Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Formation of the embryo and its parts involves morphogenesis , a form-shaping process controlled by fundamental cell behaviors that result in differential growth . Perturbation of differential growth due to a genetic mutation , teratogen exposure, or a combination of the two processes results in dysmorphogenesis and the formation of structural birth defects . Structural birth defects consist of both malformations —involving perturbation of developmental events directly involved in forming a particular structure—and deformations —involving indirect perturbation of a developing structure due to mechanical forces. Malformation can involve single organs or body parts or a constellation of organs or body parts. In the latter case, if a single cause is involved, the condition constitutes a syndrome . Understanding how development occurs requires the use of animal models in which experiments can be conducted. Because developmental mechanisms are conserved across species, the use of animal models provides insight into how normal development of the human embryo occurs and how development can be perturbed by genetic mutation or environmental insult, resulting in birth defects. The tool kit for the developmental biologist’s experiments is vast, including techniques derived from the fields of cell biology, molecular biology , and genetics , combined with the classical approaches of cut-and-paste experimental embryology . Manipulation of the mouse genome has been a particularly fruitful approach for understanding how genes function during development and for developing models for human disease and birth defects. Through experimental approaches, a small number of highly conserved signaling pathways have been identified. These pathways are used repeatedly and in various combinations throughout embryonic development. Tools originally used to study mouse embryos have been adapted for use in human embryos. This has resulted in advances in reproductive technologies, such as in vitro fertilization, and recently in the development of stem cells and organoids, which can be used potentially to regenerate diseased or damaged organs.
A first-year medical genetics fellow is on full-time service for the month of May. Early in the month, she is asked to consult on an infant with cleft lip and palate and possible brain abnormalities that were identified on prenatal ultrasound. Review of the prenatal and postnatal history is significant only for an abnormal ultrasound during the 24th week of gestation that showed dilation and possible fusion of the lateral ventricles of the brain, and for premature birth at 32 weeks after the onset of preterm labor. The family history seems negative at first, but on further discussion, the fellow elicits a history of a single central incisor in the patient’s father. The physical examination shows microcephaly (small head), ocular hypotelorism (closely spaced eyes), a flat nasal bridge, and bilateral cleft lip and cleft palate ( Fig. 5.1A ). Magnetic resonance imaging (MRI) of the brain shows fusion of the left and right frontal lobes and partial fusion of the parietal lobes characteristic of semilobar holoprosencephaly . Genetic testing discovers a deleterious mutation in one allele of the SONIC HEDGEHOG (SHH) gene in both the patient and her father. As the father and daughter carried the same mutation and both are heterozygous for this mutation, she is curious about why the child is more severely affected. It is explained that the syndrome is highly variable. Phenotypic variability may be due to differences in other genes that affect the expression or function of SHH from the wildtype allele. Alternatively, the daughter may have been exposed to a teratogen that decreased SHH expression or function from the wildtype SHH allele (see Chapter 17 for further coverage).
