Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Background
A systematic approach to side effects of medications should include consideration of the nature, severity, and timing of symptoms to facilitate optimal management of such side effects.
History
Many medications (e.g., nefazodone) previously used in psychiatric disorders, but shown to have serious adverse side effects, have been removed from the market over the years.
Clinical and Research Challenges
Rates of medication side effects may be difficult to quantify.
Though some side effects may be class effects, others are specific to individual agents.
It is difficult to predict who will suffer side effects; some side effects are idiosyncratic.
Practical Pointers
Tricyclic antidepressants are associated with cardiac effects and can be dangerous in overdose.
Use of monoamine oxidase inhibitors requires education about dietary limitations and drug–drug interactions.
Selective serotonin reuptake inhibitors and other newer antidepressants are generally well tolerated and safer in overdose than older agents, but still may cause clinically significant side effects.
Some selective norepinephrine (noradrenaline)-serotonin reuptake inhibitors have been associated with increased blood pressure (e.g., venlafaxine) and liver dysfunction (e.g., duloxetine).
Bupropion is known to lower the seizure threshold and has also been associated with an increase in panic symptoms.
Mirtazapine is associated most commonly with sedation and weight gain.
Lithium and anticonvulsant mood stabilizers are associated with a variety of side effects, including cognitive slowing, weight gain, and neurological symptoms.
Lamotrigine is the mood stabilizer most associated with the development of Stevens-Johnson syndrome, a rare but life-threatening skin disease.
Typical antipsychotics are associated with tardive dyskinesia and, of them, the high-potency typical antipsychotics commonly cause extrapyramidal symptoms.
Several atypical antipsychotics are linked to weight gain and metabolic side effects.
Most antipsychotics can cause prolongation of the QTc interval, which may increase the risk for lethal ventricular arrhythmias.
Stimulants have a propensity to cause increased heart rate and blood pressure, and their use is not recommended for patients with underlying ventricular arrhythmias.
Benzodiazepines are associated with a variety of side effects (including falls, dizziness, and ataxia).
Short-acting sedative-hypnotic agents, such as zolpidem, have been associated with various sleep-related behaviors, including eating and driving.
Side effects of psychotropics, which can range from minor nuisances to life-threatening conditions, can seriously affect the quality of a patient's life and his or her ability to comply with psychopharmacological treatments. For these reasons, it is important for clinicians who prescribe psychotropic medications to know their potential side effects and how such side effects can be managed.
Determining which medication is causing a specific side effect, and whether any medication is to blame for a given adverse effect, can be difficult. For example, more than half of all patients with untreated melancholic depression report headache, constipation, and sedation; these same symptoms are frequently attributed to side effects of antidepressant medications. A stepwise approach to the assessment of a potential side effect can help to ensure that true side effects are quickly addressed, while knee-jerk reactions that result in the discontinuation of well-tolerated treatments can be avoided. This approach ( Box 12-1 ) involves an assessment of the nature and severity of the effect, a thoughtful investigation into the causality of the effect, and the appropriate management of the symptom.
What exactly are the signs and symptoms? In some cases (e.g., drug rash) this can be easily ascertained, while in others (e.g., a severely demented patient with worsening agitation, possibly consistent with akathisia, restlessness, constipation) it may be difficult.
When did the symptom start?
Has this ever happened before?
Are there associated symptoms?
What subjective distress does the symptom cause?
What impact is it having on function and quality of life?
What medical dangers are associated with the side effect?
Did the side effect start in the context of a new medication or a dosage change?
What other medications/remedies are being taken?
Have there been other changes in medication, medical issues, diet, environment, or psychiatric symptoms?
Are the current signs and symptoms consistent with known side effects of a given medication?
