Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Upon completion of this chapter, the student should be able to answer the following questions:
How does bicarbonate (
) operate as a buffer, and why is it an important buffer of the extracellular fluid?
How does metabolism of food produce acid and alkali, and what effect does the composition of the diet have on systemic acid-base balance?
What is the difference between volatile and nonvolatile acids, and what is net endogenous acid production (NEAP)?
How do the kidneys and lungs contribute to systemic acid-base balance, and what is renal net acid excretion (RNAE)?
Why are urinary buffers necessary for the excretion of acid by the kidneys?
What are the mechanisms for H + transport in the various segments of the nephron, how are these mechanisms regulated, and how do renal tubular cells sense changes in the acid-base status of the body fluids?
How do the various segments of the nephron contribute to the process of reabsorbing the filtered
?
How do the kidneys produce new
?
How is ammonium produced by the kidneys, and how does its excretion contribute to renal acid excretion?
What are the major mechanisms by which the body defends itself against changes in acid-base balance?
What are the differences between simple metabolic and respiratory acid-base disorders, and how are they differentiated by blood gas measurements?
The concentration of H + in the body fluids is low compared with that of other ions. For example, Na + is present at a concentration some 3 million times greater than that of H + ([Na + ] = 140 mEq/L and [H + ] = 40 nEq/L). Because of the low [H + ] of the body fluids, it is commonly expressed as the negative logarithm, or pH.
Virtually all cellular, tissue, and organ processes are sensitive to pH. Indeed, life cannot exist outside a range of body fluid pH from 6.8 to 7.8 (160 to 16 nEq/L of H + ). Each day, acid and alkali are ingested in the diet. Also, cellular metabolism produces many substances that have an impact on the pH of body fluids. Without appropriate mechanisms to deal with this daily acid and alkali load and thereby maintain acid-base balance, many processes necessary for life could not occur. This chapter reviews the maintenance of whole-body acid-base balance. Although the emphasis is on the role of the kidneys in this process, the roles of the lungs and liver also are considered. In addition, the impact of diet and cellular metabolism on acid-base balance is presented. Finally, disorders of acid-base balance are considered, primarily to illustrate the physiologic processes involved. Throughout this chapter, acid is defined as any substance that adds H + to the body fluids, whereas alkali is defined as a substance that removes H + from the body fluids.
Bicarbonate (
) is an important buffer of the extracellular fluid (ECF). With a normal plasma [
] of 23 to 25 mEq/L and a volume of 14 L (for a person weighing 70 kg), the ECF potentially can buffer 350 mEq of H + . The
buffer system differs from the other buffer systems of the body (e.g., phosphate) because it is regulated by both the lungs and the kidneys. This situation is best appreciated by considering the following reaction.
As indicated, the first reaction (hydration/dehydration of CO 2 ) is the rate-limiting step. This normally slow reaction is greatly accelerated in the presence of carbonic anhydrase (CA) . a
a Carbonic anhydrase actually catalyzes the following reaction: H 2 O→H + +OH − +CO 2 →HCO 3 − +H + →H 2 CO 3
The second reaction, the ionization of carbonic acid (H 2 CO 3 ) to H + and
, is virtually instantaneous.
The Henderson-Hasselbalch equation is used to quantitate how changes in CO 2 and
affect pH:
or
In these equations the amount of CO 2 is determined from the partial pressure of CO 2 (P co 2 ) and its solubility (α). For plasma at 37°C, α has a value of 0.03. Also, pKˈ is the negative logarithm of the overall dissociation constant for the reaction in Eq. 8.1 and has a value for plasma at 37°C of 6.1. Alternatively, the relationships among
, CO 2 , and [H + ] can be determined as follows:
Inspection of Eqs. 8.3 and 8.4 reveals that the pH and the [H + ] vary when either the [
] or the P co 2 is altered. Disturbances of acid-base balance that result from a change in the [
] are termed metabolic acid-base disorders, whereas those that result from a change in the P co 2 are termed respiratory acid-base disorders. These disorders are considered in more detail in a subsequent section. The kidneys are primarily responsible for regulating the [
] of the ECF, whereas the lungs control the P co 2 .
