Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Substance use disorders are characterized by the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) as “a cluster of cognitive, behavioral, and physiological symptoms indicating that the individual continues using the substance despite significant substance-related problems”. The diagnostic criteria are further categorized into the symptom clusters of impaired control over use, compulsivity, harmful consequences, and the physiological symptoms of tolerance and withdrawal. These symptoms may persist despite clear negative consequences due to environmental, psychological, or biological reinforcers of behavior. The uncontrollable substance intake that characterizes addiction is clinically distinct from the occasional use of an addictive drug without loss of control, and current addiction research is focused on characterizing the mechanisms underpinning that distinction. This chapter will review the discoveries of neurochemical imaging studies in human subjects and explore how these findings may translate to treatments for addiction.
A brief review of the neuroanatomical structures most relevant to addiction will aid in understanding the neurochemical imaging literature. These structures have mostly been identified through research using animal models. The nucleus accumbens is located within the ventral striatum and is most closely associated with the reinforcing properties of substances. Projections from the ventral tegmental area to the nucleus accumbens shell are important for regulating motivational salience. Rewarding events increase dopaminergic release in the accumbens, which reinforces the behaviors that led to the reward. The amygdala also receives dopaminergic input from the ventral tegmental area, as well as other input from the prefrontal cortex, nucleus accumbens, brain stem, and hypothalamus. It is involved in attributing predictors to motivationally salient, or rewarding, events. The prefrontal cortex, particularly the anterior cingulate and ventral orbital cortices, is involved in reward-based decision-making, a process that appears to be strongly influenced by the predictability of a reward.
Dopamine has a central role in mediating reinforced behavior. The mesolimbic dopamine system, which connects the ventral tegmental area in the midbrain to the nucleus accumbens in the ventral striatum, is specifically involved in the reward and reinforcement value of addictive substances. When dopamine is released from the ventral tegmental area in response to novel salient stimuli, this promotes learning and diverts attention to these events. There are 5 subtypes of dopamine receptors (D1-D5), which are further classified into the D1-like (D1 and D5) and D2-like (D2, D3, D4) families. The D1-like receptors act to increase the second messenger cyclic adenosine monophosphate (cAMP), while the D2-like receptors decrease cAMP. Because of the similar properties of these receptors within their respective families and similar behavior in neuroimaging studies, they will be referred to only as D1 or D2 receptors in this chapter.
Changes in dopaminergic signaling in humans were first identified in cocaine addiction by Volkow et al., but have now been seen in many other addictions, including alcohol, opioid, tobacco, and methamphetamine use disorder. Recent studies have also found impaired dopamine systems in cannabis use disorder. Low D2 receptor binding has been hypothesized to represent a vulnerability to the rewarding effect of pharmacologic rewards over naturally occurring reinforcers.
Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) are imaging modalities that can be used to measure receptor density and changes in neurotransmitters in the human brain. The injection of a radioligand, which is a radioactive molecule that binds to a specific receptor, can be detected with PET or SPECT scanners. Radioligands have been developed for a number of different receptors in the brain, such as D1 and D2 dopamine receptors, μ-opioid receptors, serotonergic receptors, and more.
The outcome measure for these brain imaging studies is called the “binding potential” (BP). BP is a measure of the radioligand–receptor complexes in the brain, and it is defined as the product of receptor density ( B max ) and the affinity of the radioligand for the receptor (1/ K D ) such that:
However, it is also important in brain imaging to recognize that while the radioligand binds to the receptor, a small amount will bind to nonspecific proteins in the brain. This is called “nonspecific binding” and it affects the “signal to noise” of the radioligand. The most common method used to account for nonspecific binding is to use a reference region. This is a brain region that does not have the receptor being targeted and contains only the nonspecific binding of the radioligand. This is abbreviated as the BP ND because it uses the nonspecific binding in tissue as a reference region. This is calculated as:
In this equation, f ND is the free fraction of drug in the nondisplaceable reference region. For example, when imaging D2 receptors with the radiotracer [ 11 C]raclopride, the cerebellum is often used as the reference region because there are no cerebellar dopamine receptors. Other measures of binding potential are BP F and BP P , which use the equilibrium ratios of specifically bound radioligand relative to the free radioligand in tissue or the total radioligand in plasma, respectively. These other measures require more invasive procedures, such as arterial blood monitoring, to estimate the binding potential. While nomenclature can vary, these are the simplest definitions of the binding potential measures.
In addition to measuring receptor binding potential, the stimulant “challenge” method can also be used to study changes in dopamine transmission (see Fig. 1.1 ). This technique uses a PET radioligand that images the D2 receptor and scans are obtained before and after a stimulant, such as amphetamine or methylphenidate. Since the stimulant increases endogenous dopamine in the brain, there are fewer D2 receptors available to bind to the radioligand. This results in a decrease in BP, with a greater decrease in BP representing increased levels of endogenous dopamine release. This decrease is likely from internalization of the D2 receptors rather than direct competition of dopamine and the radiotracer in the poststimulant scan.
