Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Fertilizing sperm must complete maturation and capacitation before sperm-egg fusion can take place.
Sperm released from the testis and entering the epididymal duct display circular motion . After a 2-week maturation process during epididymal transit, sperm acquire forward motility , a maturation step required for fertilization.
After ejaculation, a number of sperm undergo a capacitation process in a storage site in the isthmus of the oviduct. Capacitated sperm are then guided by a combination of chemotaxis and thermotaxis from the storage site to the ovum or egg in the ampulla of the oviduct, where fertilization takes place.
Capacitation can be induced in vitro to enable in vitro fertilization. During capacitation:
Non-covalently bound epididymal and seminal glycoproteins are diluted from the sperm plasma membrane by fluids of the female reproductive tract.
The sperm influx of external bicarbonate ions stimulates the activity of a specific adenylyl cyclase (ADCY10) to maximize intracellular cyclic adenosine monophosphate (cAMP) levels, contributing to the start of capacitation.
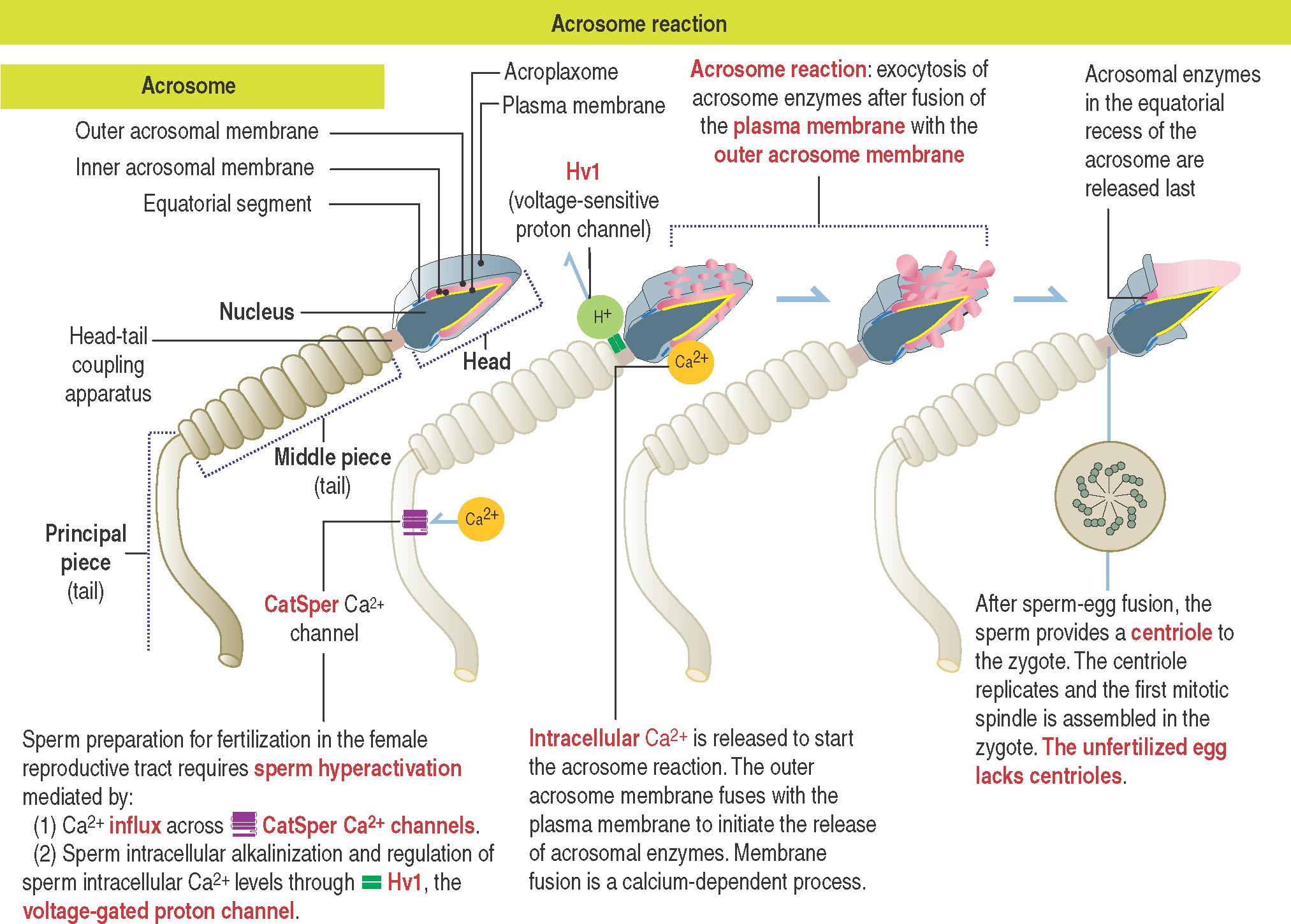
Sperm membrane permeability to Ca 2+ increases. An influx of Ca 2+ , enabled through a flagellar pH-sensitive CatSper (for Cation Sperm) Ca 2+ channel (see 21-1 ), starts in the principal piece of the sperm tail, reaching the sperm head in a few seconds.
Sperm cytoplasmic acidic pH (lower than pH 6.5) changes to an alkaline intracellular pH (pH 7.4) through the extrusion of H + through Hv1 , a voltage-sensitive proton channel . An increase in sperm intracellular pH completes capacitation.
Why are increases in Ca 2+ concentration and alkalinization so important to achieve sperm capacitation?
Increases in Ca 2+ concentration induce the exocytotic acrosome reaction in the sperm head and alkalinization triggers sperm hyperactivation (intensification of the beating of the sperm tail).
Fertilization requires that sperm complete their maturation in the epididymis and become capacitated in the oviduct. The next step is acrosome reaction.