The diet of humans contains many constituents that are either acid or alkali. In addition, cellular metabolism produces acid and alkali. Finally, alkali is normally lost each day in the feces. As described later in this chapter, the net effect of these processes is the addition of acid to the body fluids. For acid-base balance to be maintained, acid must be excreted from the body at a rate equivalent to its addition. If acid addition exceeds excretion, acidosis results. Conversely, if acid excretion exceeds addition, alkalosis results.
As summarized in Fig. 8.1 , the major constituents of the diet are carbohydrates and fats. When tissue perfusion is adequate, O 2 is available to tissues, and insulin is present at normal levels, carbohydrates and fats are metabolized to CO 2 and H 2 O. Daily, 15 to 20 moles of CO 2 are generated through this process. Normally this large quantity of CO 2 is effectively eliminated from the body by the lungs. Therefore this metabolically derived CO 2 has no impact on acid-base balance. CO 2 usually is termed volatile acid because it has the potential to generate H + after hydration with H 2 O (see Eq. 8.1 ). Acid not derived directly from the hydration of CO 2 is termed nonvolatile acid (e.g., lactic acid).
![Fig. 8.1, Overview of the acid-base balance. The lungs and kidneys work together to maintain acid-base balance. The lungs excrete CO 2 (volatile acid), and kidneys excrete acid (renal net acid excretion [RNAE] ). HA represents nonvolatile acids and reflects dietary intake, cellular metabolism, and loss of acid and alkali (e.g., HCO−3 HCO3− loss in the feces). The amount of nonvolatile acids produced by these process is referred to as net endogenous acid production (NEAP) . To maintain acid-base balance RNAE must equal NEAP. See the text for details. HA, nonvolatile acid, NaA, Na + salt of the acid. Fig. 8.1, Overview of the acid-base balance. The lungs and kidneys work together to maintain acid-base balance. The lungs excrete CO 2 (volatile acid), and kidneys excrete acid (renal net acid excretion [RNAE] ). HA represents nonvolatile acids and reflects dietary intake, cellular metabolism, and loss of acid and alkali (e.g., HCO−3 HCO3− loss in the feces). The amount of nonvolatile acids produced by these process is referred to as net endogenous acid production (NEAP) . To maintain acid-base balance RNAE must equal NEAP. See the text for details. HA, nonvolatile acid, NaA, Na + salt of the acid.](https://storage.googleapis.com/dl.dentistrykey.com/clinical/RegulationofAcidBaseBalance/0_3s20B9780323595681000081.jpg)
The cellular metabolism of other dietary constituents also has an impact on acid-base balance (see Fig. 8.1 ). For example, cysteine and methionine, which are sulfur-containing amino acids, yield sulfuric acid when metabolized, whereas hydrochloric acid results from the metabolism of lysine, arginine, and histidine. A portion of this nonvolatile acid load is offset by the production of
through the metabolism of the amino acids aspartate and glutamate. On average the metabolism of dietary amino acids yields net nonvolatile acid production. The metabolism of certain organic anions (e.g., citrate) results in the production of
, which offsets nonvolatile acid production to some degree. Overall, in persons who ingest a diet containing meat, acid production exceeds
production. In addition to the metabolically derived acids and alkalis, the foods ingested contain acid and alkali. For example, the presence of phosphate (
) in ingested food increases the dietary acid load. Finally, some
is normally lost in the feces. This loss is equivalent to the addition of nonvolatile acid to the body. Together, dietary intake, cellular metabolism, and fecal
loss result in the addition of approximately 1 mEq/kg body weight of nonvolatile acid to the body each day (50 to 100 mEq/day for most adults). This acid, referred to as net endogenous acid production (NEAP) , results in an equivalent loss of
from the body that must be replaced.
When insulin levels are normal, carbohydrates and fats are completely metabolized to CO 2 + H 2 O. However, if insulin levels are abnormally low (e.g., in persons with diabetes mellitus ), the metabolism of carbohydrates leads to the production of organic keto acids (e.g., β-hydroxybutyric acid).
In the absence of adequate levels of O 2 ( hypoxia ), anaerobic metabolism by cells also can lead to the production of organic acids (e.g., lactic acid) rather than CO 2 + H 2 O. This phenomenon often occurs in healthy persons during vigorous exercise. Poor tissue perfusion, such as occurs with reduced cardiac output, also can lead to anaerobic metabolism by cells and thus to acidosis. In these conditions the organic acids accumulate and the pH of the body fluids decreases (acidosis). Treatment (e.g., administration of insulin in the case of diabetes) or improved delivery of adequate levels of O 2 to the tissues (e.g., in the case of poor tissue perfusion) results in the metabolism of these organic acids to CO 2 + H 2 O, which consumes H + and thereby helps correct the acid-base disorder.
