Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
15
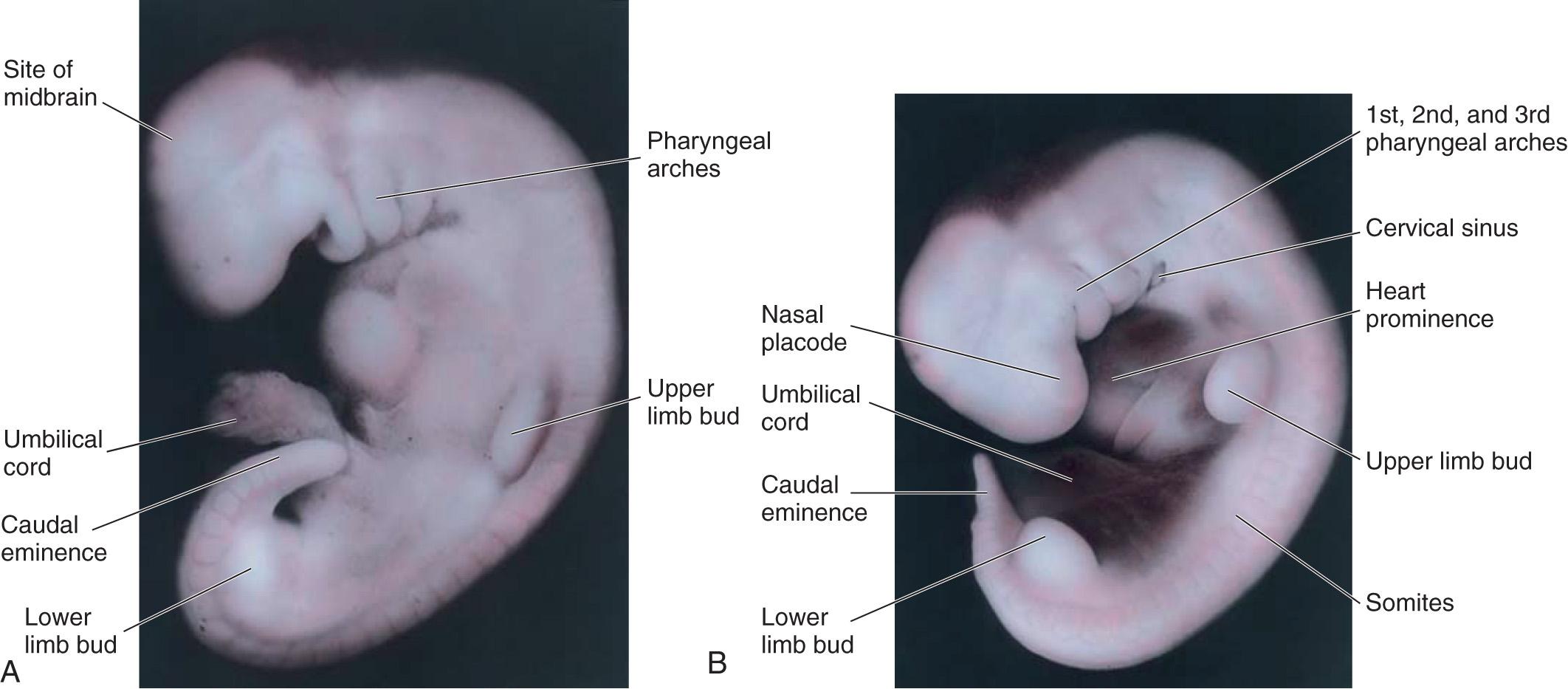
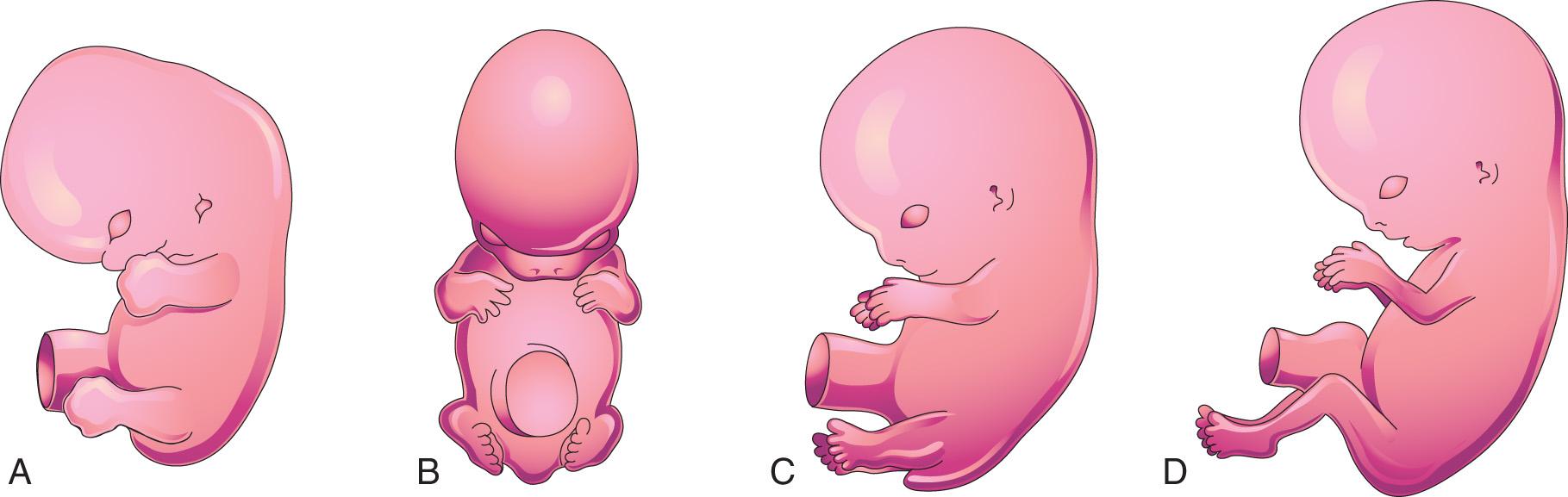
The upper limb buds are visible by day 24, and the lower limb buds appear 1 or 2 days later, with the activation of a group of mesenchymal cells in the somatic lateral mesoderm ( Fig. 16.1 A ). Homeobox (Hox) genes regulate patterning in the formation of the limbs. The limb buds form deep to a thick band of ectoderm, the apical ectodermal ridge ( AER ; Fig. 16.2 A ). The buds first appear as small bulges on the ventrolateral body wall (see Fig. 16.1 ). Each limb bud consists of a mesenchymal core of mesoderm covered by a layer of ectoderm.