![FIG. 1.1, Representative PET scan showing the decrease in D2 receptor binding following a stimulant challenge with methylphenidate 60mg PO. Methylphenidate administration decreases the amount of D2 receptors available to bind to [11C]raclopride by increasing extracellular dopamine. Values for the binding potential are shown in the color bar. FIG. 1.1, Representative PET scan showing the decrease in D2 receptor binding following a stimulant challenge with methylphenidate 60mg PO. Methylphenidate administration decreases the amount of D2 receptors available to bind to [11C]raclopride by increasing extracellular dopamine. Values for the binding potential are shown in the color bar.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/NeurochemicalImaginginAddictionHowScienceInformsPractice/0_3s20B9780323548564000018.jpg)
Thus, the use of PET imaging can provide the following information on dopamine transmission: (1) baseline binding of the D2 receptor in the human brain; and (2) dopamine release from the presynaptic neurons in response to a pharmacologic challenge with a stimulant drug.
Neurochemical imaging has been used to investigate changes in dopamine receptors (D1 and D2) as well as presynaptic dopamine release in cocaine use disorder. With chronic cocaine use, there have been consistent findings of dysfunctional dopamine signaling along multiple parts of the synapse. Fig. 1.2 illustrates the components of the synaptic cleft of a dopamine neuron.
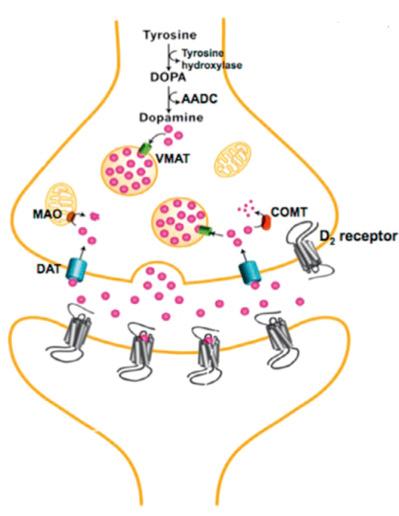
Numerous PET imaging studies have shown that DSM-IV cocaine dependence is associated with a decrease in D2 receptor binding. Volkow et al. first used [ 18 F]N-methylspiroperidol to show decreased D2 receptor binding potential in the striatum, which has been repeatedly replicated with similar findings. This decrease has been correlated with decreased cerebral glucose metabolism in the orbitofrontal cortex and cingulate measured with [ 18 F]fludeoxyglucose, suggesting associated dysregulation of inhibitory control and decision-making leading to compulsive drug taking. When the striatal substructures are differentiated into the caudate, putamen, and ventral striatum, low D2 receptor binding potential is seen across each subdivision. These findings have been found to persist after 3 months of abstinence and studies in nonhuman primates suggests that D2 receptor binding may not return to precocaine use levels until after 1 year. Additionally, imaging studies in nonhuman primates have demonstrated that this decrease is only seen in chronic and not short-term cocaine exposure.
Changes in the dopamine D1 receptor family have also been implicated in cocaine addiction. One study using [ 11 C]NNC-112 showed no difference in D1 receptor binding in persons with DSM-IV cocaine dependence compared to healthy controls. However, this same study also found that low D1 binding in the ventral striatum predicted increased cocaine self-administration, suggesting that this receptor may be associated with an increased risk of relapse.
In addition to decreased D2 receptor binding potential, several studies using the stimulant challenge method have found decreased striatal dopamine release in cocaine-dependent subjects compared to healthy controls. While the euphoric effects of stimulants are positively associated with dopamine release in healthy controls, cocaine-dependent subjects experience less subjectively positive responses to psychostimulants and there is no association between their subjective response and the amount of dopamine release. PET scans with 6-[ 18 F]-flouro- l -DOPA (FDOPA), a radiolabeled precursor to dopamine, have also been used to measure presynaptic dopamine activity. Subjects with DSM-IV cocaine dependence who were abstinent for 11–30 days had decreased FDOPA uptake when compared to healthy controls, suggesting that there is decreased dopamine synthesis during this abstinence period.
Another technique uses alpha-methyl-p-tyrosine (AMPT) to inhibit tyrosine hydroxylase. Tyrosine hydroxylase is an enzyme required for dopamine synthesis and its inhibition results in lowered endogenous dopamine. By performing a PET scan with AMPT induced dopamine depletion after a preliminary [ 11 C]raclopride scan and measuring the difference, studies have shown that endogenous dopamine is reduced in individuals with DSM-IV cocaine dependence.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here