Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
During the process of embryonic development, undifferentiated precursor cells differentiate and organize into the complex structures found in functional adult tissues. This intricate process requires cells to integrate many intrinsic and extrinsic cues for development to occur properly. These cues control the proliferation, differentiation, and migration of cells to determine the final size and shape of the developing organs. Disruption of these signaling pathways can result in human developmental disorders and birth defects. These key developmental signaling pathways are also frequently co-opted in the adult by diseases such as cancer.
Given the diverse changes that occur during embryogenesis, it may appear that a correspondingly diverse set of signaling pathways should regulate these processes. In contrast, the differentiation of many cell types is regulated through a relatively restricted set of molecular signaling pathways:
Intercellular communication: Development involves the interaction of a cell with neighboring cell directly (gap junctions) or indirectly (cell adhesion molecules).
Morphogens: These diffusible molecules specify which cell type is generated at a specific anatomic location and direct the migration of cells and their processes to their final destinations. Morphogens include retinoic acid; the transforming growth factor-β (TGF-β) superfamily of proteins, including bone morphogenetic proteins (BMPs); and WNT protein families. Table 21.1 explains gene and protein nomenclature.
| Gene | Human | Italic, all letters capitalized | PAX6 |
| Mouse | Italic, first letter capitalized | Pax6 | |
| Protein | Human | Roman, all letters capitalized | PAX6 |
| Mouse | Roman, all letters capitalized | PAX6 |
Hedgehog: The hedgehog family of morphogens signaling pathway in human cells is localized to a structure called the primary cilium. Disruption of the components of the hedgehog pathway result in a set of diseases termed ciliopathies.
Receptor tyrosine kinases (RTKs): Many growth factors signal by binding to and activating membrane-bound RTKs. These kinases are essential for the regulation of cellular proliferation, apoptosis, and migration, as well as processes such as the growth of new blood vessels and axonal processes in the nervous system.
Notch-Delta: This pathway often specifies which cell fate the precursor cells adopt.
Transcription factors: These evolutionarily conserved proteins activate or repress downstream genes that are essential for many cellular processes. Many transcription factors are members of the homeobox (HOX) or helix-loop-helix (HLH) families. Their activity can be regulated by all of the other pathways described in this chapter.
Epigenetic effects: These heritable changes in gene function do not result from a change in DNA sequence. Examples of epigenetic modifications are histone acetylation, histone methylation, microRNAs (miRNAs) and DNA methylation.
Stem cells: Embryonic stem cells can give rise to all cells and tissues in the developing organism. Adult stem cells maintain tissue homeostasis in the mature organism. These types of stem cells and induced pluripotent stem cells (iPS) are potential sources of cells for the regeneration and repair of injured or degenerating cells and organs. iPS derived from patient cells can be used to model developmental processes in vitro and to screen potential therapies. New advancements in gene editing have greatly enhanced our ability to model human diseases both in vitro and in vivo.
During embryonic development, cells receive signals from their external environment and communicate with neighboring cells. This communication directs the cell to undergo processes such as proliferation, differentiation, and migration. Two classes of proteins required for intercellular communication are discussed: gap junctions and cell adhesion molecules.
Gap junctions are a means for cells to directly communicate with one another; this is known as gap junction intercellular communication (GJIC). Although the pore size of the channels vary, only small molecules (e.g., second messengers, ions such as calcium, adenosine triphosphate [ATP]) less than 1 kDa can pass through, which excludes most proteins and nucleic acids. In the nervous and cardiac systems, gap junctions help to establish electrical cell coupling (“electrical” synapse).
Although the function of gap junctions is quite straightforward, the structure of these intercellular channels is complex and highly regulated throughout development ( Fig. 21.1 ). Each gap junction is composed of two hemichannels known as connexons. Each hexameric connexon consists of six individual connexin subunits. An individual connexin (Cx) molecule consists of four transmembrane domains. Vertebrates have more than 20 connexin molecules. Cellular and tissue functional diversity of gap junctions depends on whether individual connexons are the same (homotypic) or different (heterotypic) and whether each connexon is made from the same (homomeric) or different (heteromeric) connexin molecules.