If it appears that a specific medication is causing the side effect, options for management include:
Discontinue the medication
Decrease the dose
Change the dosing schedule (e.g., splitting up dose, taking medication during meals)
Change the preparation (e.g., to longer-lasting formulation)
Add a new medication to treat the side effect (e.g., propranolol for akathisia)
In this chapter, the most common and most dangerous side effects of psychotropics will be discussed in an effort to guide clinicians to treatment decisions and management of adverse effects. For each agent or class of agents, we will review common initial side effects, frequent long-term side effects, severe but rare adverse events, consequences of overdose, and (where applicable) withdrawal symptoms.
Tricyclic antidepressants (TCAs) have a number of common side effects that require careful management. Anticholinergic effects (including dry mouth, blurry vision, urinary hesitancy, constipation, tachycardia, and delirium) that result from the blockade of muscarinic cholinergic receptors can occur with use of TCAs. In addition, anticholinergic effects can also be dangerous to patients with pre-existing glaucoma (leading to acute angle-closure glaucoma), benign prostatic hypertrophy (leading to acute urinary retention), and dementia (leading to acute confusional states). TCAs can also cause sedation that results from blockade of H 1 histamine receptors, and orthostatic hypotension, due to blockade of alpha 1 receptors on blood vessels. All three of these side-effect clusters are more common with tertiary amine TCAs (e.g., amitriptyline, doxepin, clomipramine, imipramine) than with secondary amine TCAs (e.g., nortriptyline, desipramine, protriptyline). TCAs may also cause increased sweating. Longer-term side effects of TCAs include weight gain (related to histamine receptor blockade) and sexual dysfunction. Box 12-2 describes the management of common side effects of TCAs and other antidepressants.
Carefully consider whether the side effect is from the antidepressant.
Consider the timing, dosing, and the nature of effect, as well as the impact of concomitant medications, environmental changes, and medical conditions.
Consider drug–drug interactions as the cause of the adverse effects, rather than the effects of the antidepressant acting in isolation.
If an antidepressant appears to be causing non-dangerous side effects, consider lowering the dose (temporarily or permanently), dividing the dose, or changing medications.
Anticholinergic effects (e.g., dry mouth, urinary hesitancy, constipation): Symptomatic treatment (use hard candies for dry mouth, use laxatives for constipation); use bethanechol (25–50 mg/day) for refractory symptoms.
Sedation: Move the dose to bedtime, divide the dose, or add a psychostimulant (e.g., methylphenidate, 5–15 mg each morning) or modafinil (100–200 mg each morning).
Orthostatic hypotension: Increase fluid intake, divide the dose or move it to bedtime, or add a stimulant/mineralocorticoid.
Gastrointestinal side effects: Divide the dose, take it with meals, give it at bedtime, or use an H 2 blocker (e.g., ranitidine 150 mg twice daily).
Insomnia: Move the dose to the morning or add trazodone (25–100 mg), a sedative-hypnotic (e.g., zolpidem 5–10 mg), or another sedating agent.
Weight gain: Use diet and exercise, and consider addition of an H 2 blocker, topiramate, or sibutramine.
Sexual dysfunction: Options include switching to another agent (to bupropion, mirtazapine, or another agent not associated with sexual dysfunction), employing a drug holiday (often ineffective), or augmenting with a variety of agents (e.g., sildenafil, methylphenidate, bupropion, amantadine, buspirone, or yohimbine).
In addition to these common initial and long-term side effects, TCAs may have more serious but uncommon side effects; many of them are cardiac in nature. These agents are structurally similar to class I antiarrhythmics that are actually pro-arrhythmic in roughly 10% of the population; approximately 20% of patients with pre-existing conduction disturbances have cardiac complications while taking TCAs. TCAs are associated with cardiac conduction disturbances and their use can lead to prolongation of the PR, QRS, and QT intervals on the electrocardiogram (ECG) and have been associated with all manner of heart block. Some have suggested that effects on cardiac conduction are most severe with desipramine, while other studies have found amitriptyline and maprotiline to be most associated with torsades de pointes. Furthermore, these agents have been associated with an increased risk of myocardial infarction (MI) when compared to selective serotonin reuptake inhibitors (SSRIs). In addition to cardiac effects, other serious adverse events include the serotonin syndrome that occurs most often when TCAs are combined with other serotonergic agents, especially monoamine oxidase inhibitors (MAOIs). This syndrome can include confusion, agitation, and neuromuscular excitability (including seizures).