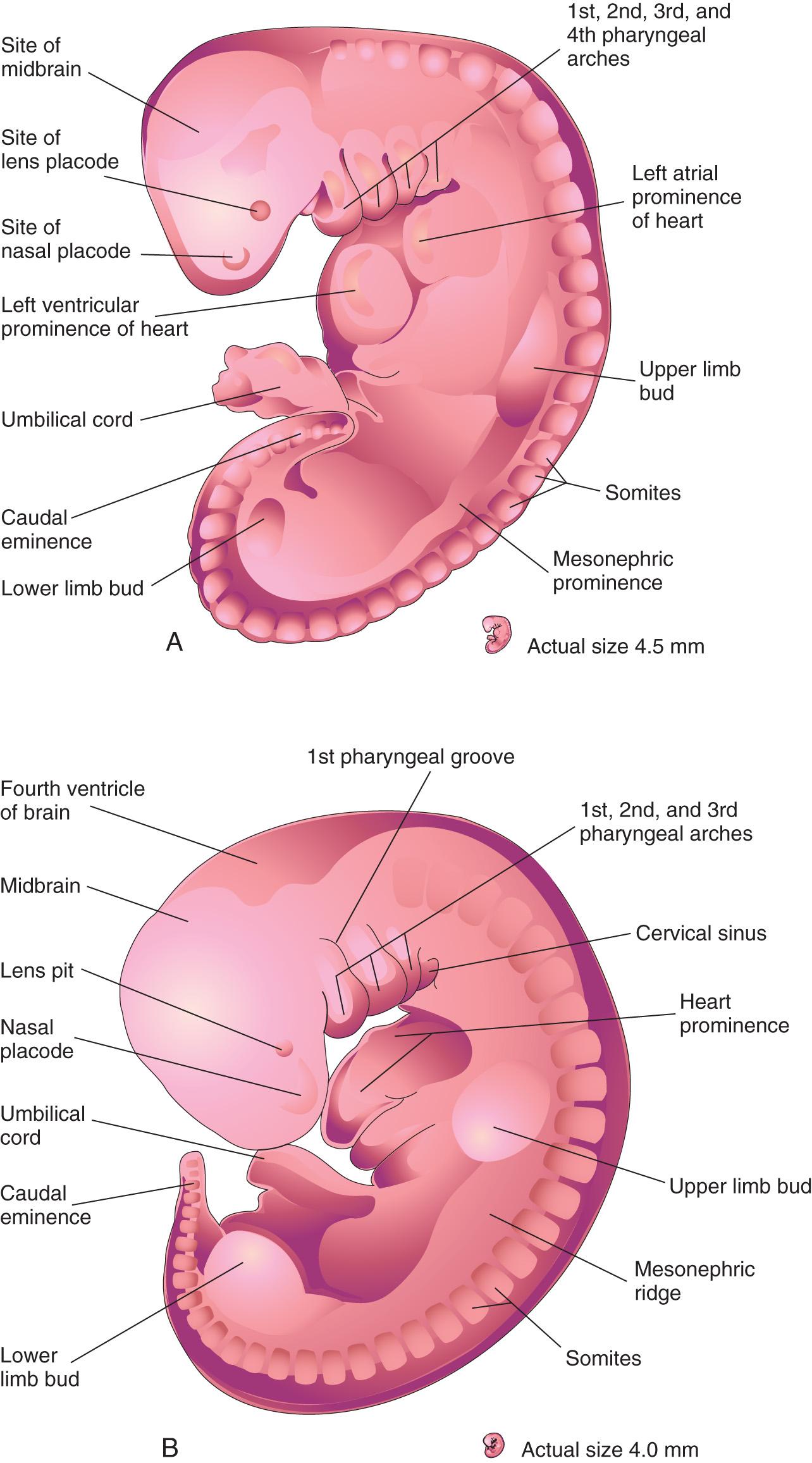
The limb buds elongate by proliferation of the mesenchyme. The upper limb buds appear disproportionately low on the embryo's trunk because of the early development of the cranial half of the embryo (see Fig. 16.1 ). The earliest stages of limb development are alike for the upper and lower limbs (see Figs. 16.1 B and 16.4 ). Later, distinct features arise because of differences in the form and function of the hands and feet.