Nonvolatile acids do not circulate throughout the body but are immediately neutralized by the
in the ECF:
This neutralization process yields the Na + salts of the strong acids and removes
from the ECF. Thus
minimizes the effect of these strong acids on the pH of the ECF. As noted previously, the ECF contains approximately 350 mEq of
. If this
was not replenished, the daily production of nonvolatile acids (≈70 mEq/day) would deplete the ECF of
within 5 days. To maintain acid-base balance the kidneys must replenish the
lost by neutralization of the nonvolatile acids, a process termed renal net acid excretion (RNAE) .
Under steady-state conditions, RNAE must equal NEAP to maintain acid-base balance. Although NEAP varies from individual to individual, and from day to day in any one individual, it is not regulated. Instead the kidneys regulate RNAE to match NEAP and in so doing replenish the
(called new
) lost by neutralization of the nonvolatile acids. In addition, the kidneys must prevent the loss of
in the urine. This latter task is quantitatively more important because the filtered amount of
is approximately 4320 mEq/day (24 mEq/L × 180 L/day = 4320 mEq/day), compared with only 50 to 100 mEq/day needed to balance NEAP.
Both the reabsorption of filtered
and the excretion of acid are accomplished primarily by H + secretion by the nephrons. b
b A small amount of HCO 3 − is reabsorbed in the proximal tubule by an apical membrane Na+-HCO 3 − symporter (NBCn2)
Thus in a single day the nephrons must secrete approximately 4390 mEq of H + into the tubular fluid. Most of the secreted H + serves to reabsorb the filtered amount of
. Only 50 to 100 mEq of H + , an amount equivalent to NEAP, is excreted in the urine. As a result of this acid excretion, the urine is normally acidic.
The kidneys cannot excrete urine more acidic than pH 4.0 to 4.5. Even at a pH of 4.0, only 0.1 mEq/L of H + can be excreted. Therefore to excrete sufficient acid, the kidneys excrete H + with urinary buffers such as inorganic phosphate (P i ). c
c The titration reaction is HPO 4 −2 +H + ↔H 2 PO 4 − . This reaction has a pK of approximately 6.8.
Other constituents of the urine also can serve as buffers (e.g., creatinine), although their role is less important than that of P i . Collectively the various urinary buffers are termed titratable acid (TA) . This term is derived from the method by which these buffers are quantitated in the laboratory. Typically alkali (OH − ) is added to a urine sample to titrate its pH to that of plasma (i.e., 7.4). The amount of alkali added is equal to the H + titrated by these urine buffers and thus is termed titratable acid.
The excretion of H + as a titratable acid is insufficient to balance NEAP. An additional and important mechanism by which the kidneys contribute to the maintenance of acid-base balance is through the synthesis and excretion of ammonium (
) . The mechanisms involved in this process are discussed in more detail later in this chapter. With regard to the renal regulation of acid-base balance, each
excreted in the urine results in the return of one
to the systemic circulation, which replenishes the
lost during neutralization of the nonvolatile acids. Thus the production and excretion of
, like the excretion of titratable acid, are equivalent to the excretion of acid by the kidneys.
In brief, the kidneys contribute to acid-base homeostasis by reabsorbing the filtered
and excreting an amount of acid equivalent to NEAP. This overall process is termed RNAE, and it can be quantitated as follows:
where
and
are the rates of excretion (mEq/day) of
and titratable acid and
is the amount of
lost in the urine (equivalent to adding H + to the body). d
d This equation ignores the small amount of free H + excreted in the urine. As already noted, urine with pH=4.0 contains only 0.1 mEq/L of H + .
Again, maintenance of acid-base balance means that RNAE must equal NEAP. Under most conditions, very little
is excreted in the urine. Thus RNAE essentially reflects TA and
excretion. Quantitatively TA accounts for approximately one-third and
for two- thirds of RNAE.
As indicated by Eq. 8.7 , RNAE is maximized when little or no
is excreted in the urine. Indeed, under most circumstances, very little
appears in the urine. Because
is freely filtered at the glomerulus, approximately 4320 mEq/day are delivered to the nephrons and are then reabsorbed. Fig. 8.2 summarizes the contribution of each nephron segment to the reabsorption of the filtered
.