What is acrosome reaction?
We have seen in Chapter 20 , Spermatogenesis, that the sperm head consists of three components:
The condensed elongated nucleus .
The acrosome , bound to the acroplaxome .
The plasma membrane .
The acrosome is a sac limited by an outer acrosomal membrane and an inner acrosomal membrane .
The acrosome sac stores hydrolytic enzymes (mainly hyaluronidase and acrosin , the latter derived from the precursor proacrosin) .
A very thin portion of the acrosome sac, extending toward the tail, is the equatorial segment (see 23-1 ). The equatorial segment of the acrosome does not participate in the acrosome reaction.
Three sequential events take place during fertilization:
The acrosome reaction .
Sperm binding to a receptor on ZP3 , a glycoprotein of the zona pellucida (ZP).
Sperm-egg fusion (see 23-2 ).
In the proximity of the ovum and in the presence of free Ca 2+ , the sperm plasma membrane fuses with the outer acrosomal membrane , an event known as acrosome reaction .
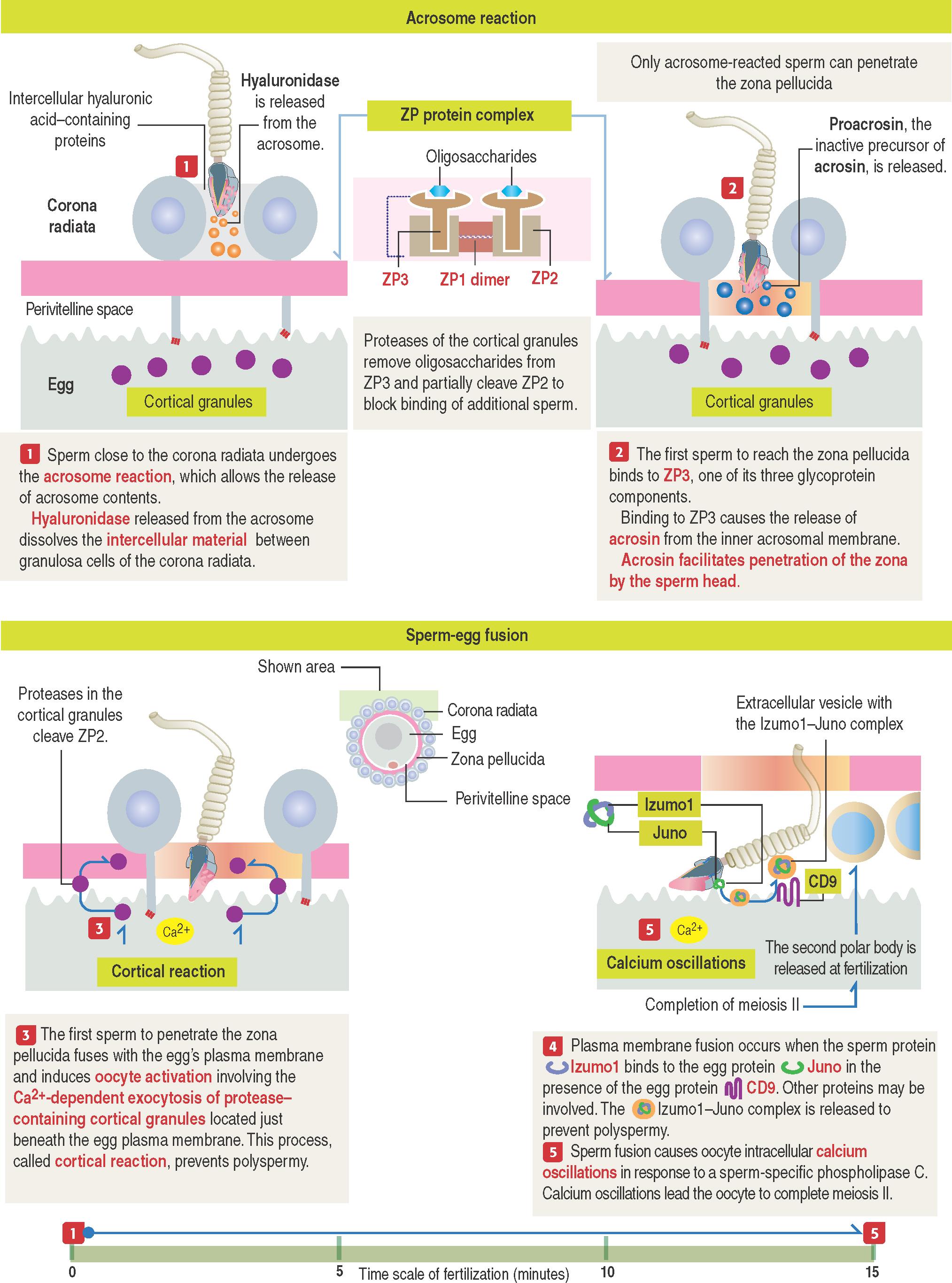
Small openings created by membrane fusion permit the leakage of hydrolytic enzymes. Hyaluronidase breaks down proteins present in the intercellular space of granulosa cells of the corona radiata. Proacrosin changes into acrosin and enables the fertilizing sperm to cross the zona pellucida.
Male infertility may occur when the acrosome reaction fails to occur or takes place before the sperm reaches the oocyte, also called egg .
After crossing the zona pellucida, the plasma membrane of the sperm (the post-acrosomal equatorial region) and plasma membrane of the egg fuse to enable the sperm nucleus to reach the cytoplasm of the oocyte. The insertion of the sperm nucleus into the cytoplasm of the egg is called impregnation .
How does sperm-egg fusion take place?
Two membrane proteins are regarded as essential for sperm-egg fusion:
Izumo1 , a protein of the immunoglobulin superfamily, is inserted in the sperm plasma membrane.