![Fig. 21.1, Gap junction intercellular communication. A , The connexin molecule consists of four transmembrane domains and two extracellular domains, and its N- and C-termini are cytoplasmic. B , Connexons, or hemichannels, are hexameric structures consisting of six connexin subunits. A gap junction can be formed from two homophilic or heterophilic connexons. Small molecules (e.g., ions, adenosine triphosphate [ATP]) less than 1 kDa can pass through an open gap junction. Fig. 21.1, Gap junction intercellular communication. A , The connexin molecule consists of four transmembrane domains and two extracellular domains, and its N- and C-termini are cytoplasmic. B , Connexons, or hemichannels, are hexameric structures consisting of six connexin subunits. A gap junction can be formed from two homophilic or heterophilic connexons. Small molecules (e.g., ions, adenosine triphosphate [ATP]) less than 1 kDa can pass through an open gap junction.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/CommonSignalingPathwaysUsedDuringDevelopment/0_3s20B9780323611541000217.jpg)
Early in development, GJIC is important for the rapid distribution of ions and other molecules essential for regionalization before the establishment of distinct boundaries and compartments. The importance of GJIC has been demonstrated in the developing chick hindbrain (rhombencephalon) by a combination of dye transfer and electrical coupling studies.
Some of the better-characterized connexins include Cx43 (heart, brain), Cx45 (heart, pancreas), Cx32 (myelin), and Cx36 (pancreas, brain). In this nomenclature system, the number following Cx refers to the molecular weight in kilodaltons (kDa) of the proteins. Mutations in Cx genes result in diseases such as the hereditary peripheral neuropathy X-linked Charcot–Marie–Tooth disease ( GJB1 , formerly CX32 ). It was previously thought that connexons had to bind to a connexon on an adjacent cell to functionally signal. However, it has since been shown that unbound connexons (hemichannels) enable the exchange of ions and small molecules between the cytoplasm and the extracellular space, especially during pathophysiologic conditions. Aberrant hemichannel activation through GJB2 (formerly CX26 ) can result in keratitis-ichthyosis-deafness syndrome.
Cell adhesion molecules have large extracellular domains that interact with extracellular matrix (ECM) components or adhesion molecules on neighboring cells. These molecules often contain a transmembrane segment and a short cytoplasmic domain that regulate intracellular signaling cascades. Two classes of molecules that have important roles during embryonic development are cadherins and members of the immunoglobulin superfamily of cell adhesion molecules.
Cadherins are critical for embryonic morphogenesis because they regulate the separation of cell layers (endothelial and epidermal), cell migration, cell sorting, establishment of well-defined boundaries, synaptic connections, and the growth cones of neurons. Cadherins mediate the interaction between the cell and its extracellular milieu (neighboring cells and ECM).
Cadherins were originally classified by their site of expression. E-cadherin (epithelial cadherin) is highly expressed in epithelial cells, whereas N-cadherin (neural cadherin) is highly expressed in neural cells.
Cadherins mediate homophilic, calcium-dependent binding. A typical cadherin molecule has a large extracellular domain, a transmembrane domain, and an intracellular tail ( Fig. 21.2 ). The extracellular domain contains five extracellular repeats (EC repeats) and has four Ca 2+ -binding sites. Cadherins form dimers that interact with cadherin dimers in adjacent cells. These complexes are found clustered in adherens junctions , which result in the formation of a tight barrier between epithelial or endothelial cells.

Through its intracellular domain, cadherins bind to p120 catenin, β-catenin, and α-catenin. These proteins connect cadherin to the cytoskeleton. E-cadherin expression is lost as epithelial cells transition to mesenchymal cells ( epithelial to mesenchymal transition [EMT] ). EMT is required for the formation of neural crest cells during development, and the same process can occur in tumors that develop from epithelial cell types.
There are more than 700 members of the immunoglobulin superfamily of cell adhesion molecules in the human genome. This large family of proteins is involved in a wide variety of cellular processes. One member of this class, the neural cell adhesion molecule (NCAM), is an abundant protein in the brain and has three isoforms that result from alternative splicing. It has a large extracellular domain that contains five immunoglobulin (Ig) repeats and two fibronectin domains (see Fig. 21.2 ). This region mediates the calcium-independent homophilic binding of NCAM to itself and heterophilic binding to other cell adhesion molecules (L1 and TAG1), RTKs (fibroblast growth factor receptor [FGFR]), or the ECM. Ligand binding induces intracellular signaling through the FYN and FAK intracellular kinases.