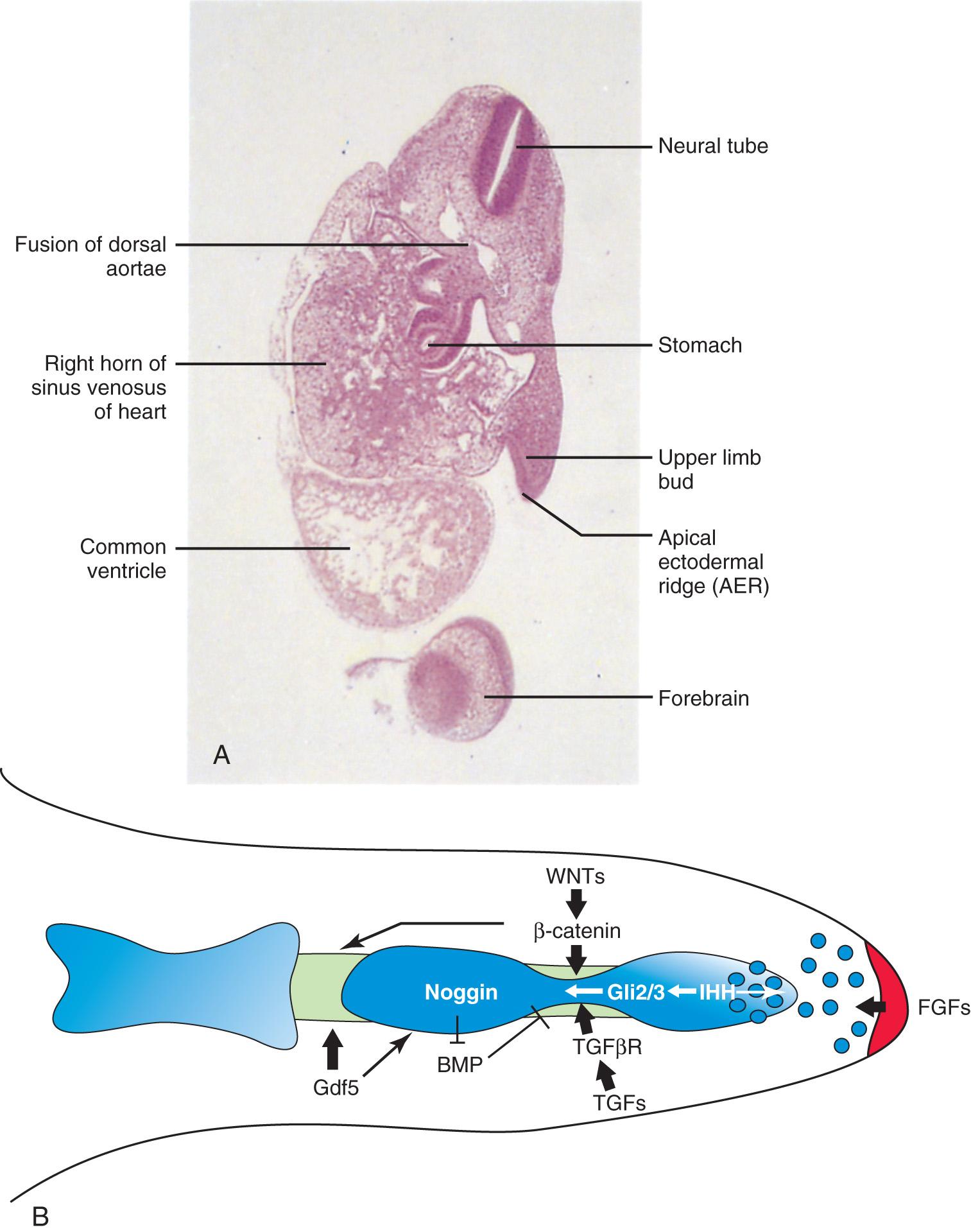
The upper limb buds develop opposite the caudal cervical segments, and the lower limb buds form opposite the lumbar and upper sacral segments. At the apex of each limb bud, the ectoderm thickens to form the AER. This ridge is a specialized, multilayered epithelial structure (see Fig. 16.2 ) that is induced by the paracrine factor, fibroblast growth factor 10 (FGF10), from the underlying mesenchyme. Transcription factors encoded by the gene BHLHA9 (Basic Helix-Loop-Helix Family Member A9) and bone morphogenetic protein (BMP) signaling is required for its formation. From recent studies, transcription factors of the T-box family of genes have been assigned a critical role in limb development .
FGF8, secreted by the AER, exerts an inductive influence on the limb mesenchyme that initiates growth and development of the limbs in a proximodistal axis. Retinoic acid promotes the formation of the limb bud by inhibiting FGF signaling. Mesenchymal cells aggregate at the posterior margin of the limb bud to form the zone of polarizing activity , an important signaling center in limb development. FGFs from the AER activate the zone of polarizing activity, which causes expression of the Sonic Hedgehog (SHH) genes.
Transcription factors encoded by the genes BHLHA9 and SHH regulate the normal patterning of the limbs along the anterior-posterior axis. Expression of WNT7A from the dorsal non-AER ectoderm of the limb bud and engrailed homeobox 1 (EN1) from the ventral aspect are involved in specifying the dorsal-ventral axis. The AER itself is maintained by inductive signals from SHH and WNT7 . It has been suggested that epiprofin, a zinc finger transcription factor, regulates WNT signaling in the limb bud (see Fig. 16.2B ).