Juno , present in the egg plasma membrane.
In the presence of CD9, Izumo1 binds to Juno to achieve sperm-egg fusion. Then, the Izumo1-Juno complex is sequestered within a membrane-bound vesicle and ejected into the perivitelline space.
This event, together with a conformation change in the molecular organization of the zona pellucida, block the binding and fusion of additional sperm, thus preventing polyspermy.
CD9 is a member of the tetraspanin family of transmembrane proteins (see Box 23-A ). The tetraspanin family consists of 33 proteins; each has four transmembrane domains. Other proteins, such as ADAMs (a disintegrin and metalloproteinase), may participate in sperm-egg fusion.
Tetraspanins , first discovered on the human leukocyte surface, have four transmembrane domains, two extracellular loops (small and large) and short intracytoplasmic N- and C-terminal tails. Tetraspanins interact with specific proteins such as integrins, receptors of the immunoglobulin superfamily and metalloproteinases.
The transmembrane domains enable the association of additional tetraspanins to assemble the tetraspanin web in which integrins are included. The large extracellular loop is involved in protein-protein interaction with laterally positioned proteins. The intracellular short tails are linked to intracellular cytoskeletal and signaling molecules.
ADAM10 (a disintegrin and metalloproteinase 10 sheddase) is a well-known binding partner of six tetraspanins of the tetraspanin (TSPAN) C8 subgroup (TSPAN5, TSPAN10, TSPAN14, TSPAN15, TSPAN17 and TSPAN33). They are named as such because their large extracellular loop contains eight conserved cysteine residues.
We discussed in Chapter 1 , Epithelium | Cell Biology, how the disintegrin domain of ADAMs participates in the shedding of the ectoplasmic portion of transmembrane proteins.
Sperm-egg fusion causes a local mild depolarization of the egg plasma membrane that generates within 5 to 20 seconds calcium oscillations across the cytoplasm of the fertilized egg. Calcium oscillations result in oocyte activation involving two fundamental steps in the process of fertilization (see Box 23-B ):
The exocytosis of the protease ovastacin from cortical granules . During this event, a vesicle is formed to release the Izumo1–Juno complex into the perivitelline space.
To trigger the secondary oocyte to complete meiosis II . The second polar body is released into the perivitelline space and the secondary oocyte achieves a haploid state. Completion of meiosis II starts the early embryogenesis developmental program as a zygote.
Oocyte activation is an important step in the process of fertilization.
Oocyte activation consists of the exocytosis of cortical granules and the release of the oocyte from meiotic arrest.
Oocyte activation involves an elevation of intracellular Ca 2+ characterized by Ca 2+ oscillations, which start soon after sperm-egg fusion.
The agent responsible for Ca 2+ oscillations within activating oocytes is a sperm-specific phospholipase C, phospholipase C zeta (PLCζ).
Abnormalities in the structure, functional ability and localization of PLCζ in sperm are associated with certain types of human male factor infertility in which oocyte activation is deficient.
Remember that the sperm contributes the centrosome responsible for assembling the first mitotic spindle of the new embryo and that mitochondria derive from the fertilized egg.
We have discussed in Chapter 22 , Folliculogenesis and Menstrual Cycle, aspects of the development of the zona pellucida, as early as during the primary follicle stage. We are revisiting the zona pellucida within the context of fertilization.
The zona pellucida has important roles in fertilization and implantation of the embryo in the endometrium. In vitro fertilization procedures overcome the inability of some sperm to penetrate the zona pellucida, a form of infertility (see Box 23-C ).
Fertilization of human sperm and eggs in vitro consists in the following steps: Pre-ovulatory oocytes (about 10 or more) are collected by laparoscopy or transvaginally guided by ultrasound imaging, following stimulation of the ovaries by gonadotropin-releasing hormone and follicle-stimulating hormone administration. Oocytes are retrieved 34 to 38 hours after injection of human chorionic gonadotropin to mimic the luteinizing hormone surge.
The oocytes are incubated overnight with motile sperm in a defined culture medium to achieve fertilization in vitro. Embryos can then be transferred to the patient.
Alternatively, in cases of a severe male infertility factor, a sperm can be injected into the oocyte by the process of intracytoplasmatic sperm injection (ICSI) .
In cases of azoospermia (absence of sperm in the ejaculate), sperm can be obtained surgically from the epididymis or the testes and used for ICSI.
Embryos can be tested in vitro for the presence of a genetic or chromosomal abnormality by a procedure known as preimplantation genetics diagnosis . A sample can be a blastomere of the embryo, a piece of the trophoderm or even the oocyte's polar body . Unaffected embryos can then be transferred to the patient.
An excess of embryos can be cryopreserved in liquid nitrogen for later use. Propanediol or dimethylsulphoxide can be used as cryoprotectant for pre-blastocyst embryos and glycerol is used for blastocysts.
The zona pellucida is composed of three glycoproteins (see 23-2 ): ZP1 , a dimer of 200 kd; ZP2 , 120 kd; and ZP3 , 83 kd. ZP2 and ZP3 interact to form a long filament, interconnected ar regular intervals by the ZP1 dimers.
There are four functional aspects related to ZP3 to keep in mind:
ZP3 is responsible for sperm binding, mediated by O -oligosaccharides linked to ZP3 with binding affinity to sperm receptors.
Only acrosome-reacted sperm can interact with ZP3 .
ZP3 is essential for species-specific sperm binding . It prevents fertilization of the egg by sperm from a different species.
After the first sperm fertilizes the egg, the protease ovastacin, released from the cortical granules of the egg, removes oligosaccharides from ZP3 and partially cleaves ZP2. This process, called cortical reaction , together with the disposal of the Izumo1–Juno complex, prevents polyspermy. Polyspermy results in non-viable zygotes. Ovastacin is an oocyte-specific member of the astacin family of metalloendoproteases.
