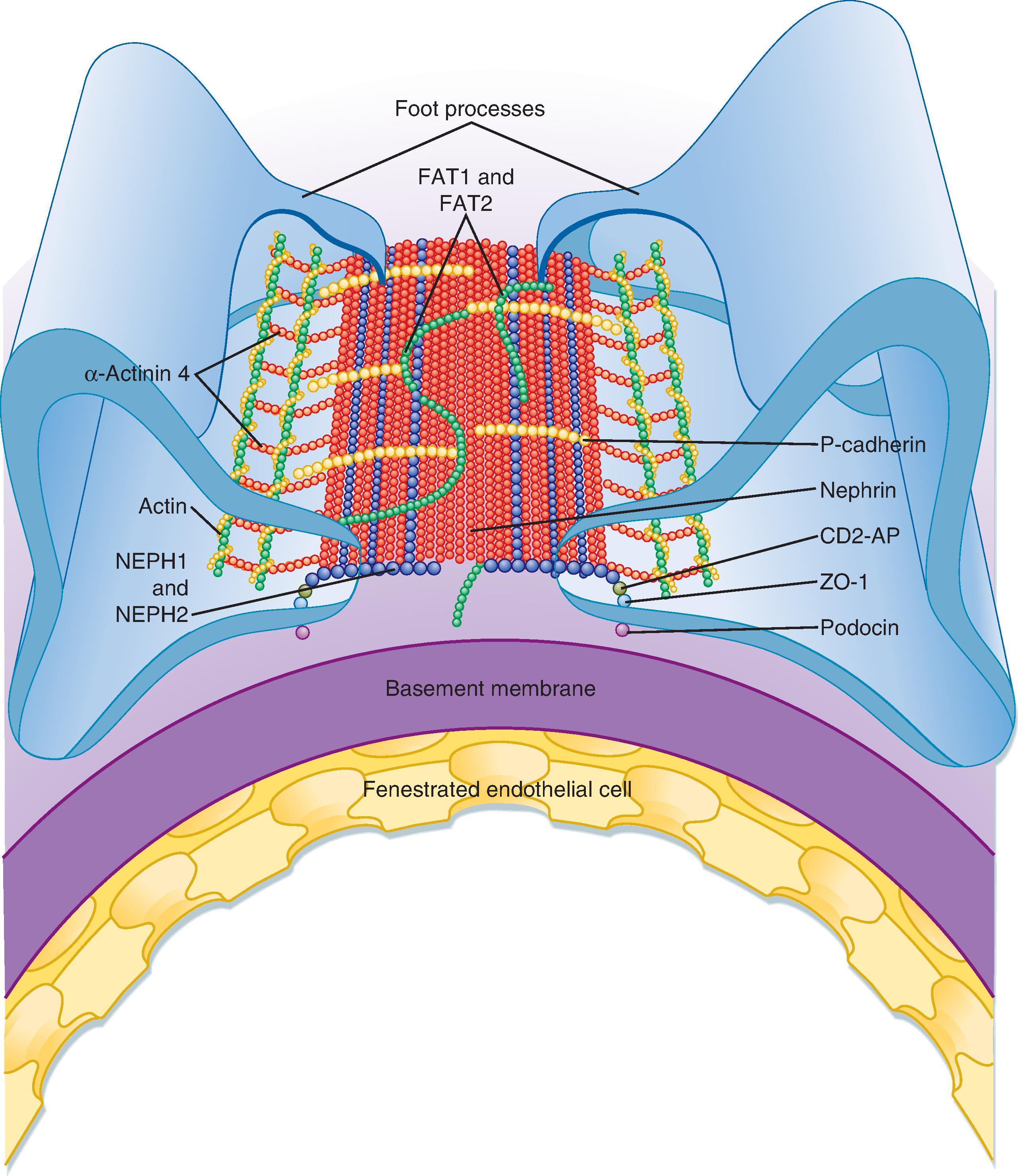
Another important component of the renal corpuscle is the mesangium, which consists of mesangial cells and the mesangial matrix (see Fig. 33.9 ). Mesangial cells, which possess many properties of smooth muscle cells, provide structural support for the glomerular capillaries, secrete extracellular matrix, exhibit phagocytic activity by removing macromolecules from the mesangium, and secrete prostaglandins and proinflammatory cytokines. Because they also contract and are adjacent to glomerular capillaries, mesangial cells may influence GFR by regulating blood flow through the glomerular capillaries or by altering the capillary surface area. Mesangial cells located outside the glomerulus (between the afferent and efferent arterioles) are called extraglomerular mesangial cells .