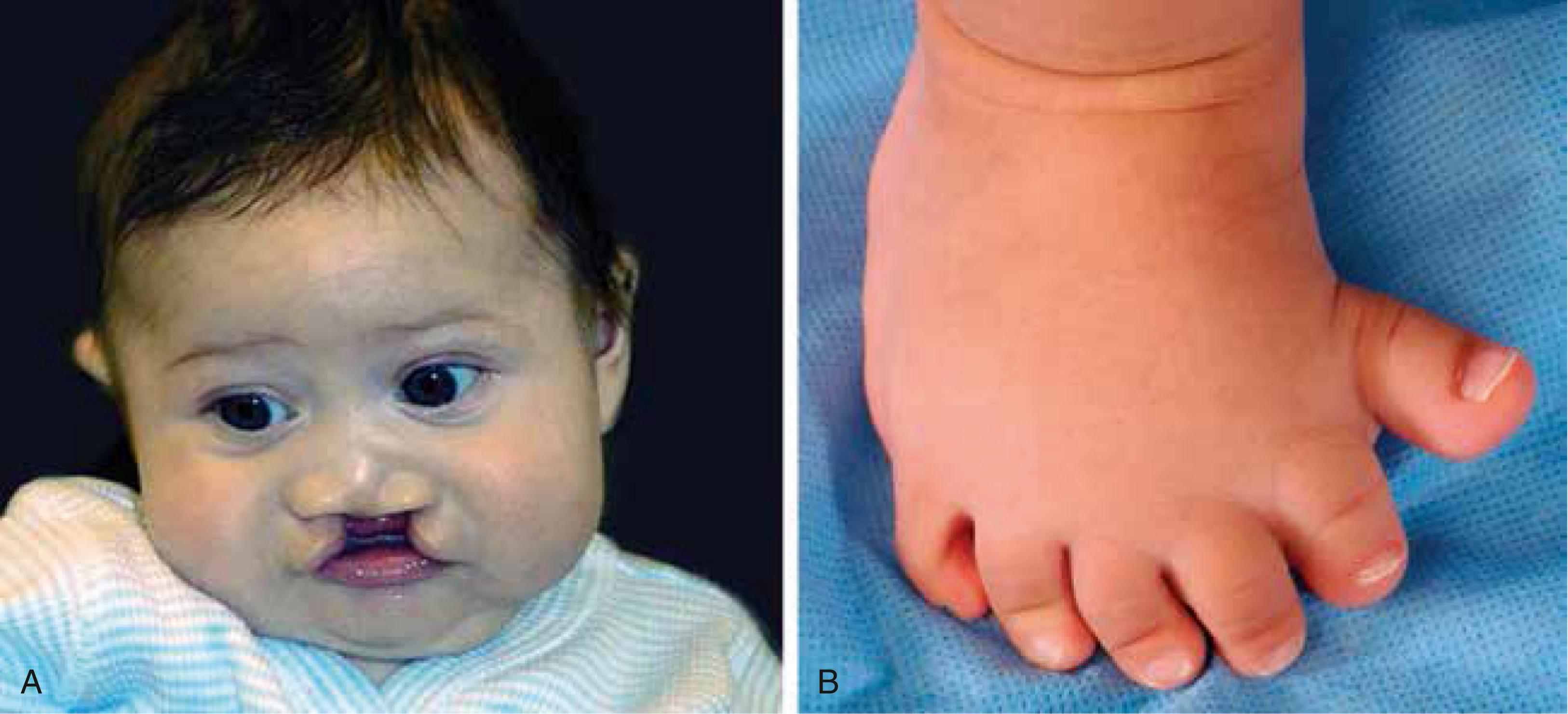
Near the end of the month, the fellow is called to the nursery to examine a newborn with limb anomalies. She finds an otherwise healthy, full-term girl with polydactyly (extra digits) of both hands and feet occurring on the thumb and great toe side (preaxial; see Fig. 5.1B ). Chromosome analysis shows a translocation involving chromosomes 5 and 7, with the chromosome 7 breakpoint occurring distant from the SHH gene, but in a region known to affect SHH expression in the limb. Disruption of these regulatory elements results in ectopic SHH expression and is known to cause preaxial polydactyly.
The fellow is impressed by the variability of manifestations caused by different defects in the same gene (SHH), with one mutation causing brain and face abnormalities and another causing limb defects.
Having described the initial steps in embryogenesis in Chapter 1, Chapter 2, Chapter 3, Chapter 4 , it is appropriate to pause to lay down the basic groundwork for understanding the concepts of normal and abnormal embryology covered in later chapters. Moreover, because these concepts have been formulated using animal models for experimental studies, it is important to understand the attributes each of these models provides for understanding human development. Finally, experimental techniques are described to explain how experiments are conducted in the field of developmental biology, and signaling pathways are covered to place molecules that control developmental events into context.
