Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The nervous system consists of complex networks of neurons that carry information from the sensory receptors in the body to the central nervous system (CNS); integrate, process, and store it; and return motor impulses to various effector organs in the body. The development of the CNS is covered in Chapter 9; this chapter covers the development of the peripheral nervous system (PNS).
The PNS and its central pathways are traditionally divided into two systems. The somatic nervous system is responsible for carrying conscious sensations and for innervating the voluntary (striated) muscles of the body. The autonomic nervous system is strictly motor and controls most of the involuntary, visceral activities of the body. The autonomic system itself consists of two divisions: the parasympathetic division , which, in general, promotes the anabolic visceral activities characteristic of periods of peace and relaxation, and the sympathetic division , which controls the involuntary activities that occur under stressful “fight or flight” conditions. Each of these systems is composed of two-neuron pathways consisting of preganglionic and postganglionic neurons . As covered in Chapter 14, Chapter 4 , the gut contains its own nervous system called the enteric nervous system .
Neurons originate from three embryonic tissues: the neuroepithelium lining the neural canal, neural crest cells , and specialized regions of ectoderm in the head and neck called ectodermal placodes . Neurons of the CNS arise from the neuroepithelium (covered in Chapter 9 ), whereas those of the PNS arise from neural crest cells and ectodermal placodes and form clusters of neurons or ganglia. In the PNS, the glial (supporting cells) arise exclusively from neural crest cells in all ganglia.
Trunk ganglia are formed by migrating neural crest cells. These ganglia include (1) sensory dorsal root ganglia that condense next to the spinal cord in register with each pair of somites and consist of sensory neurons that relay information from receptors in the body to the CNS and their supporting satellite cells ; (2) sympathetic chain ganglia that also flank the spinal cord (but more ventrally) and the prevertebral ( or preaortic) ganglia that form next to branches of the abdominal aorta and contain the peripheral (postganglionic) neurons of the two-neuron sympathetic pathways; and (3) the parasympathetic ganglia embedded in the walls of the visceral organs and containing the peripheral (postganglionic) neurons of the two-neuron parasympathetic division. The parasympathetic ganglia that reside within the gut are termed enteric ganglia .
As neural crest cells of the trunk coalesce to form spinal ganglia, somatic motor axons begin to grow out from the basal columns of the spinal cord, forming a pair of ventral roots at the level of each somite. These somatic motor fibers are later joined by autonomic motor fibers arising in the intermediolateral cell columns. The somatic motor fibers grow into the myotomes and, consequently, come to innervate the voluntary muscles. The autonomic (preganglionic) fibers, in contrast, terminate in the autonomic ganglia (sympathetic and parasympathetic), where they synapse with cell bodies of the peripheral (postganglionic) autonomic neurons that innervate the appropriate target organs.
The central (preganglionic) neurons of the sympathetic division develop in the intermediolateral cell columns of the thoracolumbar spinal cord (T1 through L2). The thinly myelinated axons of these cells leave the spinal cord in the ventral root but immediately branch off to form a white ramus , which enters the corresponding chain ganglion. Some of these fibers synapse with peripheral (postganglionic) sympathetic neurons in the chain ganglion; others pass onward to synapse in another chain ganglion or in one of the prevertebral ganglia. The unmyelinated axons of the peripheral (postganglionic) sympathetic chain ganglion neurons reenter the spinal nerve via a branch called the gray ramus .
The ganglia of the head consist of two types: cranial nerve ganglia , the neurons of which arise from either neural crest cells or ectodermal placodes, depending on the particular ganglion and cranial parasympathetic ganglia , which arise from neural crest cells.
The central (preganglionic) neurons of the parasympathetic pathways are located in the brain stem and in the spinal cord at levels S2 through S4. The parasympathetic division is, therefore, called a craniosacral system . Parasympathetic fibers from the hindbrain reach the parasympathetic ganglia of the neck and trunk viscera via the vagus nerve , whereas sacral parasympathetic fibers innervate hindgut and pelvic visceral ganglia via the pelvic splanchnic nerves .
A toddler is brought to the emergency department after biting off the right anterolateral part of his tongue. While an oral surgeon is suturing the tongue, the staff notices other suspicious injuries. These include lacerations of the gums with missing teeth ( Fig. 10.1A ), a burn on the left index finger (see Fig. 10.1B ), and multiple other small cuts and bruises. An X-ray of the face, done to investigate the broken teeth, reveals an occult fracture of the parietal bone. An inquiry is begun by child protective services (CPS).