The loss of normal podocyte structure affects the integrity of filtration barrier and increases permeability of the glomerular capillaries to proteins. The augmented permeability to proteins results in increased urinary protein excretion (proteinuria) . Thus the appearance of proteins in urine can indicate kidney disease. Urinary loss of large quantities of protein leads to hypoalbuminemia and may cause generalized edema. The triad, characterizing a condition called nephrotic syndrome , can result from mutations of genes encoding proteins essential to the integrity of filtration barrier. Nephrotic syndrome can also be caused by an infection-triggered dysregulation of the immune system that ultimately affects the filtration barrier. Mutations in several genes encoding either slit diaphragm proteins (see Figs. 33.10 and 33.11 ), including nephrin, NEPH-1 , and podocin , or intracellular proteins that functionally interact with slit diaphragm proteins, such as CD2-AP and α-actinin 4 (ACTN4), result in proteinuria and kidney disease. For example, mutations in the nephrin gene (NPHS1) in congenital nephrotic syndrome lead to abnormal slit diaphragms, causing massive proteinuria and renal failure. In addition, mutations in the podocin gene (NPHS2) cause nephrotic syndrome.
Alport syndrome is characterized by structural abnormalities and dysfunction in the glomerular basement membrane type IV collagen leading to hematuria (i.e., blood in the urine), proteinuria, and progressive loss of kidney function, and it accounts for 3% of CKD in children and 0.2% of adults with ESKD in the United States. Alport syndrome is caused by mutations in the COL4A3, COL4A4, or COL4A5 genes causing defects in the type IV collagen alpha-3, alpha-4, and alpha-5 chains, respectively. The prevailing view is that Alport syndrome is transmitted in an X-linked manner in most cases and results from mutations in the COL4A5 gene. Mutations in the COL4A3 or COL4A4 genes are transmitted in an autosomal manner and account for only a small number of Alport syndrome cases. Our understanding of the genetics of Alport syndrome has evolved in recent years. The new sequencing technologies suggest that the prevalence of all COL4A4 gene mutations , including those leading to autosomal A lport syndrome in the population, may be much higher than previously anticipated.
The juxtaglomerular apparatus is one component of an important feedback mechanism, the tubuloglomerular feedback mechanism, described later in this chapter. Structures that make up the juxtaglomerular apparatus (see Fig. 33.5 ) include:
1.the macula densa of the thick ascending limb
2.extraglomerular mesangial cells
3.renin- and angiotensin II–producing granular cells of the afferent arteriole
The cells of the macula densa represent a morphologically distinct region of the thick ascending limb. This region passes through the angle formed by the afferent and efferent arterioles of the same nephron. The cells of the macula densa contact the extraglomerular mesangial cells and the granular cells of the afferent arterioles. The granular cells of the afferent arterioles contain smooth muscle myofilaments and—importantly—manufacture, store, and release renin in response to signals associated with decreased effective circulating volume and reduced renal perfusion. Renin is involved in proteolytic generation of angiotensin II and ultimately in secretion of aldosterone (see Chapter 35 ). The juxtaglomerular apparatus is one component of the tubuloglomerular feedback mechanism involved in autoregulation of RBF and GFR.
Renal nerves regulate RBF, GFR, and salt and water reabsorption by the nephron. The nerve supply to the kidneys consists of sympathetic nerve fibers that originate in the celiac plexus. There is no corresponding parasympathetic innervation. Adrenergic fibers innervating the kidneys release norepinephrine and lie adjacent to the smooth muscle cells of the major branches of the renal artery (interlobar, arcuate, and interlobular arteries) as well as the afferent and efferent arterioles. In addition, sympathetic nerves innervate the renin-producing granular cells of the afferent arterioles. Renin secretion is stimulated by increased sympathetic activity. Nerve fibers also innervate the proximal tubule, loop of Henle, distal tubule, and collecting duct; activation of these nerves enhances Na + reabsorption by these nephron segments.
The coordinated actions of the nephron’s various segments determine the final amount of a substance that appears in urine. This represents three general processes: (1) glomerular filtration, (2) reabsorption of the substance from tubular fluid back into blood, and (3) (in some cases) secretion of the substance from blood into tubule fluid. The first step in urine formation by the kidneys is production of an ultrafiltrate of plasma across the glomerulus. The process of glomerular filtration and regulation of GFR and RBF are discussed later in this chapter. The concept of renal clearance, which is the theoretical basis for measurement of GFR and RBF, is presented in the following section. Reabsorption and secretion are discussed in subsequent chapters.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here