Putting things together, sperm maturation in the epididymis, sperm capacitation in the female reproductive tract, and acrosome reaction in the proximity of the ovulated secondary oocyte are sequential steps leading to fertilization.
Sperm reach a storage site in the isthmus region of the oviduct and a fraction of them undergoes capacitation. Sperm reach the oviduct assisted by sperm motility as well as on a passive drag by waves of muscular contractile activity of the vagina, cervix, and uterus.
Fertilization takes place in the ampulla of the oviduct.
Sperm are guided to the egg by:
A chemoattractant gradient present in the oviductal fluid and originated in the egg and granulosa cells anchored to the zona pellucida.
A temperature gradient between the storage site (34.7 o C) and the fertilization site (36.3 o C).
Contractions of the oviduct wall.
Two barriers that the fertilizing sperm confront during fertilization are the corona radiata and the zona pellucida (see 23-2 ). Enzymes released following the acrosome reaction enable the sperm to cross these barriers. The final step of fertilization is the fusion of the plasma membranes of the sperm and the secondary oocyte. Two plasma membrane proteins, Izumo1 in sperm and Juno in the oocyte, achieve sperm-egg fusion.
Recall from our discussion in Chapter 20 , Spermatogenesis, that sperm condensed chromatin lacks nucleosomes. Somatic histones have been replaced by a protamine complex during spermiogenesis. Therefore, the zygote needs to resolve differences in the chromatin state of the egg and sperm pronuclei to make sure that:
The first mitotic division can take place.
The embryo can take full control of gene expression for embryonic development by a process termed zygotic genome activation .
The calcium oscillations across the cytoplasm of the fertilized egg that we mentioned before, responsible for the completion of meiosis II, trigger the rapid removal of the protamine complex of the sperm pronucleus and DNA is re-wrapped by somatic histones donated by the egg.
One final point: remember that the embryo undergoes extensive epigenetic reprogramming, which involves DNA demethylation (see Chapter 20 , Spermatogenesis). This change is required for the zygote to acquire totipotency: the zygote is capable of developing into all the specialized cells found in the adult . The expression of cell lineage specific transcription factors starts in the blastocyst, when the outer trophoblast and the pluripotent inner cell mass acquire cellular identity.
On day 4 of pregnancy, the blastocyst is within the uterine cavity. The coordinated effect of ovarian estrogens and progesterone has already conditioned uterine receptivity for implantation, including an increase in endometrial vascular permeability at the implantation site (see Box 23-D ).
Fertilization occurs in the oviduct or fallopian tube within 24 to 48 hours after ovulation.
The development of the fertilized egg, called zygote , to the morula stage , occurs as the embryo, surrounded by the zona pellucida, travels through the fallopian tube. The morula contains compact embryonic cells called blastomeres .
The morula appears in the uterine cavity about 2 to 3 days after fertilization.
The embryo, now a blastocyst , hatches from the zona pellucida 72 hours after entering the uterine cavity and implants into the uterine wall.
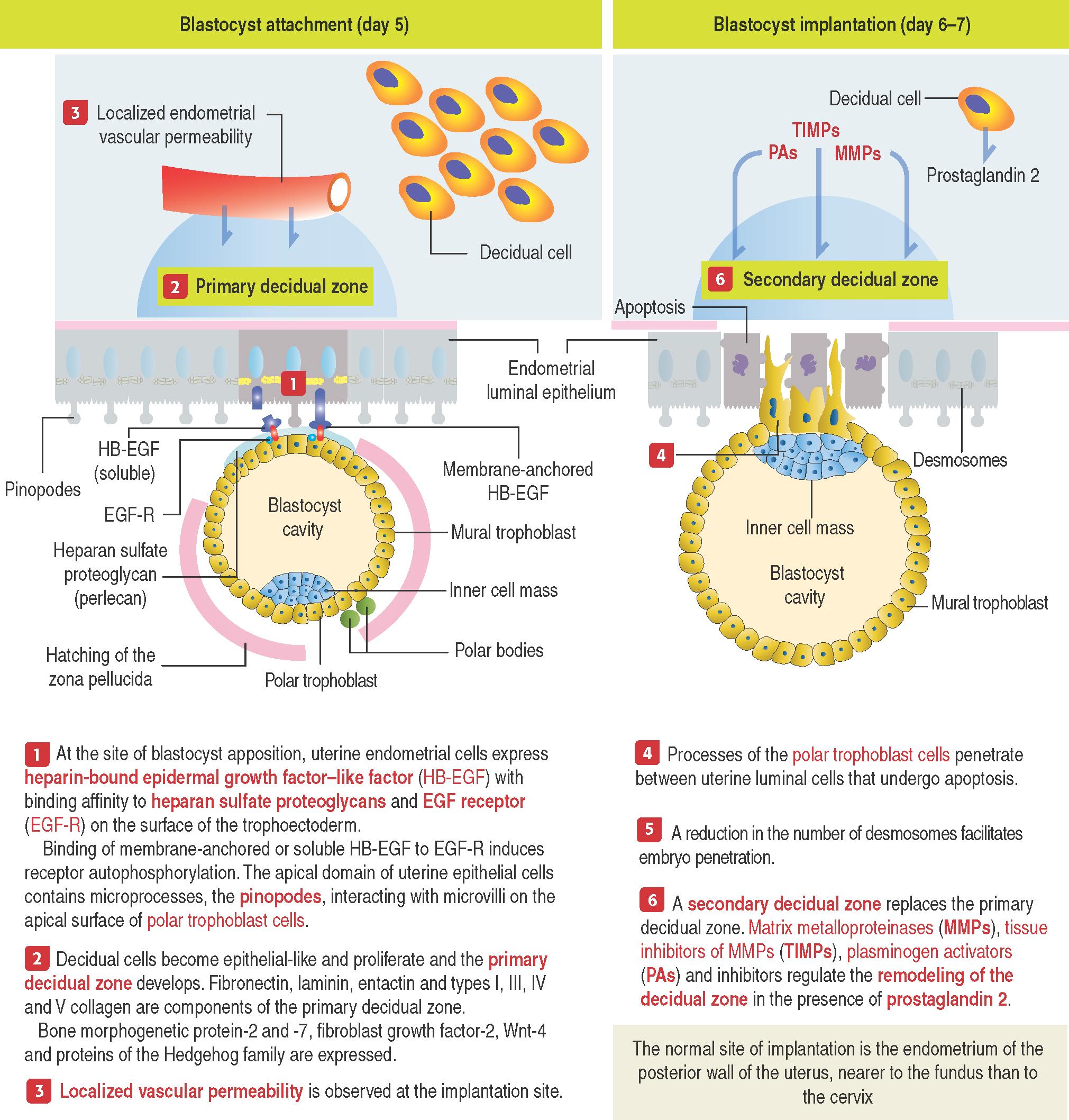
Implantation occurs 6 or 7 days after fertilization. Implantation involves two stages: (1) apposition of the blastocyst to the endometrial surface and (2) implantation of the blastocyst mediated by penetrating trophoblast cells .