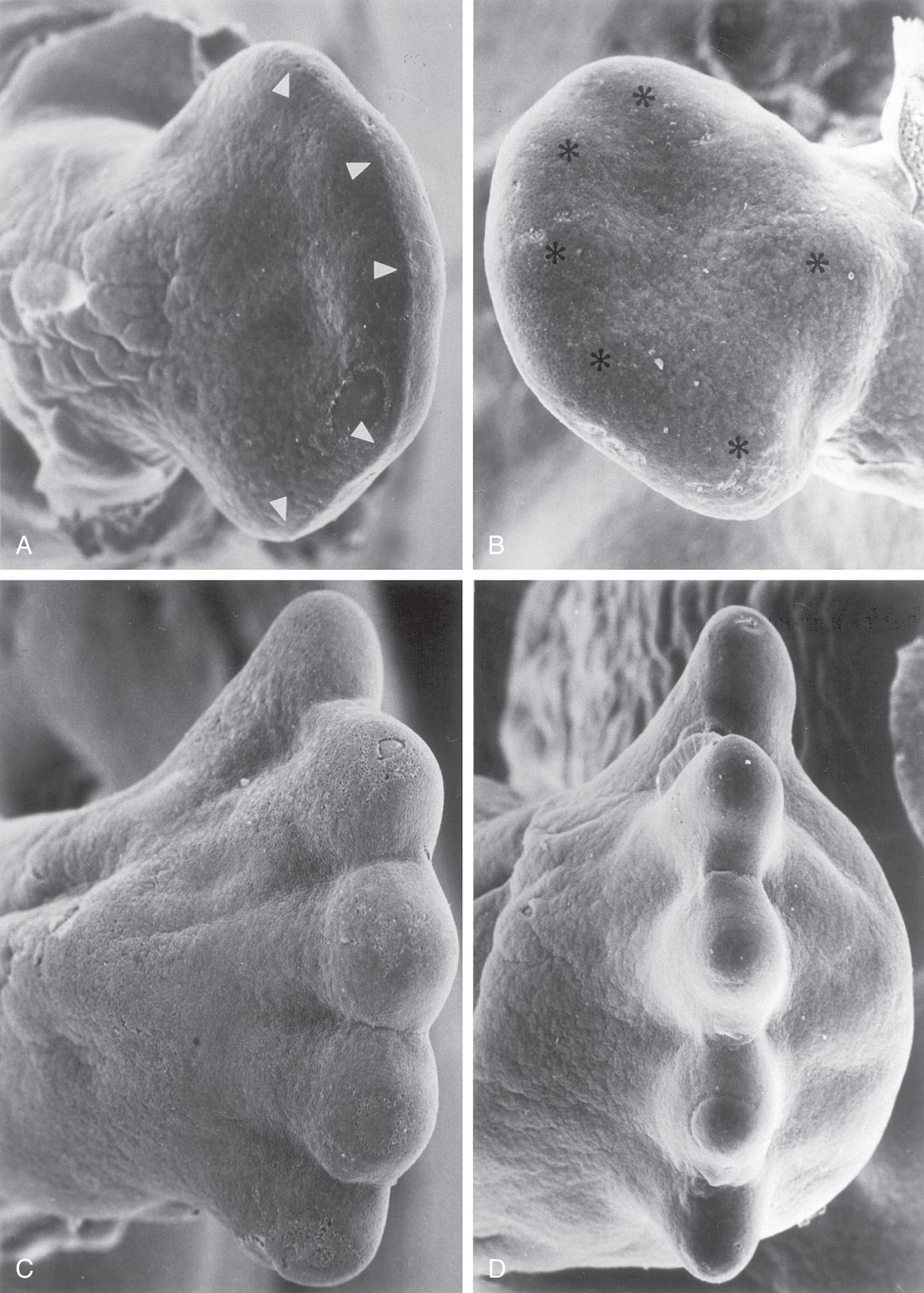
The mesenchyme adjacent to the AER consists of undifferentiated, rapidly proliferating cells, whereas mesenchymal cells proximal to it differentiate into blood vessels and cartilage bone models. The distal ends of the limb buds flatten into hand plates and foot plates ( Fig. 16.3 and Fig. 16.4 B and H ). Studies have shown that endogenous retinoic acid is also involved in limb development and pattern formation.