As covered in preceding chapters, the initially flat three-layered embryonic disc undergoes morphogenesis to form a three-dimensional embryo with a tube-within-a-tube body plan and the beginnings of rudiments that will form all of the adult organs and systems. In this chapter, we consider how morphogenesis occurs and how it goes awry during the formation of birth defects . Morphogenesis results from differential growth . Differential growth is driven by a small number of fundamental cellular behaviors , such as changes in cell shape, size, position, number, and adhesivity. If these behaviors are perturbed during embryogenesis by a genetic mutation, environmental insult (i.e., a teratogen ), or a combination of the two, differential growth is abnormal and dysmorphogenesis results with the formation of a structural birth defect.
Dysmorphogenesis can result from both malformation and deformation . Malformations consist of primary morphologic defects in an organ or body part resulting from abnormal developmental events that are directly involved in the development of that organ or body part. For example, failure of the neural groove to close results in a malformation called a neural tube defect . Similarly, failure of the digits to separate fully results in syndactyly , that is, fusion of the digits. Deformations consist of secondary morphologic defects that are imposed upon an organ or body part owing to mechanical forces, that is, deformations affect the development of an organ or body part indirectly . For example, if insufficient amniotic fluid forms (i.e., oligohydramnios), deformation of the feet can occur as the result of mechanical constraints, leading to club foot. Dysmorphogenesis can occur in an isolated organ or body part or as a pattern of multiple primary malformations with a single cause. In the latter case, the condition is referred to as a syndrome . Common examples, covered elsewhere in the text, include Down syndrome (trisomy 21) and 22q11.2 deletion syndrome—two syndromes that result from chromosomal abnormalities.