The blastocyst is entirely embedded in the receptive endometrium by day 10 after fertilization. Uterine receptivity , corresponding to days 20 to 24 of a regular 28-day menstrual cycle, is defined by the optimal state of endometrial maturation for the implantation of the blastocyst. Uterine receptivity consists of a vascular and edematous endometrial stroma, secretory endometrial glands and apical microprocesses, the pinopodes , on the apical domain of the luminal endometrial lining cells.
Differentiated syncytiotrophoblast cells invade the primary decidua (interstitial invasion) , as well as local uterine blood vessels (endovascular invasion) .
The uteroplacental circulation is established when syncytiotrophoblast cells erode the wall of the maternal blood vessels (see Box 23-E).
On day 5, the blastocyst hatches from the zona pellucida and exposes the polar trophoblast to the endometrium. If zona pellucida hatching fails to occur, the embryo will not implant.
The receptive time of the endometrium for the incoming embryo, called the implantation window , lasts 4 days (days 20-23 of the menstrual cycle).
The implantation of the blastocyst involves the following steps (see 23-3 ):
An initial unstable adhesion of the blastocyst to the endometrial surface, called apposition . Apposition is followed by a more stable adhesion phase.
The decidualization of the endometrial stroma. Failure of the uterine stroma to undergo decidualization can lead to spontaneous abortion.
Implantation of the embryo requires the interaction of trophoblast cells with a receptive endometrium .
The receptive endometrium and the invading trophoblasts display the following conditions:
The apical surface of the endometrial epithelial cell is coated with bound and soluble forms of heparin-bound epidermal growth factor–like factor (HB-EGF) , a member of the transforming growth factor-α family.
The surface of the trophoblast cells harbors epidermal growth factor receptor (EGF-R) and is coated with heparan sulfate proteoglycan . Binding of membrane-anchored or soluble HB-EGF to EGF-R induces receptor autophosphorylation and heparan sulfate proteoglycan (also called perlecan) to bind strongly to HB-EGF. Apposition and adhesion between the blastocysts and the uterine luminal epithelium have been established.
Then, cytoplasmic processes of trophoblast cells interact with pinopodes , small processes on the apical surface of the endometrial epithelial cells. Trophoblast processes penetrate the intercellular spaces between endometrial cells as the number of desmosomes linking the endometrial cells decreases and endometrial cells undergo apoptosis .
As you recall, fibroblasts in the endometrial stroma underwent decidualization during the secretory phase of the menstrual cycle.
This primary decidual zone is remodeled by the action of metalloproteinases into a secondary decidual zone that houses and protects the implanting embryo.
Soon after implantation, the trophoblast differentiates into two cell layers (see 23-3 ):
An inner layer of mitotically active mononucleated cytotrophoblast cells .
An outer layer of multinucleated syncytiotrophoblast cells at the embryonic pole, facing the endometrium. Syncytiotrophoblast cells arise from the fusion of cytotrophoblast cells .