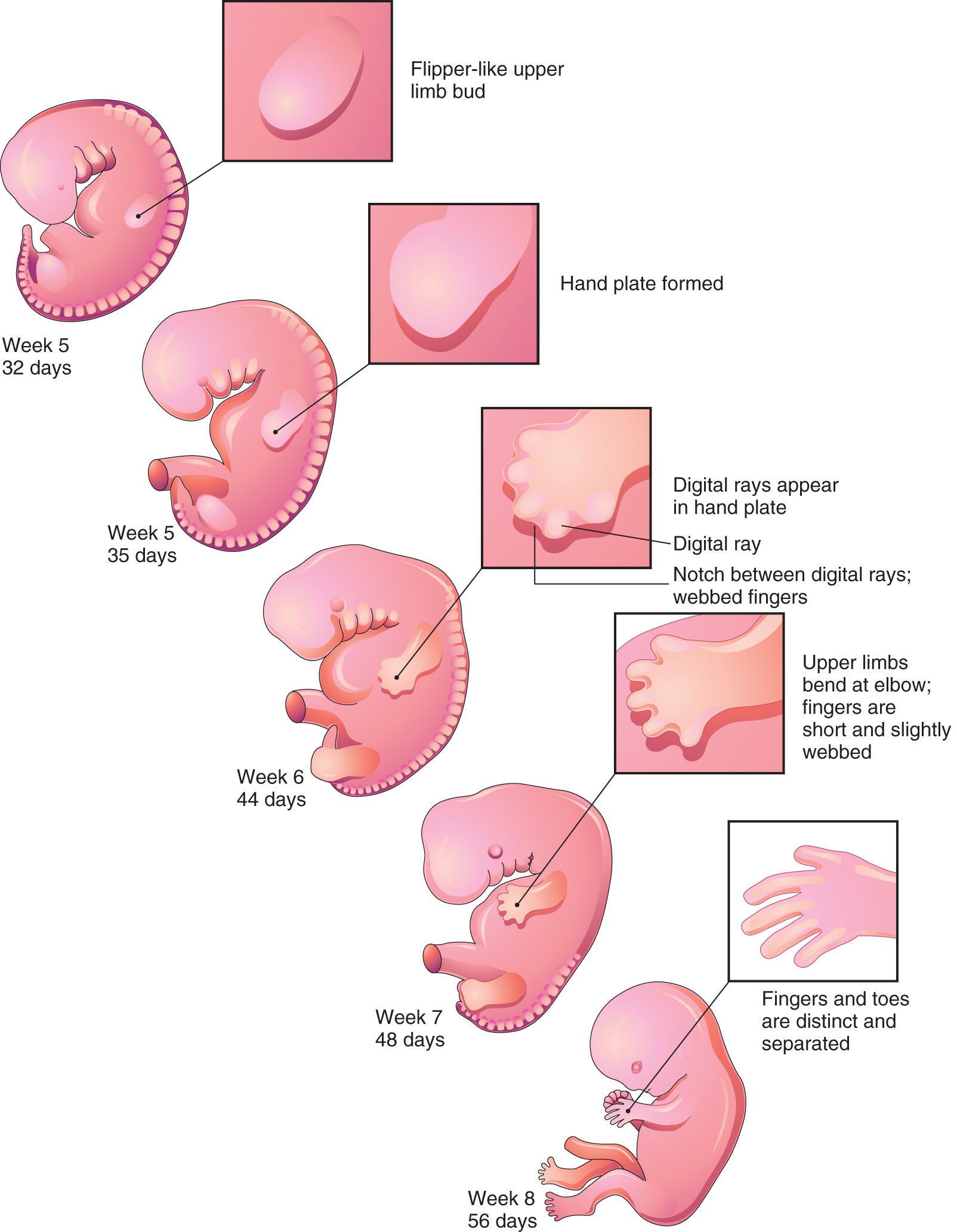
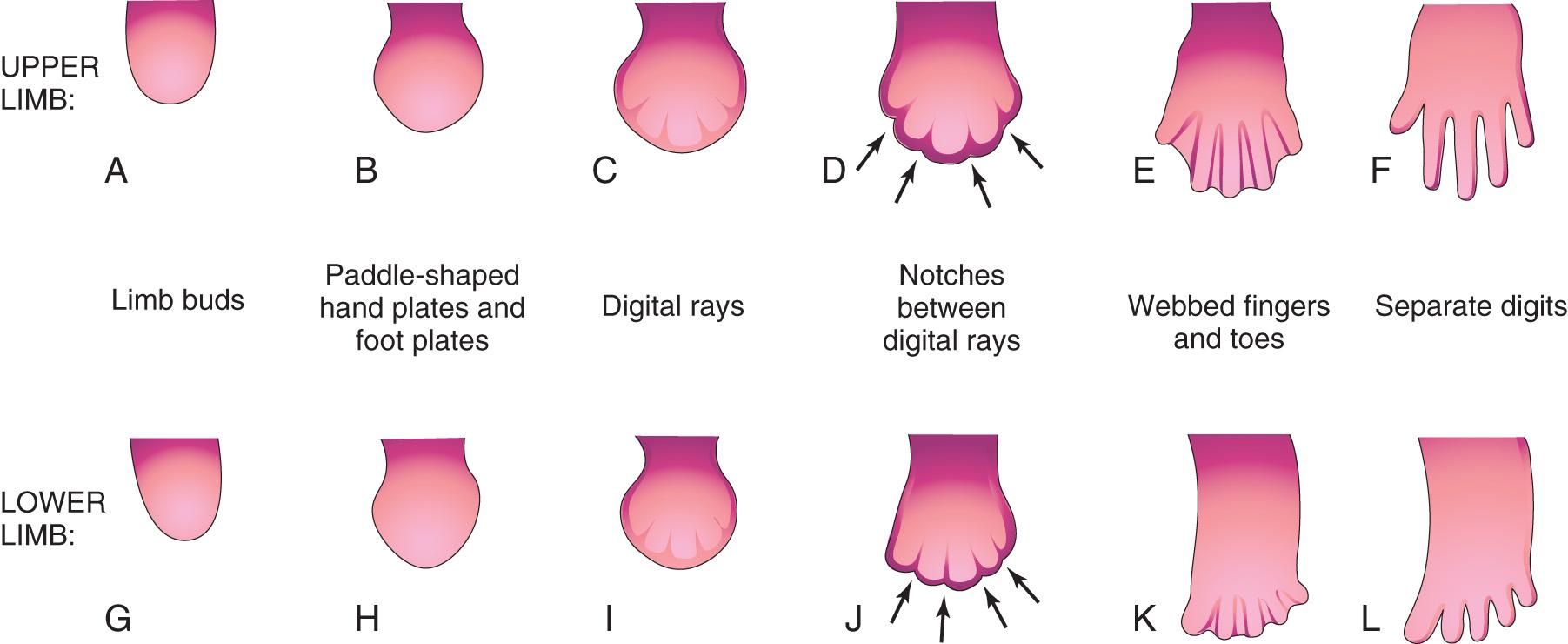
By the end of the sixth week, mesenchymal tissue in the hand plates has condensed to form digital rays (see Figs. 16.3 and 16.4 C ). These mesenchymal condensations outline the pattern of the digits (fingers) in the hand plates. During the seventh week, similar condensations of mesenchyme condense to form digital rays and digits (toes) in the footplates (see Fig. 16.4 I ).