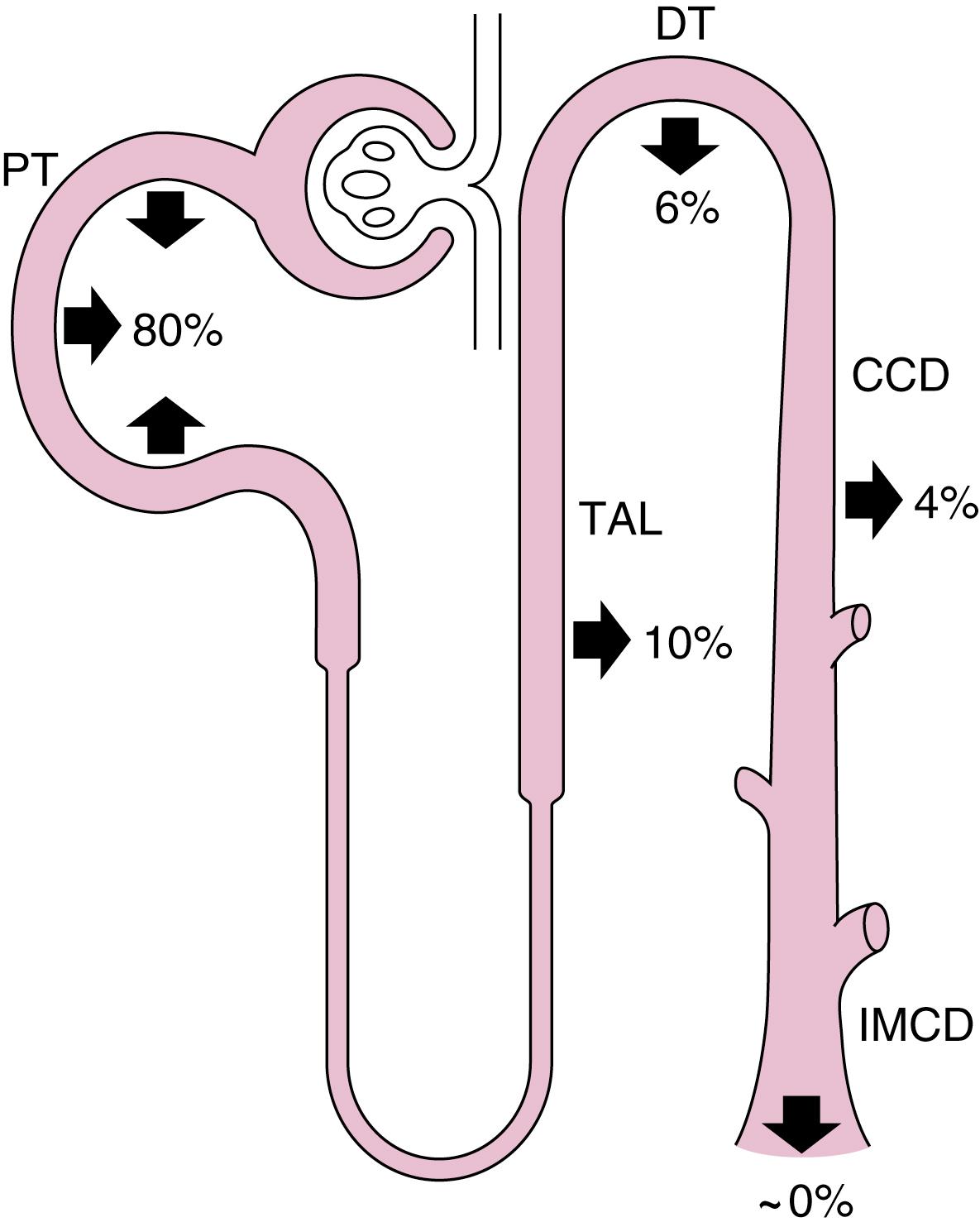
The proximal tubule reabsorbs the largest portion of the filtered
. Fig. 8.3 summarizes the primary transport processes involved. H + secretion across the apical membrane of the cell occurs by both a Na + -H + antiporter and H + –adenosine triphosphatase (V-type H + -ATPase). The Na + -H + antiporter (NHE3) is the predominant pathway for H + secretion (accounts for approximately two- thirds of
reabsorption) and uses the lumen-to-cell [Na + ] gradient to drive this process (i.e., secondary active secretion of H + ). Within the cell, H + and
are produced in a reaction catalyzed by carbonic anhydrase (CA-II). The H + is secreted into the tubular fluid, whereas the
exits the cell across the basolateral membrane and returns to the peritubular blood.