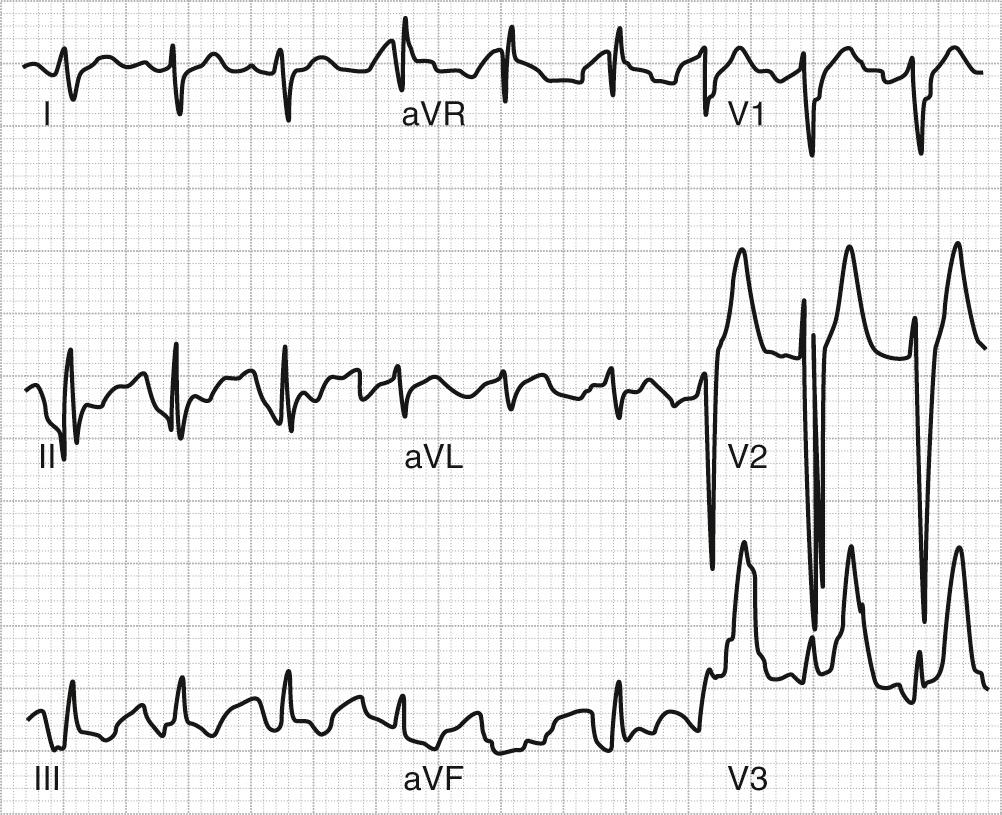
Adverse effects associated with TCA overdose include the exacerbation of standard side effects (e.g., severe sedation, hypotension, anticholinergic delirium). Ventricular arrhythmias and seizures can also result from TCA overdose. TCA overdose is frequently lethal, with death most often occurring via cardiovascular effects. Figure 12-1 shows an ECG (with characteristic QRS interval widening) of a patient following TCA overdose. A withdrawal syndrome (manifested by malaise, nausea, muscle aches, chills, diaphoresis, and anxiety) can occur following abrupt discontinuation of TCAs; the syndrome is thought to result from cholinergic rebound.