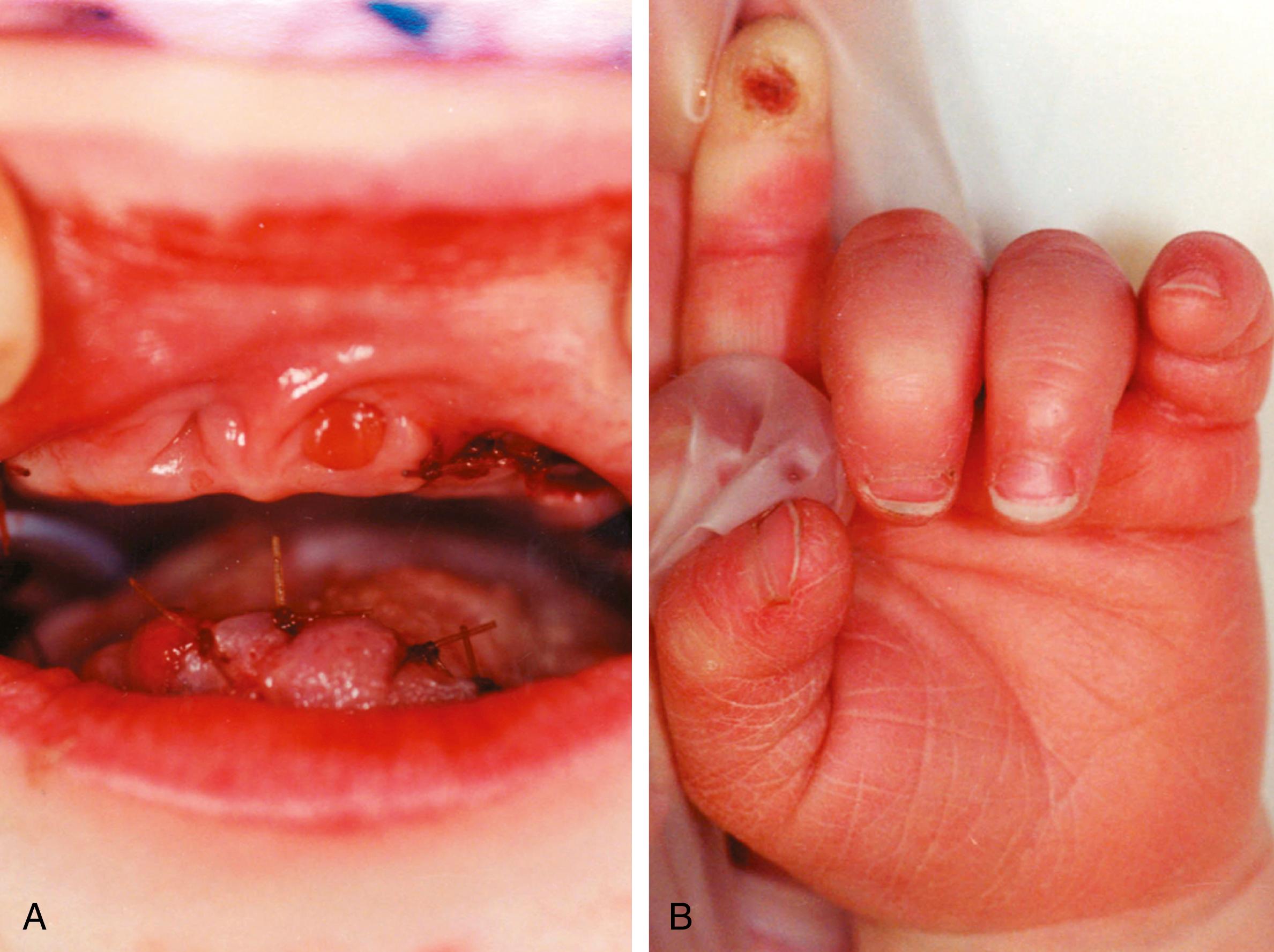
The parents claim that the injuries are all “self-inflicted,” and describe the boy as having “no pain.” They explain that the broken teeth are a result of biting on toys, and that the burned finger occurred when the child touched a hot grill. He does not cry with any of these significant injuries, including the bitten tongue, and they express their surprise when the skull fracture is discovered. His medical records show that he has been admitted to the hospital several times with high fever and presumed sepsis (severe infection) that was treated with antibiotics. The family has noticed that he becomes flushed and lethargic in the heat, and they have never seen him sweat. The boy cries little during the procedure to repair his tongue and is indifferent to the needle sticks needed to obtain laboratory tests. The CPS investigation uncovers no evidence of abuse. The family has two older children who are healthy and well cared for.