movement out of the cell across the basolateral membrane is coupled to other ions. The majority of
exits through a symporter that couples the efflux of Na + with 3
(sodium bicarbonate cotransporter, NBC1; note: the splice variant in the kidney is NBCe-1A). Some
exits the cell by other transporters, but they are not as important as the Na + -
symporter NBC1. As noted in Fig. 8.3 , carbonic anhydrase (CA-IV) also is present in the brush border and basolateral membrane of the cell. The brush border enzyme catalyzes the dehydration of H 2 CO 3 in the luminal fluid to CO 2 and H 2 O, whereas the enzyme localized to the basolateral membrane facilitates the exit of
from the cell. The movement of CO 2 (and H 2 O) into and out of the cell occurs via aquaporin 1 (AQP1), which is present in both the luminal and basolateral membranes. The net effect of these various transporters is that
disappears from the luminal fluid and
is added to peritubular blood (i.e., net
reabsorption occurs). It has been found that a small amount of
is reabsorbed by an apical membrane Na + -
symporter (NBCn2).

The cellular mechanism for
reabsorption by the thick ascending limb of the loop of Henle is similar to that in the proximal tubule. H + is secreted by an Na + -H + antiporter (NHE3) and a V-type H + -ATPase. As in the proximal tubule, the Na + -H + antiporter is the predominant pathway for H + secretion.
exit from the cell involves both an electroneutral Na + -
symporter (NBCn1, which transports Na + and
in a 1:1 ratio) and a Cl − -
antiporter (anion exchanger, AE2). Some
may also exit the cell through Cl – channels present in the basolateral membrane.
The distal tubule e
e Here and in the remainder of the chapter we focus on the function of intercalated cells. The early portion of distal tubule, which does not contain intercalated cells, also reabsorbs HCO 3 − . The cellular mechanism appears to involve an apical membrane Na + /H + antiporter (NHE2) and a basolateral Cl − /HCO 3 − antiporter (AE2).
and collecting duct reabsorb the small amount of
that escapes reabsorption by the proximal tubule and loop of Henle. Fig. 8.4 shows the cellular mechanism of H + and
transport by intercalated cells in the distal tubule and collecting duct (see also Chapter 2 ).
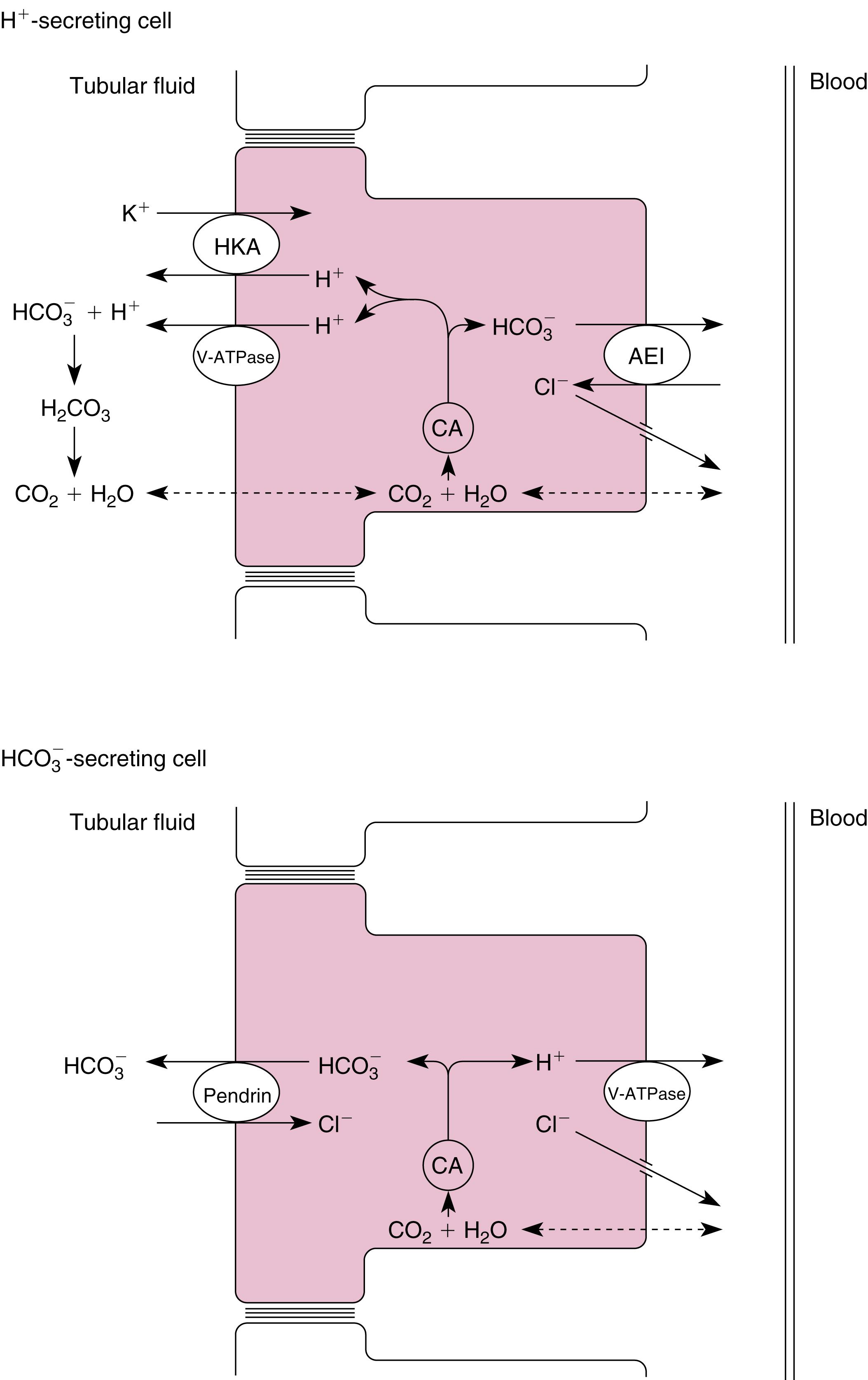
One type of intercalated cell secretes H + (reabsorbs
) and is called the A- or α-intercalated cell . Within this cell, H + and