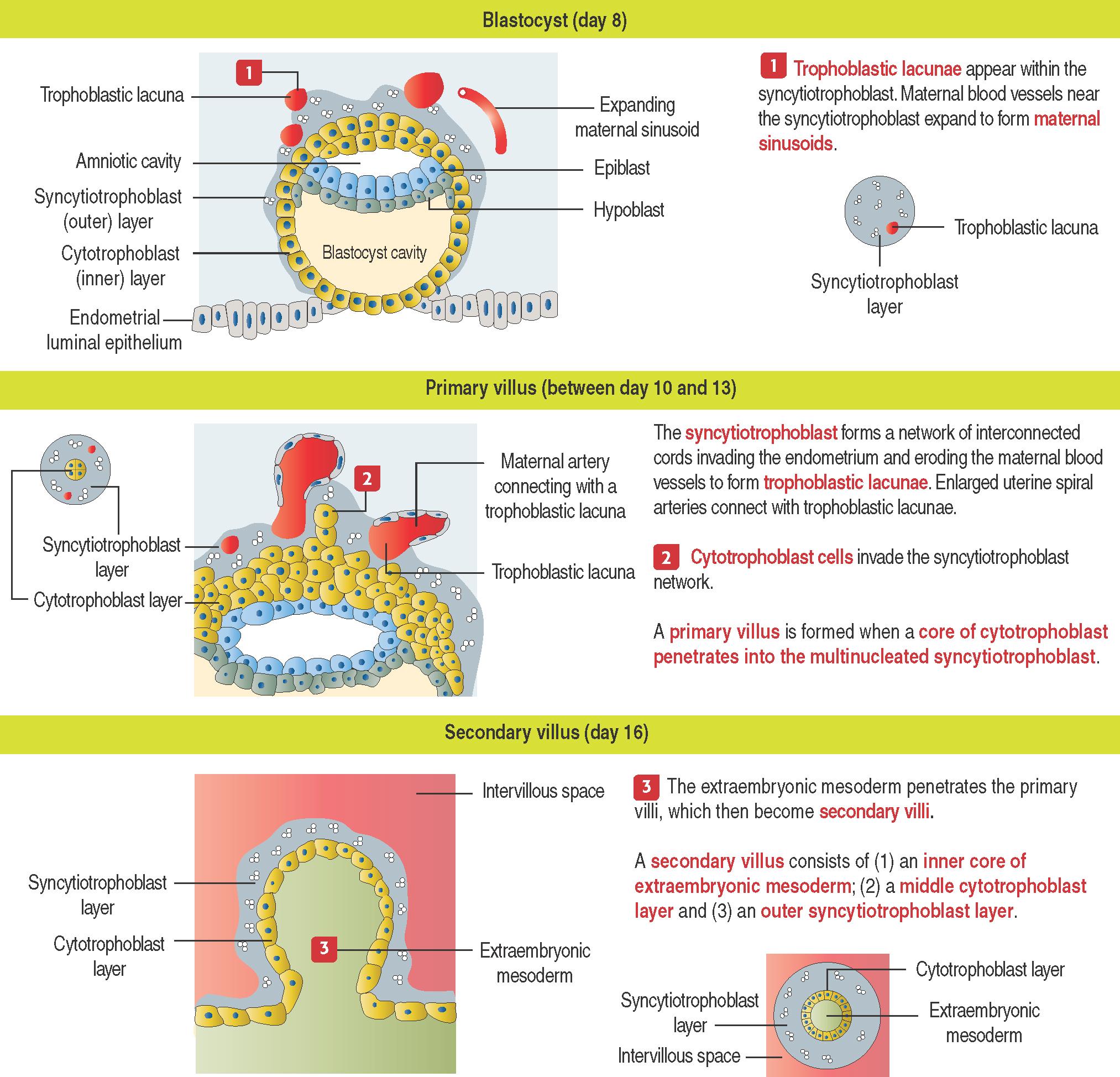
The syncytiotrophoblast produces proteolytic enzymes , penetrating the primary decidua, and the entire blastocyst is rapidly surrounded by the endometrium. Invasion of the endometrium at the edge of the myometrium is called interstitial invasion .
The blastocyst has a cavity containing fluid and the eccentric inner cell mass , which gives rise to the embryo and some extraembryonic tissues.
The mural trophoblast cells, proximal to the inner cell mass, begin to develop the chorionic sac . The chorionic sac consists of two components: the trophoblast and the underlying extraembryonic mesoderm .
Proteases released by the syncytiotrophoblast erode the branches of the spiral uterine arteries to form spaces or lacunae of maternal blood within the syncytiotrophoblast cellular mass (see 23-4 ).
This endometrial eroding event, called endovascular invasion , marks the initiation of the primitive uteroplacental circulation .
Decidualization allows an orderly access of trophoblastic cells to the maternal nutrients by modulating their invasion of uterine spiral arteries.
The syncytiotrophoblast begins the secretion of human chorionic gonadotropin (hCG) into the maternal lacunae. The secretion of estrogens and progesterone by the corpus luteum is now under the control of hCG, an LH equivalent.
On the maternal side, decidual cells, close to the mass of invading syncytiotrophoblast cells, deisintegrate and release glycogen and lipids. Glycogen and lipids, together with products of the endometrial glands and maternal blood in the lacunae, constitute the initial nutrients for embryonic development.
The decidual reaction provides an immune-protective environment for the development of the embryo. The decidual reaction involves:
The production of immunosuppressive substances (mainly prostaglandins) by decidual cells to inhibit the activation of natural killer cells at the implantation site.
Infiltrating leukocytes in the endometrial stroma that secrete interleukin-2 to prevent maternal tissue rejection of the implanting embryo.
Syncytiotrophoblasts do not express major histocompatibility complex (MHC) class II . Therefore, the syncytiotrophoblast cannot present antigens to maternal CD4 + T cells.
At the end of the second week, cytotrophoblast cells proliferate under the influence of the extraembryonic mesoderm and extend into the syncytiotrophoblast mass, forming the villi .
There are three different types of chorionic or placental villi:
Primary villi .
Secondary villi .
Tertiary villi .
The blastocyst has two distinct cell populations: (1) trophoblast cells , derived from the trophoectoderm surrounding the blastocyst; and (2) the inner cell mass , which gives rise to the embryo.
Trophoblast cells (the collective designation of cytotrophoblast cells and syncytiotrophoblast cells) are always the outermost layer of fetal cells covering the mesenchyme and fetal capillaries of the chorionic villi.
The wall of maternal blood vessels is infiltrated and ruptured by trophoblast cells. Maternal blood is released into the intervillous space and the outer layer of the chorionic villi (syncytiotrophoblast cells) is immersed in maternal blood like a sponge in a container of blood.
The extravillous cytotrophoblast cells replace the endothelium and tunica media of the uterine spiral arteries (see 23-5 ), which deliver blood, at low pressure, to the intervillous space. Basal straight arteries are not involved in these changes.