NCAM undergoes polysialylation (PSA), a unique post-translational glycosylation modification. PSA-NCAM is abundant early in neural development and becomes restricted to areas of neural plasticity and migration in the adult. It is thought that PSA decreases the adhesiveness of NCAM and facilitates migration. NCAM regulates neurite outgrowth, axonal pathfinding, survival, and plasticity.
Extrinsic signals guide the differentiation and migration of cells during development and thereby dictate the morphology and function of developing tissues (see Chapter 5 ). Many of these morphogens are found in concentration gradients in the embryo, and different morphogens can be expressed in opposing gradients in the dorsal-ventral, anterior-posterior, proximal-distal, and medial-lateral axes. The fate of a specific cell can be determined by its location along these gradients. Cells can be attracted or repelled by morphogens depending on the particular set of receptors expressed on the cell surface.
The anterior (rostral, head)–posterior (caudal, tail), or anteroposterior, axis of the embryo is crucial for determining the correct location for structures such as limbs and for the patterning of the nervous system. For decades, it has been clinically evident that alterations in the level of vitamin A (retinol) in the diet (excessive or insufficient amounts) can lead to the development of congenital malformations (see Chapters 17 and 20 ).
The bioactive form of vitamin A is retinoic acid , which is formed by the oxidation of retinol to retinal by retinol dehydrogenases and the subsequent oxidation of retinal by retinal aldehyde dehydrogenase. Free levels of retinoic acid can be further modulated by cellular retinoic acid–binding proteins that sequester retinoic acid. Retinoic acid also can be actively degraded into inactive metabolites by enzymes such as CYP26 ( Fig. 21.3 ). Normally, retinoic acid posteriorizes the body plan. Excessive retinoic acid levels or inhibition of retinoic acid degradation both lead to a truncated body axis in which structures have a more posterior nature. Insufficient retinoic acid or defects in enzymes such as retinal aldehyde dehydrogenase lead to a more anteriorized structure.
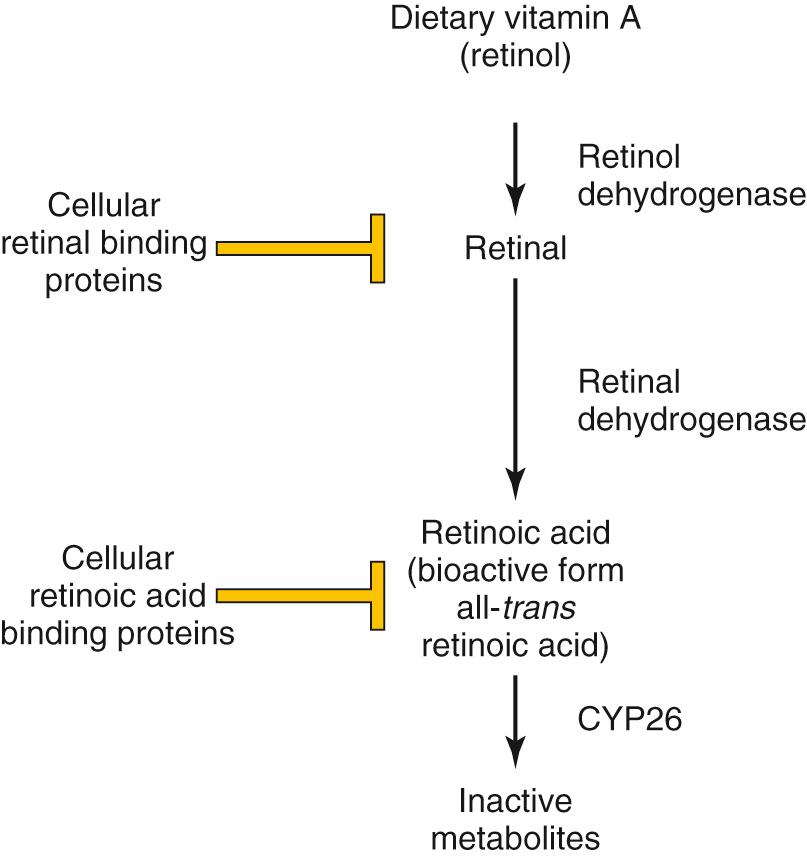
At a molecular level, retinoic acid binds to its receptors inside the cell and activates them. Retinoic acid receptors are transcription factors, and their activation regulates the expression of downstream genes. Crucial targets of retinoic acid receptors in development are the HOX genes. Due to their profound influence on early development, retinoids are powerful teratogens, especially during the first trimester.