Neurology is consulted, and they obtain a skin biopsy that shows a paucity of small nerve fibers in the skin and absence of innervation of the sweat glands. Based on the clinical history and on these histologic findings, the diagnosis of congenital insensitivity to pain with anhidrosis (CIPA; anhidrosis means lack of sweating) is made. Confirmatory sequencing of the NTKR1 gene uncovers two deleterious mutations, each carried by one parent. NTKR1 is a receptor for nerve growth factor and is required for the development of nociceptive (pain) sensory innervation of the skin, and for autonomic innervation of the eccrine sweat glands.
As covered in Chapter 9 , the nervous system of vertebrates consists of two major structural divisions: a central nervous system (CNS) and a peripheral nervous system (PNS) . The CNS consists of the brain and spinal cord. The development of the CNS is covered in Chapter 9 . The PNS consists of all components of the nervous system outside of the CNS. Thus, the PNS consists of cranial nerves and ganglia, spinal nerves and ganglia, autonomic nerves and ganglia, and the enteric nervous system. The development of the PNS is covered in this chapter.
As covered in Chapter 9 , the nervous system of vertebrates consists of two major functional divisions: a somatic nervous system and a visceral nervous system . The somatic nervous system innervates the skin and most skeletal muscles (i.e., it provides both sensory and motor components). Similarly, the visceral nervous system innervates the viscera (organs of the body) and smooth muscle and glands in the more peripheral part of the body. The visceral nervous system is also called the autonomic nervous system . It consists of two components: the sympathetic division and the parasympathetic division . The somatic and visceral nervous systems are covered both in Chapter 9 (CNS components) and in this chapter (PNS components).
Both divisions of the autonomic nervous system consist of two-neuron pathways. Because the peripheral autonomic neurons reside in ganglia, the axons of the central sympathetic neurons are called preganglionic fibers and the axons of the peripheral sympathetic neurons are called postganglionic fibers . This terminology is used for both sympathetic pathways and parasympathetic pathways (covered later in the chapter). Sometimes preganglionic fibers are also called presynaptic fibers , and postganglionic fibers, postsynaptic fibers , because the axons of the preganglionic fibers synapse on the cell bodies of postganglionic neurons in the autonomic ganglia.
![]()
Animations are available online at StudentConsult.
Chapter 3, Chapter 4 describe how, during neurulation, the rudiment of the central nervous system arises as a neural plate from the ectoderm of the embryonic disc and folds to form the neural tube (the rudiment of the brain and spinal cord). The PNS arises from the neural tube and two groups of cells outside of the neural tube: neural crest cells and ectodermal placodes (the ectodermal placodes are covered further in Chapter 18 ). The PNS develops as an integrated system, essentially in cranial-to-caudal sequence. However, for the sake of simplicity, the development of the trunk (associated with the spinal cord) and of cranial (associated with the brain) portions of the PNS will be covered separately. The sympathetic division of the autonomic nervous system arises in association with the trunk (thoracolumbar levels of the spinal cord), whereas the parasympathetic division of the autonomic nervous system arises in association with the brain and caudal spinal cord (craniosacral levels of the CNS).
As previously mentioned, the PNS arises from both neural crest cells and ectodermal placodes. How are these structures determined in the early embryo and to what extent are they able to change their fate? Induction of neural crest cells is covered in Chapter 4 , and induction of ectodermal placodes is covered in Chapter 18 . Hence, here we will consider the plasticity of neural crest cells and ectodermal placodes.