Other syndromes can result from teratogen exposure. A common example is fetal alcohol syndrome , also known as fetal alcohol spectrum disorder . This disorder affects 2 in 1000 live-born infants ( Fig. 5.2 ). Fetal alcohol syndrome is most prevalent in alcoholic women, especially those in their third or fourth pregnancies, suggesting that maternal health status interacts with alcohol to produce the syndrome. Nevertheless, consumption of amounts of alcohol as low as 80 g/day (i.e., between two and three shots of a grain liquor such as rum) by a non-alcoholic woman during the first month of pregnancy can cause significant defects, and it has been suggested that even a single binge may be teratogenic. In addition, chronic consumption of even small amounts of alcohol even in later pregnancy can be dangerous, as it may affect development of the fetal brain, resulting in behavioral and cognitive deficiencies that may last a lifetime. Thus, there is no known safe level of alcohol consumption during pregnancy.
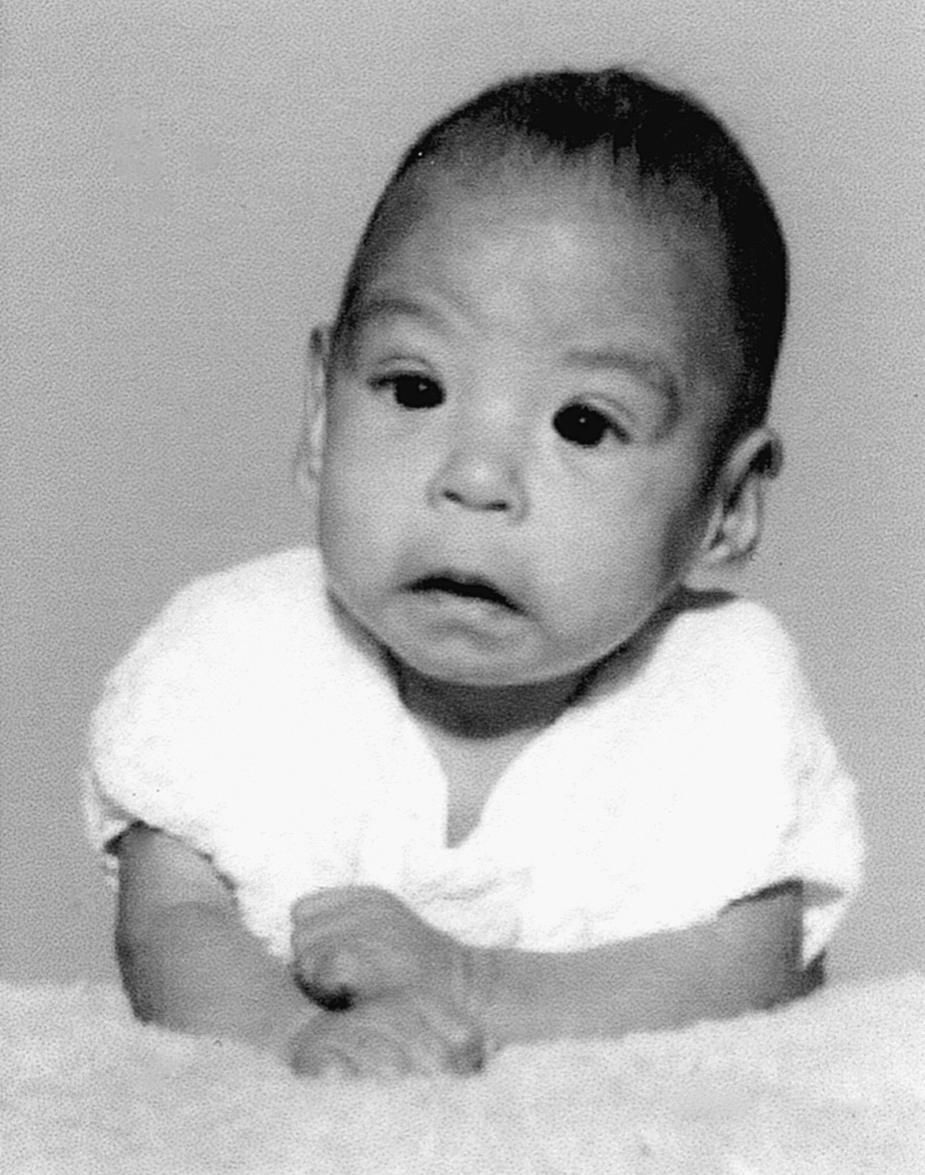
Common components of the disorder include defects of brain and face development, namely, microcephaly (small head), short palpebral fissures (eye openings), epicanthal folds (folds over eye lids), a low nasal bridge with a short nose, flat midface, minor external ear anomalies, and jaw anomalies, including a thin upper lip with indistinct philtrum and micrognathia (small jaw) (see Fig. 5.2 ).
The aim of research in developmental biology/embryology is to understand how development occurs at tissue, cellular, and molecular levels. This aim speaks largely to our innate curiosity to understand nature and how it works. An additional aim is to understand how normal development can go awry, resulting in birth defects, particularly in humans. Understanding how both normal development and abnormal development occur could lead to ways to detect (diagnose), prevent, and cure birth defects. Thus, this aim speaks to our desire to prevent and relieve human suffering.