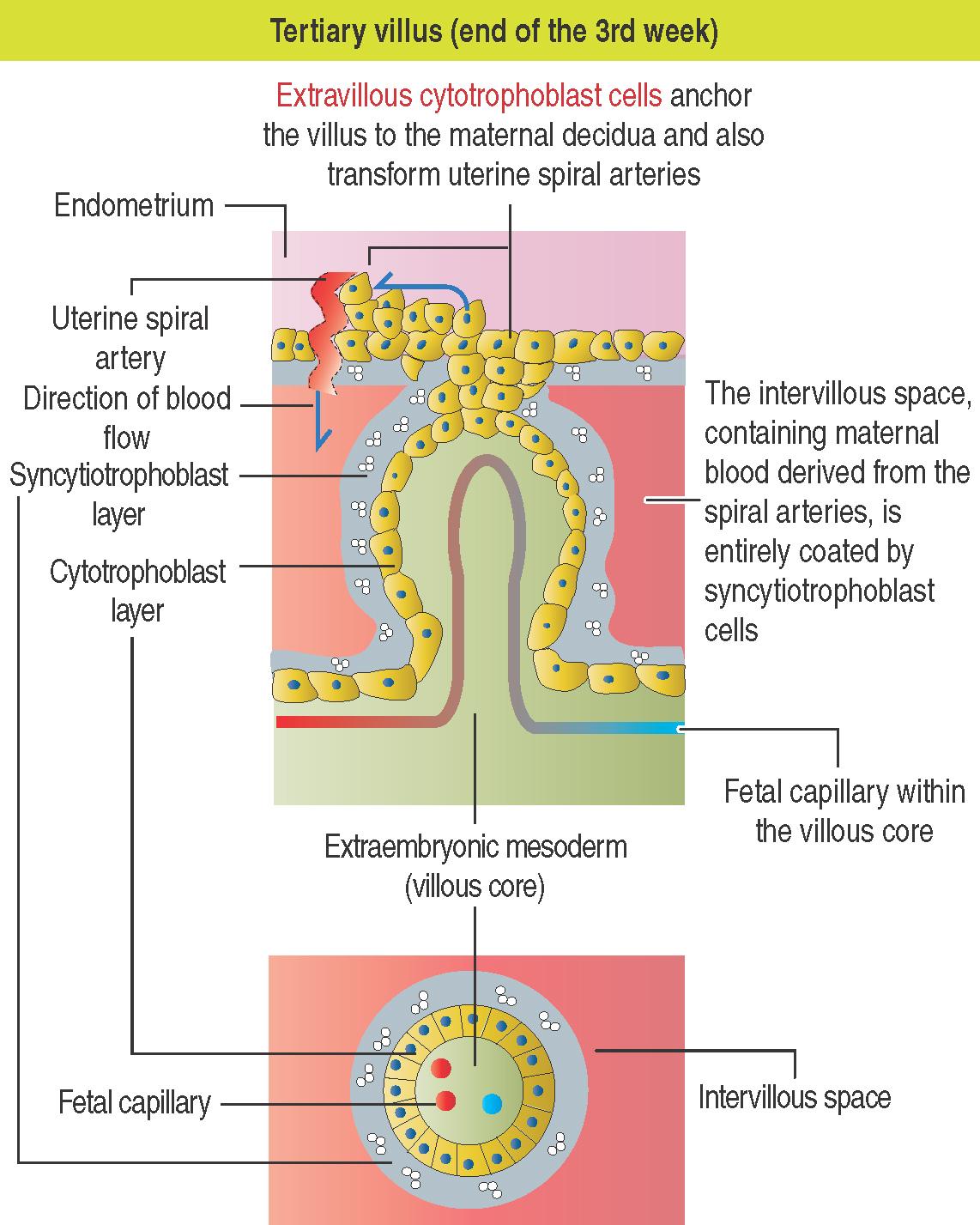
If extravillous cytotrophoblast cell replacement of the wall of the uterine spiral arteries is incomplete, the development of the transformed arteries is deficient and blood flow is deficient.
Pre-eclampsia occurs when there is a reduced development of the branches of the chorionic villus tree and limited fetal growth.
Primary villi (see 23-4 ) represent the first step in the development of the chorionic villi of the placenta. A primary villus is formed by a core of cytotrophoblast cells covered by syncytiotrophoblast.
Early in the third week, the extraembryonic mesoderm extends into the primary villi, forming the secondary villi (see 23-4 ).
A secondary villus consists of a core of extraembryonic mesoderm surrounded by a middle cytotrophoblast layer and an outer syncytiotrophoblast layer.
Soon after, cells of the extraembryonic mesoderm differentiate into capillary and blood cells and tertiary villi are developed.
A tertiary villus (see 23-5 ) is formed by a core of extraembryonic mesoderm with capillaries, surrounded by a middle cytotrophoblast layer and an outer layer of syncytiotrophoblast. The difference between the secondary and tertiary villi is the presence of capillaries in the latter. The capillaries in the tertiary villi interconnect to form arteriocapillary networks leading to the embryonic heart.
The placenta and embryonic-fetal membranes (amnion, chorion, allantois and yolk sac) protect the embryo-fetus and provide for nutrition, respiration, excretion and hormone production during development. The membranes are formed by the embryo.

The mature placenta (see 23-6 ) is 3 cm thick, has a diameter of 20 cm and weighs about 500 g.
The fetal side of the placenta is smooth and associated with the amniotic membrane.
The maternal side of the placenta is partially subdivided into 10 or more lobes by decidual septa derived from the decidua basalis and extending toward the chorionic plate. The decidual septa do not fuse with the chorionic plate. Each lobe contains 10 or more stem villi and its branches.
The 50- to 60-cm-long and 12-mm-thick and twisted umbilical cord is attached to the chorionic plate and contains two umbilical arteries (transporting deoxygenated blood) and one umbilical vein (transporting oxygen-rich blood).