Heterotopic transplantation studies have revealed that both neural crest cells and ectodermal placodes are highly plastic at the time of their formation. In these studies, small groups of prospective neural crest cells or small patches of preplacodal ectoderm are transplanted from their normal site of origin to an ectopic site. Typically, quail tissues are transplanted heterotopically to chick embryos, so that donor and host tissues can be specifically traced during subsequent development (covered in Chapter 5 ; see Fig. 5.6 ). Preplacodal cells generally are transplanted from one prospective placode to another (e.g., lens to otic or vice versa), where they readily adapt to their new environment and change their fate, that is, they exhibit plasticity. Neural crest cells are generally transplanted from one craniocaudal level to another, including the placement of trunk neural crest cells in the head and vice versa. As covered in Chapter 4 , neural crest cells give rise to a large number of cell types, including cartilage, bone, melanocytes, endocrine tissues, PNS neurons, and glial cells. Only head (cranial) neural crest cells are capable of forming bone and cartilage in transplantation studies, although isolated trunk neural crest cells subjected to various signaling molecules in vitro are capable of forming cartilage in some cases. Thus, neural crest cells at the time of their migration also display considerable plasticity. Furthermore, studies labeling individual migratory trunk and cranial post-migratory neural crest cells have shown that a single cell can give rise to several cell types. Thus, even post-migratory neural crest cells have plasticity.
As just covered, neural crest cells give rise to a large number of different cell types, and, consequently, they have properties similar to stem cells (embryonic stem cells are covered in Chapter 5 ). This stem-like cell nature not only occurs within migrating neural crest cells but also continues in their progeny (i.e., in tissues and organs formed by neural crest cells) as individual cells. For example, pluripotent neural crest cells (i.e., stem-like cells) have been identified in the embryonic chick dorsal root ganglion, sympathetic ganglion, and cardiac outflow tract. Neural crest cell stem-like cells are present also in the mammalian embryonic sciatic nerve, and in the embryonic and adult gut. However, the developmental potentials of these cells are more restricted than for migrating neural crest cells, and they vary according to the location of the cells.