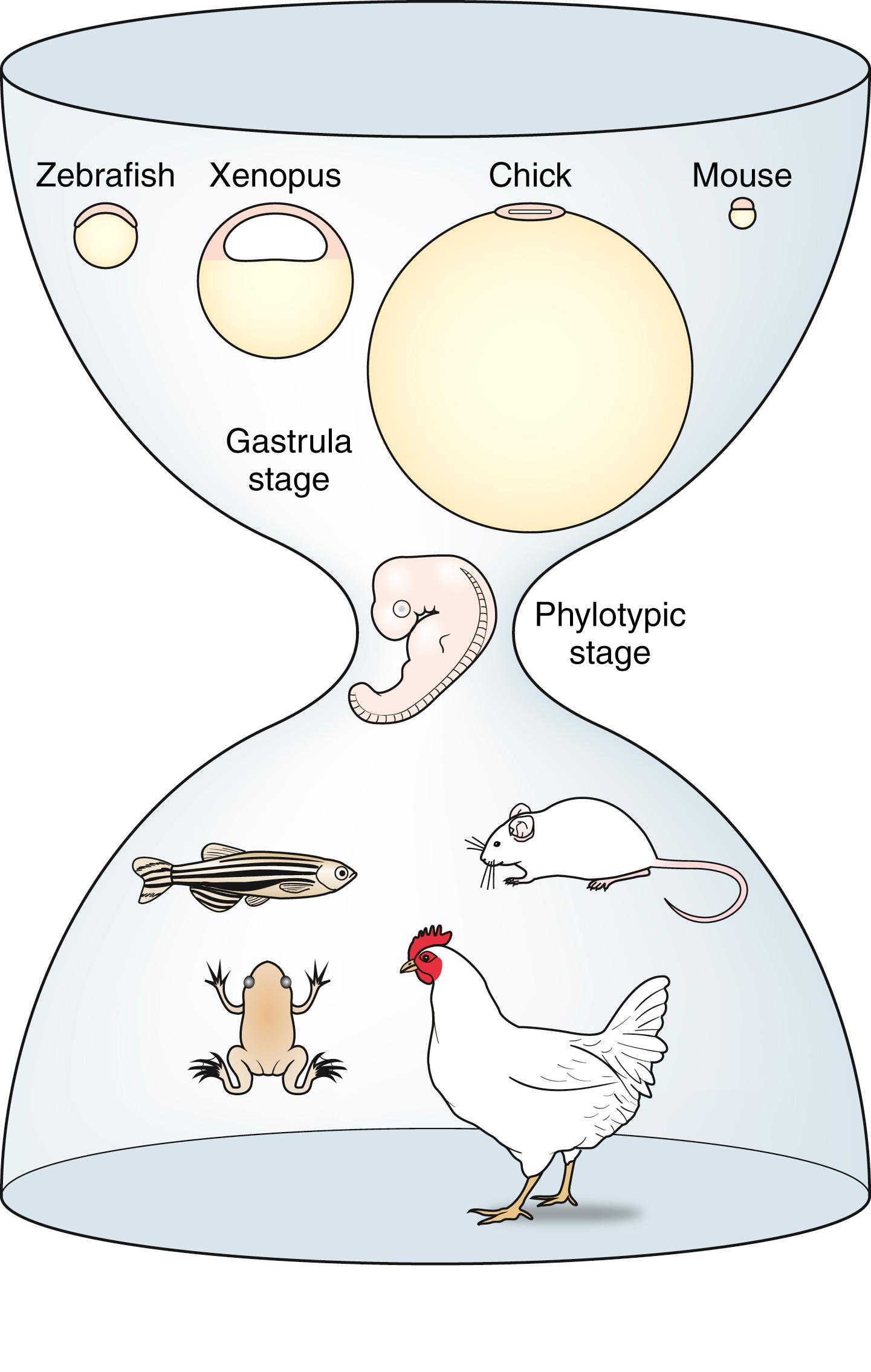
Although the only perfect organism for studying how the human embryo develops is the human embryo, animal models provide useful surrogates because of the principle that developmental mechanisms are highly conserved from organism to organism ( Fig. 5.3 ). Five animal models have been especially useful for deciphering mechanisms and principles of embryogenesis: one invertebrate and four vertebrates. In vertebrates, similarity among the animal models is most pronounced at the phylotypic stage of development when embryos are starting to undergo organogenesis. At this stage of development, the basic body plan has been established and embryos are characterized by the presence of somites, pharyngeal arches, ventral heart tube, head, eyes, and tail ( Fig. 5.4 ). Many conserved genes that establish the patterning of the embryo and organs (e.g., Hox genes) are also expressed during the phylotypic stage. Individual animal models provide complementary information, which, when compared across animal models, provides considerable insight into how the human embryo develops. Amphibians and zebrafish are also used to understand the ability to regenerate organs such as the limbs, tail, lens, and heart. All of these models are practical to obtain, use, and maintain in the laboratory, and all can be acquired and used throughout the year (i.e., they are not seasonal breeders). The unique strengths of each of these organisms for understanding mechanisms of development are covered in the following.