Selective serotonin reuptake inhibitors (SSRIs) are generally well tolerated. The most common side effects of SSRIs include gastrointestinal side effects (e.g., nausea, diarrhea, heartburn) that likely result from interactions with serotonin receptors (primarily 5-HT 3 that line the gut), central nervous system (CNS) activation (e.g., anxiety, restlessness, tremor, insomnia), and sedation that appear within the first few days of treatment. Gastrointestinal side effects may be most common with sertraline and fluvoxamine, CNS activation with fluoxetine, and sedation with paroxetine. Headache and dizziness can also occur early in treatment. These symptoms often improve or resolve within the first few weeks of treatment. Rarely, akathisia or other extrapyramidal symptoms (EPS) may occur (sertraline appears to be the most frequent offender due to its dopamine-blocking properties). Nausea, insomnia, and somnolence are adverse effects that most often lead to discontinuation of SSRIs. It is notable that the rate of discontinuation due to side effects is higher with fluvoxamine than with other SSRIs.
Longer-term side effects associated with SSRIs include sexual dysfunction, which occurs in 30% or more of SSRI-treated patients; SSRI-induced sexual dysfunction occurs in both men and women, affecting both libido and orgasms. Weight gain, fatigue, and apathy are infrequent long-term side effects; weight gain may occur more frequently with paroxetine. The syndrome of inappropriate antidiuretic hormone secretion (SIADH) can occur with all SSRIs, though it may be more frequent with fluoxetine. Finally, SSRIs may increase the risk of bleeding, primarily due to the effects of these agents on serotonin receptors of platelets, resulting in decreased platelet activation and aggregation; it appears that the bleeding risk associated with use of SSRIs is similar to that of low-dose non-steroidal anti-inflammatory agents. SSRIs are relatively safe in overdose. The serotonin syndrome can occur when these agents are combined with other serotonergic compounds, or, very rarely, when used alone.
SSRIs are generally considered safe in terms of cardiovascular side effects. Recently, concern has arisen regarding the possibility of SSRIs leading to prolongation of the QTc interval and increasing the risk for torsades de pointes, a potentially lethal ventricular arrhythmia. In fact, all SSRIs have been associated in case reports with QTc prolongation at therapeutic doses and in overdose. In particular, citalopram has been shown to have a modest QT-prolonging effect, which resulted in a recommendation from the Food and Drug Administration (FDA) in August 2011 to limit the maximum daily dose of citalopram to 40 mg (20 mg in patients with hepatic impairment or those older than 60 years) because of the increased risk of QTc prolongation at higher doses, and to declare its use contraindicated in patients with congenital long-QT syndrome. Less stringent recommendations were issued in March 2012, but citalopram remains not recommended for use at doses greater than 40 mg per day. No QTc-related recommendations have been issued for other SSRIs, though escitalopram appears to have a more modest, dose-dependent effect on prolongation of the QTc interval.
Finally, abrupt withdrawal from SSRIs can lead to a withdrawal syndrome characterized by several somatic symptoms. The syndrome includes disequilibrium (dizziness, vertigo, and ataxia), flu-like symptoms (headache, lethargy, myalgias, rhinorrhea, and chills), gastrointestinal symptoms (nausea, vomiting, and diarrhea), sensory disturbances (paresthesias and sensations of electrical shock), and sleep disturbances (insomnia, fragmented sleep, and vivid, often frightening, dreams). In addition, a number of psychological symptoms (e.g., agitation, irritability, anxiety, crying spells) are associated with SSRI discontinuation.
Symptoms of the discontinuation syndrome typically begin 1 to 3 days after withdrawal of an SSRI, though when associated with a longer–half-life agent (fluoxetine in particular), symptoms may begin as long as 7 to 10 days after its discontinuation. Symptoms usually resolve within 2 weeks. If the original antidepressant is restarted, or another SSRI is substituted, the symptoms resolve, usually within 24 hours of re-initiation. This syndrome is most common with SSRIs with shorter half-lives (paroxetine and fluvoxamine); it rarely occurs with fluoxetine, whose metabolite has a half-life of more than 1 week. The syndrome is thought to result from diminished synaptic serotonin levels at serotonin receptors that have been desensitized in the context of serotonin reuptake inhibition.