At the tip of each digital ray, a part of the AER induces development of the mesenchyme into the mesenchymal primordia of the bones (phalanges) in the digits (see Fig. 16.6 C and D ). The intervals between the digital rays are occupied by loose mesenchyme. These intervening regions of mesenchyme soon break down, forming notches between the digital rays ( Fig. 16.5 , and see Fig. 16.3 and Fig. 16.4 D and F ). As the tissue breakdown progresses, separate digits (fingers and toes) are formed by the end of the eighth week ( Fig. 16.6 , and see Fig. 16.4 E , F , K , and L ).
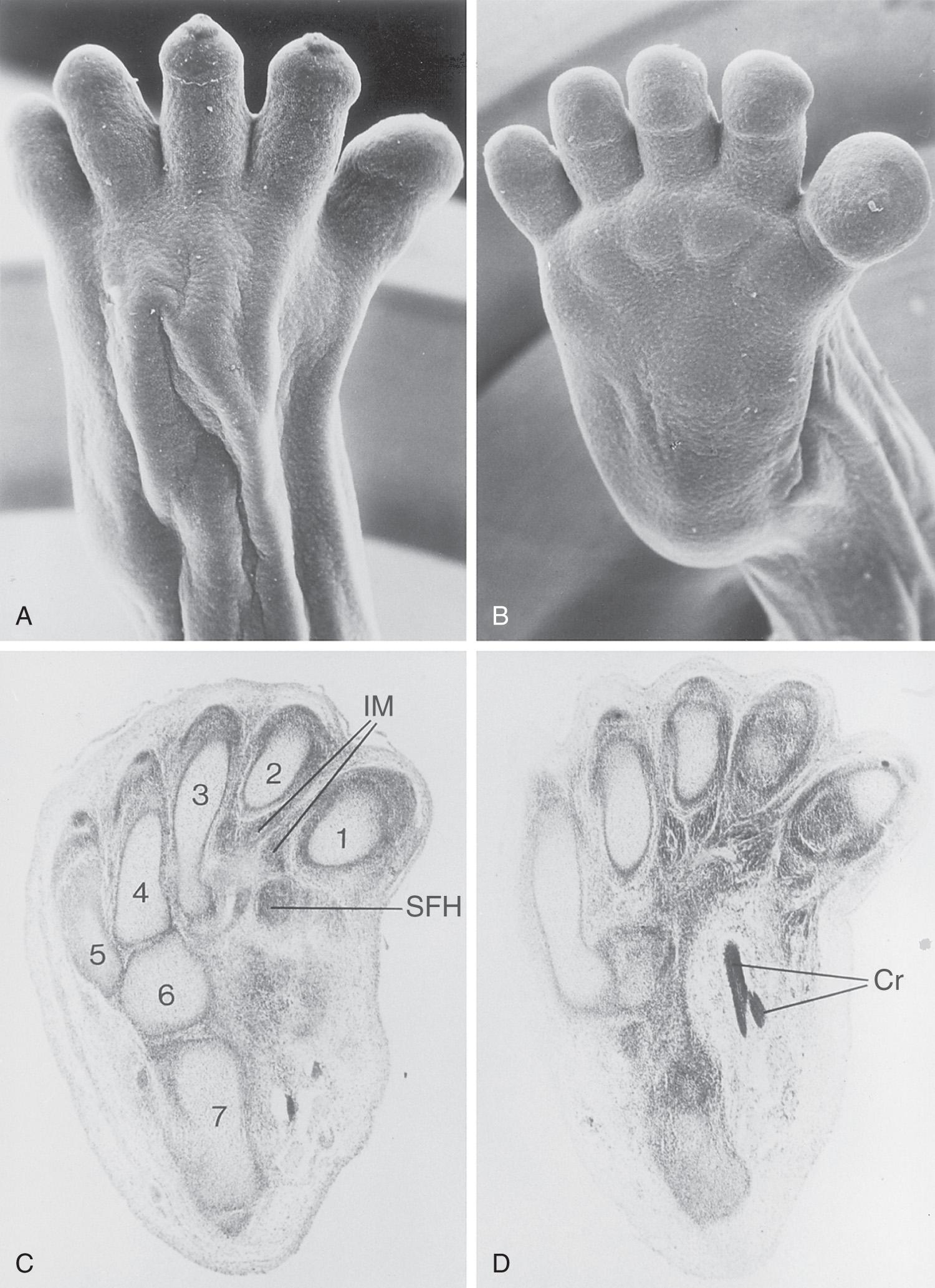
Molecular studies indicate that the earliest stages of limb patterning and digit formation involve the expression of the patched 1 gene (PTCH1) , which is essential for the downstream regulation of multiple Hox genes and the SHH pathway. Gradual apoptosis (programmed cell death) through both the apoptosis-inducing factor (AIF) and caspase-3 mediated pathways is responsible for the tissue breakdown in the interdigital regions. Antagonism between retinoic acid signaling and transforming growth factor-β (TGF-β) appears to control the interdigital apoptosis and digit formation. Blockade of these cellular and molecular events could account for syndactyly or webbing of the fingers or toes (see Fig. 16.14 C and D ).