Members of the TGF-β superfamily include TGF-β, BMPs, activin, and nodal. These molecules contribute to the establishment of dorsoventral patterning, cell fate decisions, and formation of specific organs, including the nervous system, kidneys, skeleton, and blood (see Chapter 5, Chapter 16, Chapter 17 ).
In humans, there are three TGF-β isoforms (TGF-β 1 , TGF-β 2 , and TGF-β 3 ). Binding of these ligands to heterotetrameric (four-subunit) complexes, consisting of specific type I (inactive kinase domain) and type II TGF-β receptor subunit (TβR-II) (constitutively active) transmembrane serine-threonine kinase receptors results in intracellular signaling events ( Fig. 21.4 ). When TGF-β ligands bind to their respective membrane-bound type II receptor, a type I receptor is recruited and transphosphorylated, and its kinase domain is activated, subsequently phosphorylating intracellular receptor-associated SMAD proteins (R-SMADs).
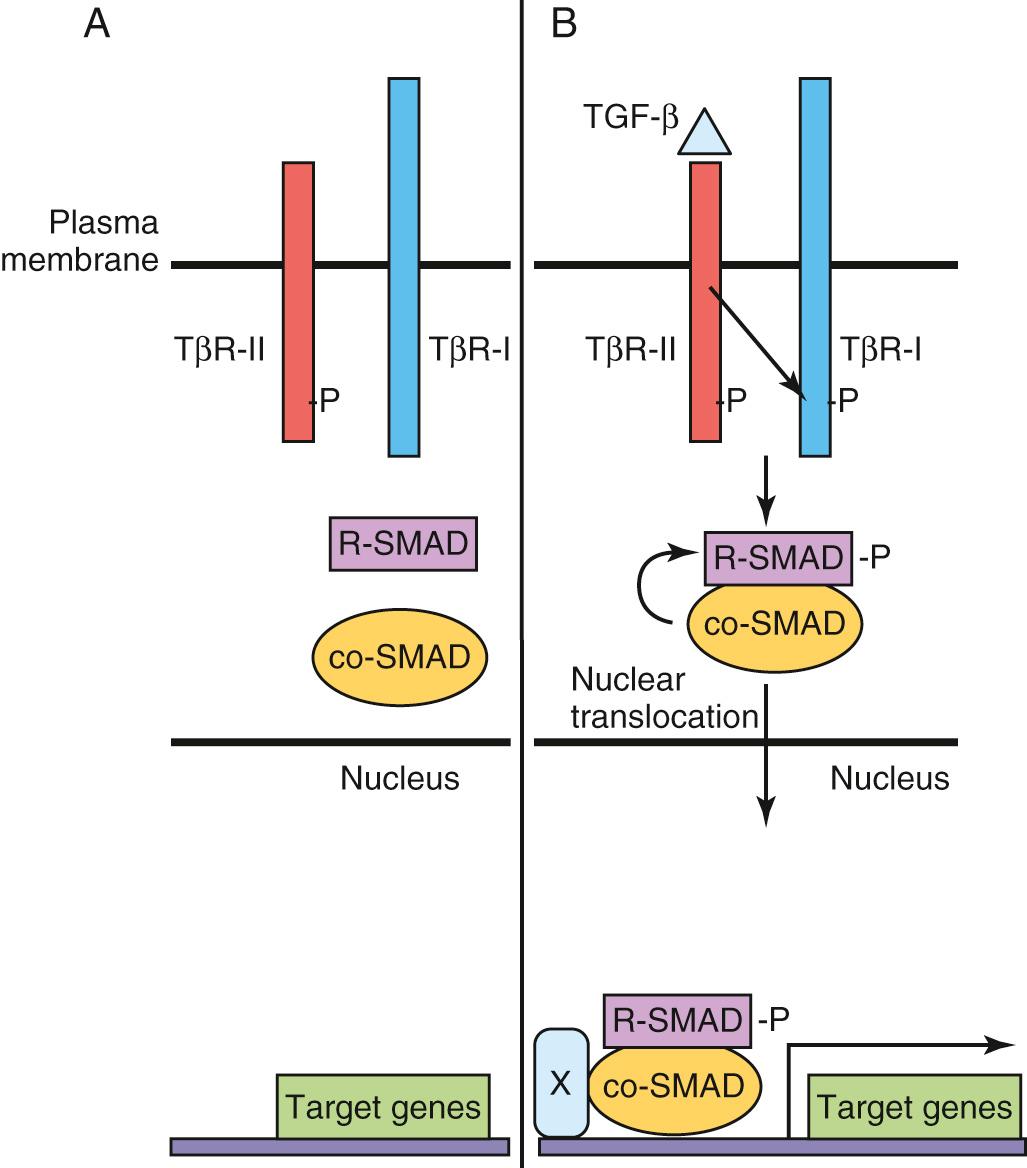
The SMADs are a large family of intercellular proteins that are divided into three classes: receptor activated (R-SMADs 1–3, 5, and 8), common partner (co-SMADs such as SMAD4), and inhibitory SMADs (I-SMADs such as SMAD6 and SMAD7). R-SMAD/SMAD4 complexes translocate to the nucleus and regulate target gene transcription by interacting with other proteins or function as transcription factors by directly binding to DNA.
Inhibitory SMAD proteins block the actions of I-SMAD by several mechanisms, such as preventing phosphorylation of R-SMADs by TβR-I, induction of R-SMAD degradation, and transcriptional repression. TβR-I activation is a highly regulated process that involves membrane-anchored coreceptors and other receptor-like molecules that can sequester ligands and prevent their binding to respective TβR-II receptors. Dominant negative forms of TβR-II have inactive kinase domains and cannot transphosphorylate TβR-I, thereby blocking downstream signaling events. The diversity of TGF-β ligand, TβR-I and TβR-II, coreceptor, ligand trap, and R-SMAD combinations contributes to particular developmental and cell-specific processes, often in combination with other signaling pathways.