The developing field of genetics was greatly enhanced in the early 20th century using Drosophila melanogaster , the common fruit fly. Drosophila offers several advantages for understanding mechanisms of development. Through saturation mutagenesis using chemicals such as EMS (ethyl methane sulfonate) and subsequent screening to identify unique phenotypes, mutations have been identified in virtually every gene ( Drosophila has 13,639 predicted genes). The powerful process of using random mutations in unknown genes to identify perturbed developmental events (i.e., thereby resulting in phenotypes ), followed by identification and cloning of the mutated gene, is referred to as the forward genetic approach . In addition, several techniques have been developed for gene overexpression or underexpression in Drosophila , allowing experimental analyses of gene function during development.
A surprising finding of the genomic era has been the realization that the genomes of fruit flies and humans are highly similar. Orthologs of about 60% of the genes expressed during Drosophila embryogenesis have been identified in other animal models, as well as in humans, although total gene number in humans is about double that in Drosophila (it is estimated that humans have 20,000 to 25,000 genes). Vertebrates, including humans, typically have multiple family members orthologous to each identified Drosophila gene. Thus, for example, in Drosophila , there are 3 Fgf ligand genes (branchless, pyramus, and thisbe) and 2 Fgf receptor genes (breathless and heartless), whereas in mammals, there are 22 Fgf genes and 4 Fgf receptor genes (Fgfs and Fgf receptors are covered later in this chapter; branchless and breathless are covered in Chapter 11 in the “In the Research Lab” entitled “ Drosophila Tracheal System Development”).
The zebrafish model, Danio rerio , enables the use of mutagenesis and phenotype screening to directly study vertebrate development. Using N-ethyl-N-nitrosourea (ENU) mutagenesis, mutant embryos can be identified and studied developmentally; more than 8000 mutations have been identified using the forward genetic approach. Such study is greatly facilitated by the fact that zebrafish embryos are transparent, so internal structures can be readily visualized without the need in many cases for histologic study. Also, like Drosophila , zebrafish embryos develop rapidly, progressing from fertilization to free swimming fry in about 2 days.
The cells (blastomeres) of cleaving zebrafish embryos are relatively large and can be injected with lineage tracers or RNAs for gene misexpression studies. Morpholinos (stabilized antisense DNA; covered later in the chapter) can be injected to knock down gene expression, and they can also be injected in mutant embryos to study the combined effects of loss of function of multiple genes. In addition, transgenic approaches, including generating gene knock ins and knock outs (covered later in the chapter), have been developed in zebrafish. Knock-in approaches include the generation of fluorescent protein reporter lines, which can be used to determine the fate of cells by lineage analyses or, as the zebrafish is transparent, to carry out live imaging of cell behaviors in vivo. The zebrafish genome has been sequenced and is estimated to contain 30,000 to 60,000 genes (genome duplications have occurred during zebrafish evolution).
The field of experimental embryology began in the 19th century with the use of a variety of amphibian—frog and salamander—embryos. However, during the past few decades, Xenopus laevis , the South African clawed toad, has become the amphibian of choice for developmental biologists. Amphibian embryos readily tolerate microsurgical manipulation, so-called cutting-and-pasting experimental embryology (covered later in the chapter). In addition, because cells (blastomeres) of cleaving embryos are relatively large, as they are in zebrafish, they can be injected with lineage tracers, or molecular constructs to express genes or inhibit gene expression. In fact, probably the most precise fate maps produced to date using this lineage tracing approach are for X. laevis . X. laevis , like the models already covered, develops relatively rapidly, progressing from the fertilized egg to the tadpole in about 4 days.
Because genome duplication has occurred in X. laevis , this species is largely tetraploid (approximately one-third of the duplicated genes have been lost). Thus, it is difficult to use X. laevis for gene manipulation studies due to genetic redundancy. However, another species of Xenopus , Xenopus tropicalis , is diploid, and it has been possible to use this species to generate transgenic animals , that is, animals in which the genome has been modified using molecular genetic techniques. Sequencing of the X. tropicalis genome has been completed, greatly enhancing the value of Xenopus as a model system.