It is surprising to note that pluripotent neural crest cells that can give rise to all cranial neural crest cell derivatives have been isolated from the bulge of adult mammalian hair follicles. The follicular bulge is an epidermal structure of the hair follicle that serves as a niche for keratinocyte stem cells, which form new epidermis, sebaceous gland, and hair (covered in Chapter 7 ). Thus, the bulge contains a mixed population of stem-like cells consisting of both keratinocyte stem cells and neural crest cell stem-like cells. Highly motile neural crest cell–derived stem-like cells ( epidermal neural crest cell stem-like cells ) emigrate from bulge explants dissected from adult hair follicles ( Fig. 10.2 ). Remarkably, more than 88% of these migrating cells are pluripotent stem cells that can generate all cranial neural crest derivatives.
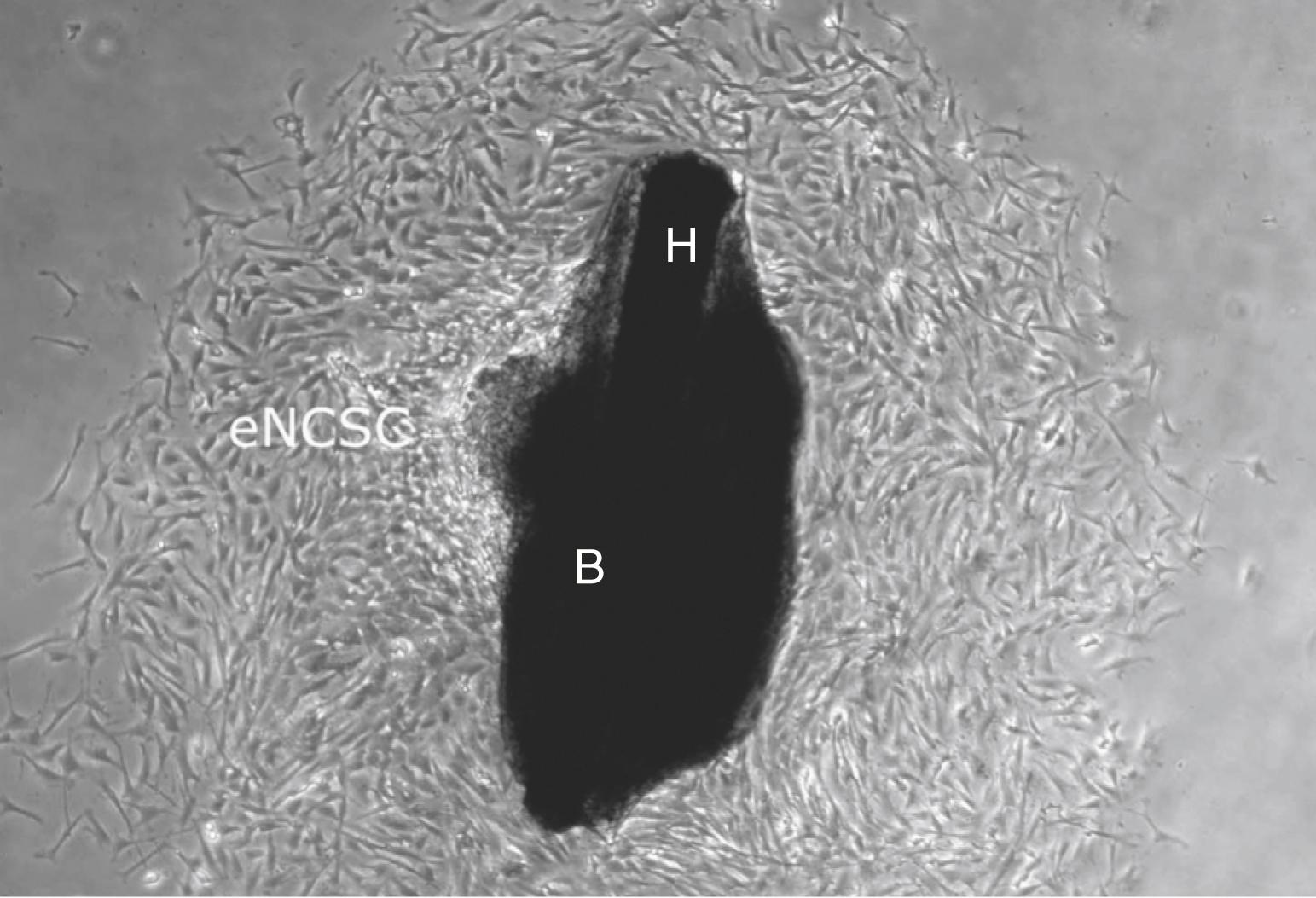
Because of their existence in humans, their accessibility, and their high degree of physiologic plasticity, neural crest cell stem-like cells in the periphery of the adult organism are promising candidates for cell replacement therapy.
The majority of defects in the peripheral nervous system arise from alterations in neural crest cell behaviors and, therefore, can be classified as neurocristopathies (see Chapter 14, Chapter 17, Chapter 4 for additional discussion and other examples of neural crest disorders).
Neurofibromatosis type 1 (NF1; also known as von Recklinghausen disease) is a prevalent familial tumor disposition that affects 1 in 3500 individuals worldwide. It is a progressive disease with multiple deficits, including benign and malignant tumors of the peripheral and central nervous systems. The gene mutated in NF1, NEUROFIBROMIN, is a tumor suppressor gene that inactivates the proto-oncogene RAS. Patients with NF1 are heterozygous for the inactivating mutations of the NEUROFIBROMIN gene. Thus, RAS function is upregulated in NF1 patients. In addition, inhibitors of fibroblast growth factor (Fgf) Ras Mapk signaling (see Chapter 5 ) such as spred1, a member of the sprouty/spred family of proteins, result in an NF1–like condition when mutated.
One hallmark of NF1 is the presence of six or more large (greater than 0.5 cm in children; greater than 1.5 cm in adults) café au lait spots — pigmented birth marks (frequently, one or two café au lait spots are present in unaffected individuals). These are non-symptomatic. A second, problematic hallmark is the presence of numerous benign cutaneous tumors, called neurofibromas . These tumors contain multiple cell types, including Schwann cells, neurons, endoneurial fibroblasts (i.e., fibroblasts within the endoneurial covering of nerve fibers), and mast cells. As covered in Chapter 4 , and later in this chapter (see “In the Research Lab” entitled “Patterning and Migration of Sympathetic Precursor Cells and Sympathetic Ganglia”), the first three of these cell types are derived from neural crest cells. Evidence suggests that the second wild-type allele is lost in NF1 patients through subsequent somatic deletion (the so-called two-hit hypothesis ), which leads to certain types of tumors (e.g., malignant peripheral nerve sheath tumor). However, because of the infrequency of somatic deletion and the frequency of neurofibromas developing in NF1 patients, second mutations are likely not required for neurofibroma formation.