are produced by the hydration of CO 2 ; this reaction is catalyzed by carbonic anhydrase (CA-II). H + is secreted into the tubular fluid by two mechanisms. The first mechanism involves an apical membrane H + -ATPase (V-type). The second mechanism couples the secretion of H + with the reabsorption of K + via an H + -K + -ATPase similar to that found in the stomach and colon. The
exits the cell across the basolateral membrane in exchange for Cl − (via a Cl − -
antiporter, AE1) and enters the peritubular capillary blood. Cl – exit from the cell across the basolateral membrane occurs via a Cl − channel and via a K + -Cl – symporter (KCC4).
A second population of intercalated cells secretes
rather than H + into the tubular fluid and is called the B- or β-intercalated cell . In these intercalated cells the H + -ATPase (V-type) is located in the basolateral membrane and a Cl − -
antiporter is located in the apical membrane (see Fig. 8.4 ). However, the apical membrane Cl − -
antiporter is different from the one found in the basolateral membrane of the H + -secreting intercalated cell and has been identified as pendrin . The activity of the
-secreting intercalated cell is increased during metabolic alkalosis, when the kidneys must excrete excess
. However, under most conditions, H + secretion predominates in these segment. f
f Traditionally it was believed that intercalated cells were only involved in acid-base transport. However, NaCl reabsorption is also carried out by the B-type intercalated cell. NaCl reabsorption occurs by the tandem operation of the apical membrane Cl −/ HCO 3 − antiporter (pendrin) and an apical membrane Na+/Cl − 2HCO 3 − antiporter (NDCBE). This mechanism of NaCl reabsorption is inhibited by thiazide diuretics (see Chapter 10).
The apical membrane of collecting duct cells is not very permeable to H + , and thus the pH of the tubular fluid can become quite acidic. Indeed, the most acidic tubular fluid along the nephron (pH = 4.0 to 4.5) is produced there. In comparison, the permeability of the proximal tubule to H + and
is much higher, and the tubular fluid pH falls to only 6.5 in this segment. As explained later, the ability of the collecting duct to lower the pH of the tubular fluid is critically important for the excretion of urinary titratable acids and
.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here