15
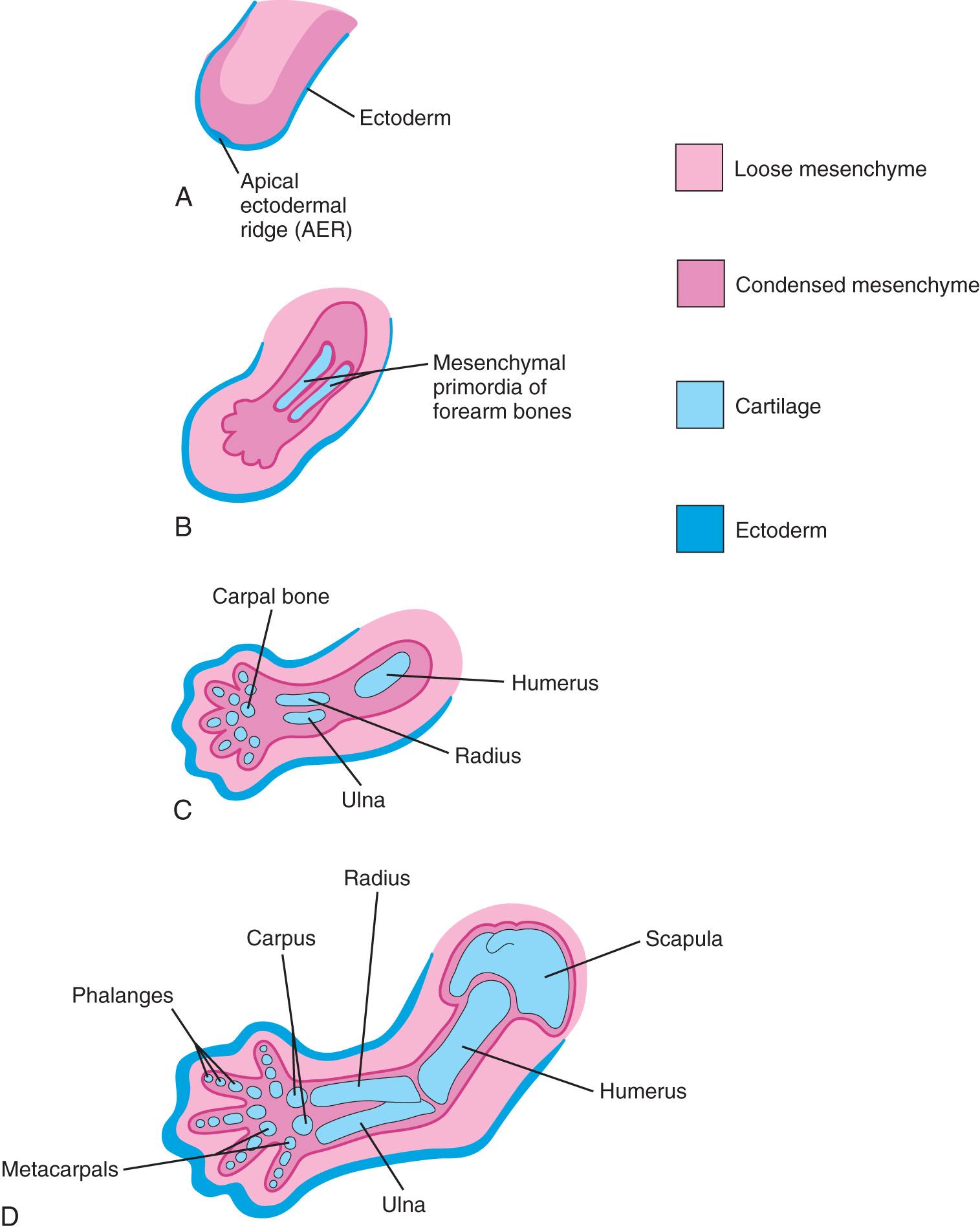
As the limbs elongate, mesenchymal models of the bones are formed by cellular aggregations (see Fig. 16.7 B ). Chondrification centers appear in the fifth week. By the end of the sixth week, the entire limb skeleton is cartilaginous ( Fig. 16.7 ; see Chapter 14 , Fig. 14.13 D and E ). Osteogenesis of long bones begins in the seventh week from primary ossification centers in the middle of the cartilaginous models of the long bones. Ossification centers are present in all long bones by the 12th week (see Chapter 14 , Fig. 14.14 A ).
From the dermomyotome regions of the somites, myogenic precursor cells migrate into the limb buds and later differentiate into myoblasts. The c-Met receptor tyrosine kinase (encoded by the gene MET ) plays an essential role in regulating this process. As the long bones form, the myoblasts aggregate and form a large muscle mass in each limb bud (see Chapter 15 , Fig. 15.1 ). In general, this muscle mass separates into dorsal (extensor) and ventral (flexor) components. The mesenchyme in the limb bud also gives rise to ligaments and blood vessels.
Early in the seventh week, the limbs extend ventrally. Originally, the flexor aspect of the limbs is ventral, and the extensor aspect is dorsal; the preaxial and postaxial borders are cranial and caudal, respectively (see Fig. 16.10 A and D ). The developing upper and lower limbs rotate in opposite directions and to different degrees ( Figs. 16.8 and 16.9 ):
The upper limbs rotate laterally through 90 degrees on their longitudinal axis; as a result, the future elbows come to point dorsally, and the extensor muscles lie on the lateral and posterior aspects of the limb.
The lower limbs rotate medially through almost 90 degrees; therefore, the future knees come to face ventrally, and the extensor muscles lie on the anterior aspect of the limb.
Developmentally, the radius and tibia are homologous bones, as are the ulna and fibula; likewise, the thumb and great toe are homologous digits. Synovial joints appear at the beginning of the fetal period (ninth week), coinciding with functional differentiation of the limb muscles and their innervation.
15
There is a strong relationship between the growth and rotation of the limbs and their cutaneous segmental nerve supply. Motor axons arising from the spinal cord enter the limb buds during the fifth week and grow into the dorsal and ventral muscle masses. Sensory axons enter the limb buds after the motor axons and use them for guidance. Neural crest cells, the precursors of Schwann cells, surround the motor and sensory nerve fibers in the limbs and form the neurolemma (sheath of Schwann) and myelin sheaths (see Chapter 17 , Fig. 17.11 ).