Venlafaxine's initial side effects are similar to those of the SSRIs. Nausea and CNS activation appear to occur somewhat more commonly than with the SSRIs; in addition, dry mouth and constipation may be associated with venlafaxine use despite lack of effects on muscarinic cholinergic receptors. Increased blood pressure, presumably related to effects on norepinephrine (noradrenaline), can occur with immediate-release venlafaxine, with 7% of patients taking 300 mg per day or less and 13% taking doses greater than 300 mg having elevation of blood pressure; this resolves spontaneously in approximately one-half of cases. The extended-release (XR) formulation appears to be associated with lower rates of hypertension. Sexual dysfunction occurs at approximately the same rate as occurs with SSRIs. Venlafaxine does not appear to have substantial adverse effects on the cardiovascular system, though at least one study has suggested the possibility of QTc prolongation. Among more serious side effects, SIADH and serotonin syndrome have been reported with venlafaxine.
Overdose of venlafaxine generally causes symptoms similar to those of SSRI overdose. However, venlafaxine, according to one large epidemiological study in the United Kingdom, has been associated with a high rate of death in overdose (possibly via seizure and cardiovascular effects); other (smaller) studies have not found an increased lethality with overdose. Finally, due to the short half-life of venlafaxine, the discontinuation syndrome reported with SSRIs is common in patients who abruptly stop taking this antidepressant.
In general, duloxetine's common side effects are similar to those of SSRIs. Nausea, dizziness, headache, and insomnia may be somewhat more frequent than with SSRIs, but overall this agent is well tolerated. Duloxetine does not appear to have significant effects on blood pressure or other cardiovascular parameters, including the QTc interval. Sexual dysfunction appears in concert with duloxetine use, but may be less common than with SSRIs such as paroxetine. Duloxetine has not shown significant affinity for histaminic or cholinergic receptors, and thus sedation, weight gain, and anticholinergic effects are uncommon.
With respect to more severe adverse effects, SIADH has been reported with duloxetine use, and serotonin syndrome may develop with this agent because of its significant serotonin reuptake inhibition. Increased levels of hepatic transaminases develop in a small percentage of patients taking duloxetine; this is usually asymptomatic, but patients with chronic liver disease or cirrhosis have experienced elevated levels of bilirubin, alkaline phosphatase, and transaminases, and currently it is recommended that duloxetine should not be given to patients who consume substantial amounts of alcohol or who exhibit evidence of chronic liver disease. Duloxetine does not appear to have increased rates of death in cases of overdose. A discontinuation syndrome similar to that seen with SSRIs and venlafaxine can occur with abrupt withdrawal of duloxetine, though it is probably less likely than with venlafaxine or paroxetine due to its longer half-life.
Tranylcypromine and phenelzine are the most commonly used oral MAOIs and are both irreversible inhibitors. Tranylcypromine is associated with anxiety, restlessness, insomnia, and tremor, while phenelzine is more associated with sedation, mild anticholinergic effects, and orthostatic hypotension (though this last effect can occur with both agents). Both agents are associated with headache, dry mouth, and gastrointestinal side effects. With regard to long-term side effects, weight gain and sexual dysfunction can occur with all MAOIs, though perhaps more commonly with phenelzine. MAOIs can result in symptoms of pyridoxine deficiency, including paresthesias and weakness. Finally, MAOIs have been associated with elevated liver transaminases, though true hepatotoxicity is exceedingly rare.
Hyperadrenergic crises, characterized by occipital headache, nausea, vomiting, diaphoresis, tachycardia, and severe hypertension, can occur in patients taking MAOIs. These most commonly occur when tyramine-containing foods are consumed or when adrenergic agonists (such as sympathomimetics) are taken in combination with MAOIs. Box 12-3 lists tyramine-containing foods that should be avoided by patients on MAOIs. Serotonin syndrome can also occur with MAOIs when these agents are taken with SSRIs, TCAs, or other serotonergic agents ( Box 12-4 lists medications that should be avoided by patients taking MAOIs). MAOI overdose is quite dangerous, with rates of death higher than those for SSRIs and other newer antidepressants ; with serotonin syndrome, neuromuscular excitability, seizures, arrhythmias, and cardiovascular collapse are all possible.