The umbilical vessels are embedded in embryonic connective tissue , called Wharton's jelly (see Chapter 4 , Connective Tissue). Embryonic connective tissue cushions the umbilical cord blood vessels to ensure steady blood flow by preventing twisting and compression. The cord is lined by amniotic epithelium.
Blood collected from the vein of the severed umbilical cord from a newborn baby (following detachment from the newborn) contains stem cells , including hematopoietic stem cells, useful for transplantation to patients with leukemia, lymphoma and anemia.
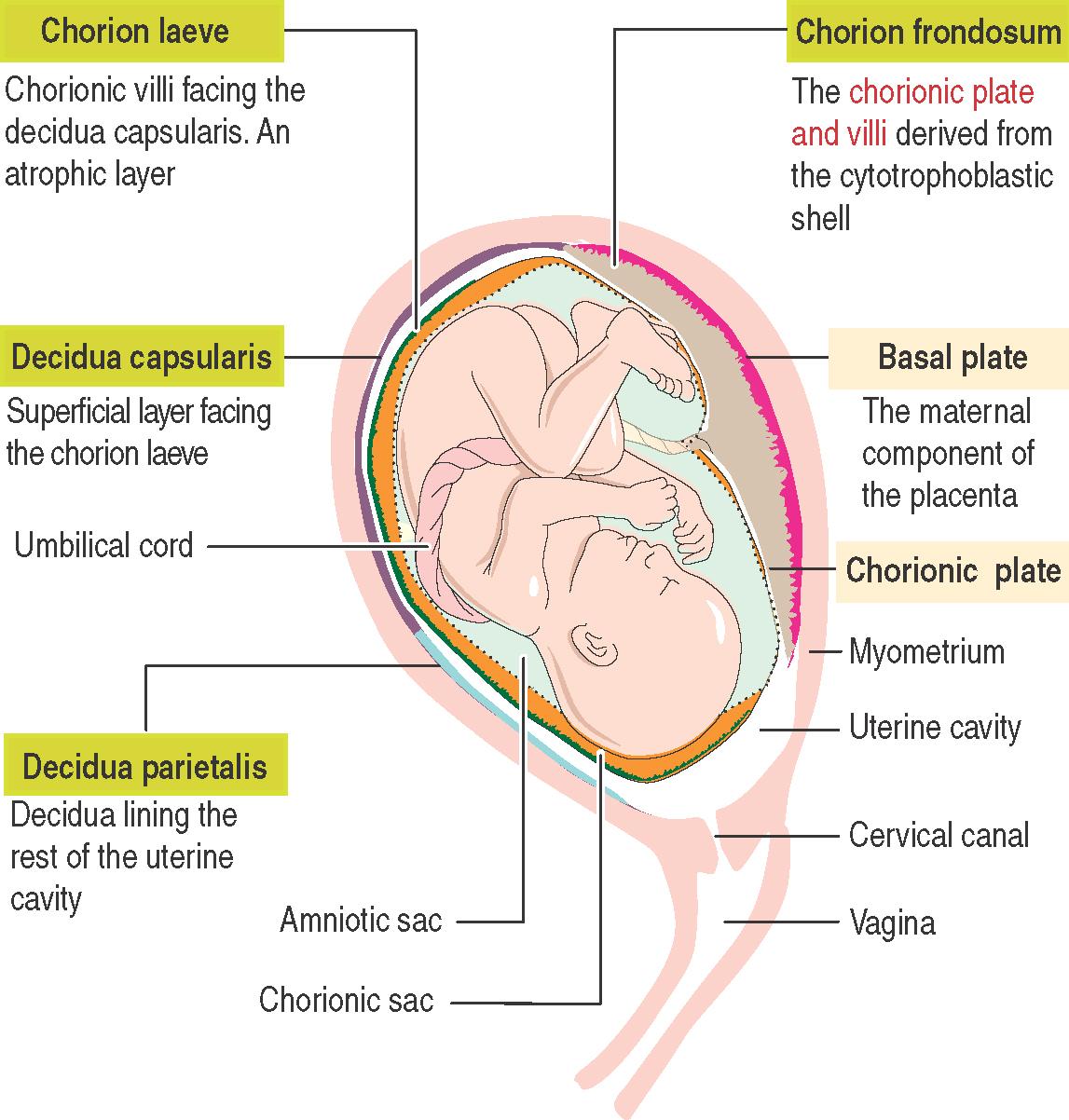
The placenta consists of a maternal component, the decidua , and a fetal component, the chorion .
The decidua (Latin deciduus , falling off; a tissue shed at birth) is the endometrium of the gravid uterus.
There are three regions of the decidua , named according to their relationship to the developing fetus:
The decidua basalis is the maternal component of the placenta. Chorionic villi facing the decidua basalis are highly developed and form the chorion frondosum (bushy chorion).
The decidua capsularis is the superficial layer covering the developing fetus and its chorionic sac.
The decidua parietalis is the rest of the decidua lining the cavity of the uterus not occupied by the fetus.
The fetal component is the chorion , represented by the chorion frondosum: the chorionic plate and derived chorionic villi .
Chorionic villi facing the decidua capsularis undergo atrophy, resulting in the formation of the chorion laeve (smooth chorion).
The intervillous space between the maternal and fetal components contains circulating maternal blood. Arterial blood, derived from the open ends of the cytotrophoblast-transformed spiral arteries, flows into the intervillous space. Blood flows back into the uterine veins.
Extravillous cytotrophoblast cells, anchoring the villi to the decidua, invade the wall of the maternal spiral arteries, replace their endothelial lining and induce apoptosis of the surrounding smooth muscle cells. Cytotrophoblasts cells gradually change into an endothelial cell-type and vascular remodeling controls the flow of blood into the intervillous space .
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here