During the fifth week, peripheral nerves grow from the developing brachial and lumbosacral limb plexuses into the mesenchyme of the limbs ( Fig. 16.10 B and E ). The spinal nerves are distributed in segmental bands, supplying both dorsal and ventral surfaces of the limbs. A dermatome is the area of skin supplied by a single spinal nerve and its spinal ganglion; however, cutaneous nerve areas and dermatomes show considerable overlap.
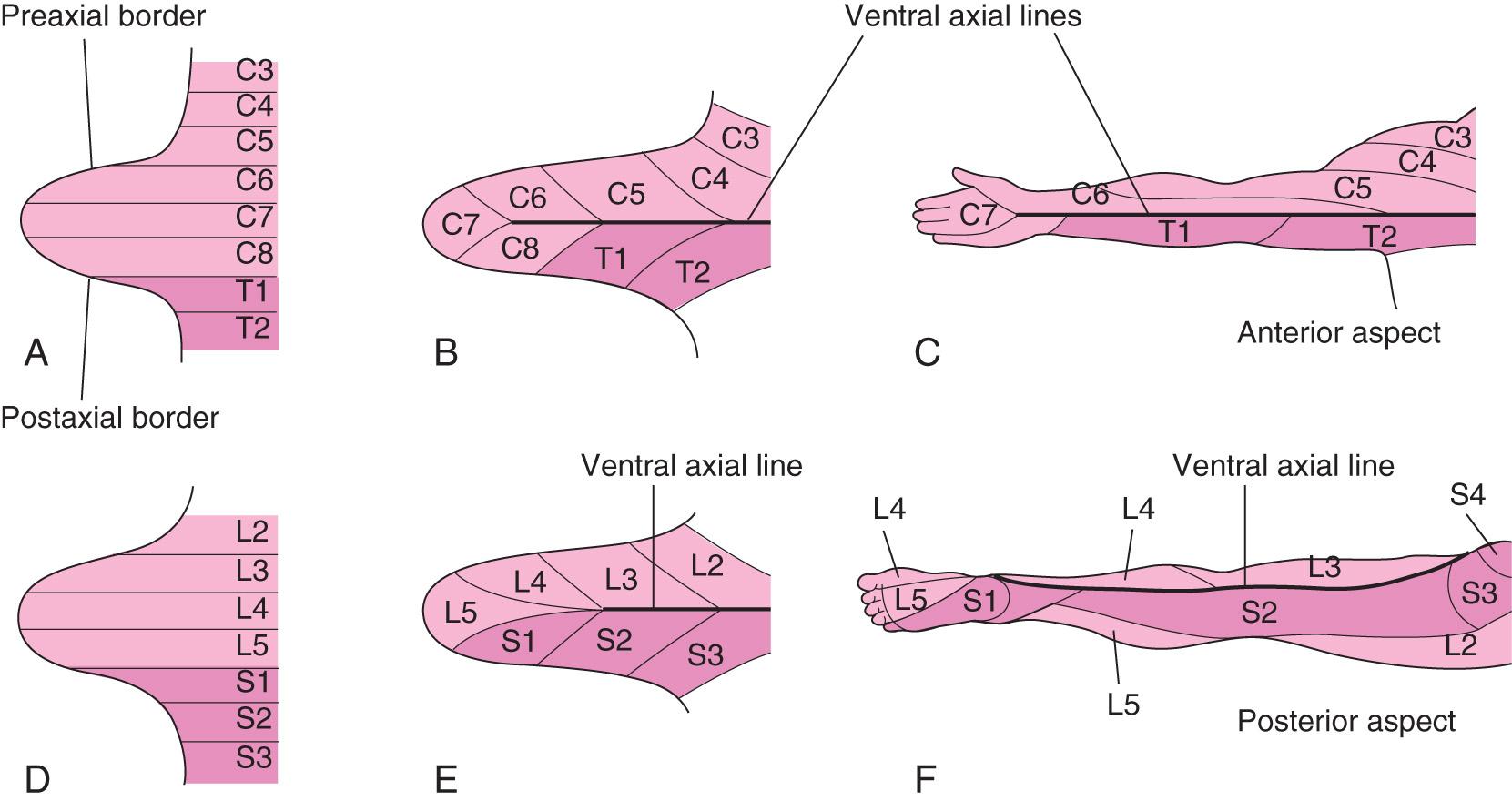
As the limbs elongate, the cutaneous distribution of the spinal nerves migrates along the limbs and no longer reaches the surface in the distal parts of the limbs. Although the original dermatomal pattern changes during growth of the limbs, an orderly sequence of distribution can still be recognized in the adult (see Fig. 16.10 C and F ). In the upper limb, the areas supplied by spinal nerves C5 and C6 adjoin the areas supplied by T2, T1, and C8, but the overlap between them is minimal at the ventral axial line.
A cutaneous nerve area is the area of skin supplied by a peripheral nerve. If the dorsal root supplying the area is cut, the dermatomal patterns indicate that there may be a slight deficit in the area indicated. However, because there is overlapping of dermatomes, a particular area of skin is not exclusively innervated by a single segmental nerve. The limb dermatomes may be traced progressively down the lateral aspect of the upper limb and back up its medial aspect. A comparable distribution of dermatomes occurs in the lower limbs, which may be traced down the ventral aspect and then up the dorsal aspect. As the limbs descend, they carry their nerves with them; this explains the oblique courses of the nerves arising from the brachial and lumbosacral plexuses.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here