All matured or aged cheeses (fresh cottage, cream, ricotta, and processed cheese are tolerated)
Pizza, lasagne, and other foods made with cheese
Fermented/dried meat (e.g., pepperoni, salami, or summer sausage)
Improperly stored meat and fish
Fava or broad bean pods
Banana peel (banana and other fruit are tolerated, but do not use more than ![]() pound of raspberries)
pound of raspberries)
All tap beer (two or less cans/bottles of beer or 4 oz glasses of wine per day)
Sauerkraut
Soy sauce and other soybean products (soy milk is acceptable)
Marmite yeast extract (other yeast extract are acceptable)
SSRIs
TCAs
Mirtazapine
Nefazodone
Vilazodone
Trazodone
Buspirone
Lithium
Dextromethorphan
Tramadol
Methadone
Carbamazepine
Sumatriptan and related compounds
Cocaine
MDMA
St. John's wort
SAMe
Linezolid
Dopamine
L-dopa
Psychostimulants
Bupropion
Amphetamine
Cold remedies or weight loss products containing pseudoephedrine, phenylpropanolamine, phenylephrine, or ephedrine
Other
Meperidine (may cause seizures and delirium)
MDMA, 3,4-methylenedioxy-N-methylamphetamine; SAMe, S-adenosyl-methionine; SSRIs, selective serotonin reuptake inhibitors; TCAs, tricyclic antidepressants.
The transdermal MAOI (selegiline) patch does not require dietary modification at its lowest dose. At this lowest dose (6 mg/24 hours) transdermal selegiline appears to have an incidence of orthostatic hypotension, gastrointestinal side effects, weight gain, and sexual dysfunction that is greater than placebo, but lower than with orally-ingested MAOIs; skin irritation at the patch sites has been the most common adverse effect. At doses above 6 mg/24 hours, dietary modification is required, and at all doses concomitant medications that increase catecholamines or serotonin should be avoided as with the oral MAOIs.
Bupropion, an agent that does not directly affect serotonin neurotransmission, has initial side effects that differ from those of SSRIs and dual-action agents. Its most common and important initial side effects include headache, dizziness, dry mouth, anxiety, restlessness, anorexia, nausea, and insomnia. Long-term side effects are rare; rates of sexual dysfunction and weight gain are equal to placebo (weight loss can occur in some patients), and this agent has minimal cardiovascular effects, even in overdose. The most serious side effect associated with bupropion use is seizure. The risk of seizure with the immediate-release preparation is 0.1% at doses less than 300 mg/day, and 0.4% at doses from 300 to 400 mg/day; the risk of seizure may be lower with longer-acting preparations, but guidelines to keep the total daily dose at or below 450 mg/day remain. In addition, maximum single doses should not exceed 150 mg for the immediate-release form and 200 mg for the sustained-release form. The longest-acting preparation, Wellbutrin XL, can be given as a single dose of up to 450 mg. Because of its increased risk of seizure, bupropion should not be used in those patients with a history of seizures, or in those at increased risk of seizures (e.g., those with eating disorders, head trauma, or alcohol abuse). Overdose is infrequently life-threatening, though seizures, arrhythmias, and death have occurred in overdose. There is no withdrawal syndrome associated with abrupt discontinuation of bupropion.