Both paracrine and/or cell-autonomous events are known to trigger neurofibroma formation. For example, the onset of puberty and of pregnancy is often associated with a major increase in the number and size of neurofibromas. Both circumstances involve hormonal changes and an increase in subcutaneous adipose tissue deposits. Therefore, potentially hormonal and/or paracrine mechanisms may be responsible for tumorigenesis in some NF1 patients. In this regard, there is an interesting convergence of two observations: first, hair follicles from normal-looking skin of NF1 patients are often surrounded by numerous S100-positive neural crest cell–derived Schwann cells, or Schwann cell progenitors; and second, as covered in the preceding “In the Research Lab” entitled “Plasticity of Precursor Cells of PNS,” hair follicles contain neural crest cell stem-like cells. Therefore, it is conceivable that mitogens produced by adipocytes and/or female hormones promote proliferation of neural crest stem-like cells of hair follicles and/or of the multipotential Schwann cell progenitors of NF1 patients, leading to neurofibroma formation.
In NF1, neuromas can grow uncontrollably, putting pressure on affected nerves and resulting in nerve damage, pain, and loss of nerve function. As no specific treatment for the disease is known, it is managed surgically by removing neuromas that are painful or rapidly growing, as the latter may become cancerous. Tumors can also occur in structures such as the optic nerves, subsequently resulting in blindness.
Neuroblastomas are pediatric solid tumors of the sympathetic ganglia and adrenal medulla, which typically arise within the first decade of life. The blastoma contains two cell types—neuroblasts and Schwann cells—but it is clinically and molecularly heterogeneous with variable outcome. Some neuroblastomas spontaneously resolve whereas others metastasize and have a poor prognosis. Activating mutations in anaplastic lymphoma kinase (Alk) or loss-of-function of the transcription factor, Phox2b, which is required for differentiation of the sympathetic lineage, have been identified in a subset of tumors. Amplification or activation of Myc, which drives proliferation and maintains neural crest cells in a progenitor state, has been identified in over half of the more aggressive neuroblastomas.
The process of neurogenesis occurs similarly in the CNS and the PNS and involves a series of steps in which multipotential precursor cells (i.e., stem cells or stem-like cells) become progressively restricted in their fate over time. During this process, cells generally transform from multipotential precursors (e.g., capable of forming all types of neurons and glia) to restricted neuronal (or glial) precursors (e.g., capable of forming only neurons or only glia, but not both) to differentiated cell types (i.e., a specific type of neuron). Within the CNS, these precursors arise from the neural plate; within the PNS, they arise from neural crest cells and ectodermal placodes. Initially, cells in these rudiments rapidly divide to expand the number of cells in the population. However, over time the division of these cells becomes asymmetric such that one daughter cell derived from a particular mitotic division remains mitotically active and undifferentiated, whereas the other daughter cell becomes postmitotic, migrates away from its site of generation, and begins to differentiate.
Several genes play essential roles in regulating neurogenesis. These include both positive regulators and negative regulators. Examples of the former include the basic helix-loop-helix (bHLH) transcription factors known as the proneural genes . In vertebrates, these include genes such as Mash (the mammalian ortholog of Drosophila achaete-scute genes). Other vertebrate proneural genes include Math, NeuroD, and the neurogenins (the latter three are vertebrate — mammalian in the case of Math — orthologs of Drosophila atonal genes). Expression of these proneural genes is both sufficient and necessary for the formation of neurons. Examples of the negative regulators of neurogenesis include members of the notch signaling pathway (covered in Chapter 5 ). Through a process called lateral inhibition (covered in Chapters 10 and 18), which involves notch signaling, a neuronal precursor cell inhibits its neighbors from differentiating as neurons (e.g., by secreting a notch ligand such as delta, which binds to the neighbor’s notch receptors). Lateral inhibition thus regulates the number of neurons born in any one region of the developing nervous system and allows for the generation of supporting glial cells. Nevertheless, many more neurons are actually born than required. Hence, through a subsequent process of programmed cell death , the number of definitive neurons is reduced to the characteristic number for each area of the CNS and PNS.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here