The sonic hedgehog gene (SHH) was the first identified mammalian ortholog of the Drosophila hedgehog gene (Hh) . SHH and other related proteins, including desert hedgehog and Indian hedgehog, are secreted morphogens critical to early patterning, cell migration, and differentiation of many cell types and organ systems (see Chapter 5 ).
In Drosophila , cells have various thresholds for response to the secreted Hh signal. The primary receptor for Shh is Patched (PTCH in humans, PTC family in mice), a 12-transmembrane domain protein that in the absence of Shh inhibits Smoothened (Smo), a seven-transmembrane domain G protein–linked protein, and downstream signaling to the nucleus. However, in the presence of Shh, Patched (Ptc) inhibition is blocked, and downstream events follow, including nuclear translocation of Gli (Gli1, Gli2, Gli3) with transcriptional activation of target genes such as Ptc1 , Engrailed , and others ( Fig. 21.5 ).

Other membrane-bound SHH coreceptors have been identified with key roles in ventral neural patterning, including BOC, GAS1, and the LDL receptor-related protein 2 (LRP2; in mammals). Individually, these coreceptors act to enhance SHH signaling. BOC and GAS1 each interact with the SHH canonical receptor PTC/PTCH to form distinct receptor complexes essential for cell proliferation mediated by SHH. The role of BOC is especially important for commissural axonal guidance during development and in medulloblastoma progression. In contrast, LRP2 promotes the internalization and subsequent degradation of PTC/PTCH upon SHH binding thereby removing PTC/PTCH inhibition of Smo. Hedgehog interacting protein (HHIP) is also a coreceptor, but it functions to blunt Indian hedgehog signaling by sequestering Indian hedgehog and thus preventing Indian hedgehog from binding to PTC/PTCH.