The most common initial side effects associated with mirtazapine include sedation and increased appetite due to histamine receptor blockade; sedation may be less prevalent at higher doses (i.e., 30 mg/day or greater) than at lower doses due to recruitment of noradrenergic effects at higher doses. Less frequently, dry mouth, constipation, and dizziness have been associated with mirtazapine use; orthostatic hypotension can occur occasionally. Gastrointestinal side effects, anxiety, insomnia, and headache are all less common than with use of most other antidepressants. Weight gain is the most common long-term side effect, and elevated lipids occur in approximately 15% of patients who use mirtazapine. Rare, but more serious, side effects have included an increase in liver transaminases (in approximately 2% of patients), and neutropenia (in 0.1%). Mirtazapine appears to be associated with less sexual dysfunction than the SSRIs and it has minimal cardiovascular effects; it has no withdrawal syndrome. There is also a low mortality rate in overdose.
Trazodone is associated with significant sedation. Other common initial side effects can include dry mouth, nausea, and dizziness; orthostasis is much more common than with nefazodone, with reports of syncope. Weight gain and sexual dysfunction are rare, and trazodone is not associated with hepatotoxicity. Trazodone has, very infrequently, been associated with cardiac arrhythmias and QTc prolongation, possibly due to effects on potassium channels, and it should be used with caution in patients with a propensity for, or a history of, arrhythmias. Priapism occurs in approximately 1 in 6,000 male patients who take trazodone; this effect usually occurs within the first month of treatment. In overdose, sedation and hypotension are the most common adverse effects; isolated trazodone overdose is rarely fatal. There is no discontinuation syndrome.
Vilazodone, a newer antidepressant, has diarrhea, nausea, and headache as its most common side effects, all of which are typically transient. The dose is typically titrated incrementally to avoid gastrointestinal side effects. Dry mouth, dizziness, insomnia, and abnormal dreams have also been reported. Vilazodone has not been shown to have adverse cardiac effects and appears to have minimal effects on weight gain. Though purported to cause less sexual dysfunction than other antidepressants, the FDA reports that vilazodone does not meet the minimal criteria to make this claim.
Lithium has many associated adverse effects, summarized in Box 12-5 . Gastrointestinal and neurological side effects (e.g., sedation, tremor), along with increased thirst, often occur early in therapy, while effects on thyroid and renal function occur more chronically. Box 12-6 summarizes potential treatments for lithium-induced side effects; change in dosing and formulation can reduce gastrointestinal side effects, while other side effects require additional therapies. Lithium has some effects on sino-atrial node transmission and (less commonly) an atrio-ventricular conduction. However, it has not been commonly associated with other effects on the cardiovascular system, and cardiac disease (aside from sick sinus syndrome) is not an absolute contraindication for lithium use. Lithium has a low therapeutic index and lithium toxicity (whether intentional or unintentional) can lead to a variety of symptoms, including neurological (e.g., severe sedation, tremor, dysarthria, delirium, anterograde amnesia, myoclonus, seizure), gastrointestinal (e.g., nausea, vomiting), and cardiovascular (e.g., arrhythmia) effects; renal function often is impaired, and dialysis may be required to treat lithium toxicity if supportive measures and intravenous (IV) normal saline to aid lithium excretion are ineffective. Lithium does not have a characteristic discontinuation syndrome, but rapid withdrawal of lithium therapy is associated with substantially higher rates of relapse than when lithium is tapered.
Sedation
Tremor (fine action tremor)
Ataxia/incoordination
Cognitive slowing
Nausea and vomiting
Diarrhea
Abdominal pain
Weight gain
Renal
Polydipsia/polyuria
Nephrogenic diabetes insipidus
Interstitial nephritis
Acne
Psoriasis
Edema
T-wave inversion on electrocardiogram
Sinoatrial node slowing
Atrioventricular blockade
Other
Hypothyroidism
Leukocytosis
Hypercalcemia
Propranolol (10–30 mg twice or three times daily); primidone is a second-line option (25–100 mg per day)
Gastrointestinal
Change the formulation to a longer-lasting oral preparation or lithium citrate syrup
Amiloride (5–20 mg/day); hydrochlorothiazide (50 mg/day) is a second-line option (halve the lithium dose and follow lithium levels closely)
Spironolactone (25 mg/day); follow lithium levels closely
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here