Chick, or Gallus gallus domesticus , embryos, like Xenopus embryos, can be readily manipulated microsurgically during development. Because the chick is a warm-blooded organism (as is the human) and because it can be so readily manipulated during development, it has become, over the past several decades, the favored workhorse for studies utilizing cut-and-paste experimental embryology approaches (covered later in the chapter). Although transgenic GFP-expressing embryos have been generated for fate-mapping studies, the chicken is not used extensively for genetic studies. As compared with the other models covered earlier in the chapter, development of the chick embryo is relatively slow, taking about 21 days from fertilization to hatching.
The chicken genome has been sequenced, enhancing the use of this organism for understanding molecular mechanisms of development. It is estimated that the chicken genome contains about 25,000 genes. Techniques have been developed for overexpressing proteins locally at specific times during chick development (e.g., using small beads coated with growth factors, injecting engineered viruses, injecting transfected cells); overexpressing genes using whole-embryo electroporation (through techniques such as sonoporation and lipofection) to target plasmids expressing the gene of interest to desired tissues in the chick embryo; and using RNAi or morpholinos (covered later in the chapter) to knockdown gene expression (typically introduced through whole-embryo electroporation).
The laboratory mouse, Mus musculus , was originally used for genetic studies, and hundreds of naturally occurring mutations have been identified and are available for study. The time of gestation of the mouse is similar to that of the chick, ranging from 19 to 21 days after fertilization.
Like the forward-genetic approach used in Drosophila and zebrafish, ENU-induced mouse mutants have been generated. However, the main strength of the mouse model is the availability of techniques to make transgenic mice (covered later in the chapter). Using homologous recombination, it is possible to inactivate (knock out) any gene of interest or to replace one gene with another (knock in). About 30% of mouse genes have been knocked out by this approach. This is called the reverse-genetic approach, which starts with a known gene and mutates it to determine its function during development. In a variation of this approach using conditional transgenics, it is now possible to use tissue-specific promoters to drive expression of a transgene (including reporter genes) in specific tissues (or to knock out the gene in specific tissues only) enhancing the precision of the experiment. The mouse genome has been sequenced and is predicted to contain about 30,000 genes.
Developmental toxicologists usually choose animal models different from those used by developmental biologists because the goal in their studies is different: to predict the risk to humans of exposure to drugs and potential environmental pollutants. Thus, such studies are concerned with similarities between animal models and human placentation (i.e., How similar is placental function?) or pharmacodynamics (i.e., How similar is the metabolism of drugs?). Following the thalidomide-induced birth defects in the 1960s, new guidelines were introduced and now at least two animal models must be used in toxicity studies (see Chapter 20 for further coverage). Rodents or rabbits are typically used for initial studies, and if the results of these studies warrant further testing, non-human primate models are used for later studies.
Understanding how normal development and abnormal development occur requires a detailed understanding of what happens during development—that is, a detailed understanding of descriptive embryology . However, descriptive embryology alone cannot reveal how development occurs. Descriptive embryology provides a catalog of developmental events, which, when carefully studied and reflected upon, can lead to the formulation of hypotheses about how a developmental event occurs. The investigator then designs and conducts tests of the formulated hypotheses. Hypotheses are tested through a series of experiments (specific manipulations that usually perturb a developmental process) as compared with controls (non-specific manipulations used to ensure that results obtained from particular manipulations are specific and not artifactual). Through this approach, hypotheses are refuted, modified, or supported (never truly proven to be correct, but often proven to be incorrect). The cycle continues as new hypotheses are crafted, based on additional data obtained through experiments, leading to new experimental tests of their veracity.
Conducting experiments on developing model embryos constitutes the science of experimental embryology . Classically, experimental embryology has been used to define the tissue and cellular basis of development through a series of microsurgical manipulations. More recently, experimental embryology has merged with cell biology, molecular biology, and genetics, allowing investigators to define the molecular-genetic basis of development.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here