The SHH protein is modified post-translationally by the addition of cholesterol and palmitate moieties to the N- and C-termini, respectively. These lipid modifications affect SHH's association with the cell membrane, formation of SHH multimers, and movement of SHH, altering its tissue distribution and concentration gradients. One of the best explained activities of SHH in vertebrate development is its role in patterning the ventral neural tube (see Chapters 4 and 17 ). SHH is secreted at high levels by the notochord. The concentration of SHH is highest in the floor plate of the neural tube and lowest in the roof plate, where members of the TGF-β family are highly expressed. The cell fates of four ventral interneuron classes and motor neurons are determined by relative SHH concentrations and by a combinatorial code of homeobox and basic HLH (bHLH) genes.
The requirement of SHH signaling for many developmental processes is underscored by the discovery of human mutations of members of the Shh pathway and the corresponding phenotypes of genetically modified mice, in which members are inactivated (loss of function or knockout) or overexpressed (gain of function). Mutations of SHH and PTCH have been associated with holoprosencephaly, a congenital brain defect resulting in the fusion of the two cerebral hemispheres; anophthalmia or cyclopia (see Chapter 18 ); and dorsalization of forebrain structures. In sheep, this defect can also result from exposure to the teratogen cyclopamine, which disrupts SHH signaling (see Fig. 21.5 ). Some patients with severe forms of the inborn error of cholesterol synthesis, the autosomal recessive Smith–Lemli–Opitz syndrome, have holoprosencephaly (see Chapter 20 ).
GLI3 mutations are associated with autosomal dominant polydactyly syndromes (see Chapter 16 ), such as the Greig and Pallister–Hall syndromes. Gorlin syndrome, which often is caused by germline PTCH mutations, is a constellation of congenital malformations mostly affecting the epidermis, craniofacial structures (see Chapter 9 ), and the nervous system. These patients are significantly predisposed to basal cell carcinomas, especially after radiation therapy, and a few develop malignant brain tumors (medulloblastomas) during childhood. Somatic mutations of PTCH , SUFU , and SMO have been identified in patients with sporadic medulloblastomas not associated with Gorlin syndrome.
In vertebrates, the Shh signaling pathway is closely linked to primary cilia (see Fig. 21.5 , inset) and their constituent intraflagellar transport (IFT) and basal body proteins. Primary cilia are sometimes referred to as nonmotile cilia. IFT proteins act upstream of the GLI activator (GLI-A) and repressor (GLI-R) proteins and are necessary for their production. Mutations involving genes encoding basal body proteins, such as KIAA0586 (formerly TALPID3) and oral-facial-digital syndrome 1 (OFD1) , affect SHH signaling in knockout mice. A group of human cilia-related diseases called ciliopathies result from disruption of primary cilia function and includes rare genetic diseases and more common disorders such as autosomal recessive polycystic kidney disease. To date, almost 40 ciliopathies have been described involving up to 200 genes. Although there may be some overlap (as with many congenital heart defects and left–right asymmetries), diseases of primary, nonmotile cilia are usually distinguished from disorders affecting motile cilia (found in sperm and in epithelial cells lining the airways, ventricles of the brain, and oviducts). Manifestations of diseases affecting motile cilia include hydrocephalus, lung infections, and infertility. Table 21.2 lists some common ciliopathies involving primary, nonmotile cilia and affected organ systems.
| Disease | Organ Systems Involved |
|---|---|
| Bardet–Biedl syndrome | Multisystemic |
| Oral-facial-digital syndromes | Multisystemic |
| Oculocerebrorenal syndrome of Lowe | Multisystemic |
| Meckel–Gruber syndrome | Brain, kidney, skeleton |
| Holoprosencephaly | Brain, eye |
| Cone–rod dystrophy | Eye |
| Leber congenital amaurosis | Eye |
| Hearing loss | Ear |
| Polycystic kidney disease | Kidney |
| Nephronophthisis | Kidney |
| Situs inversus | Heart, organ laterality |
| Greig cephalopolysyndactyly syndrome | Skeleton |
| Orofaciodigital syndrome | Skeleton |
| Ellis van Creveld syndrome | Skeleton |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here