Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Chronic kidney disease (CKD) is defined as abnormal measurements of the actual or estimated glomerular filtration rate (GFR) for a minimum of 3 months ( Box 50.1 ), or situations where the GFR is normal but pathology in the kidney is still present, such as radiographically imaged cysts in polycystic kidney disease or isolated proteinuria in early glomerular disease (see Chapter 51). The most commonly reported causes of CKD and end-stage kidney disease (ESKD) ( Table 50.1 ) are diabetic nephropathy ( Fig. 50.1 ) and hypertensive nephrosclerosis, although the diagnosis of “hypertensive nephrosclerosis” has recently been revisited in reference to APOL1 genetic abnormalities (Chapter 36). However, many other conditions can cause CKD, including primary glomerular diseases (e.g., IgA nephropathy, membranous glomerulopathy), secondary glomerular diseases (e.g., lupus nephritis, amyloidosis), and tubulointerstitial, vascular, cystic, and hereditary kidney diseases. Each of these has specific pathophysiologic mechanisms for kidney damage; therefore, the treatments developed for these diseases are unique and aimed at controlling or reversing the underlying disease process.
Chronic kidney disease (CKD) is currently defined by a reduction in glomerular filtration rate over a period of time or evidence of kidney damage.
The most commonly reported causes of CKD are diabetes mellitus and hypertension, and less frequent causes are primary glomerular, tubulointerstitial, and cystic diseases.
The pathophysiology of chronic kidney damage is related to the underlying disease, but it is accelerated by glomerular hypertension, systemic hypertension, inflammation, and fibrosis.
Risk factors for progression are hypertension, proteinuria, and recurrent acute kidney injury.
Treatment for CKD is disease specific, but several generalized methods can be applied to almost all kidney diseases. The goal is slowing or reversing progression with therapies aimed at correcting the pathophysiologic patterns. These involve blocking the renin-angiotensin-aldosterone system (RAAS) with medications, SGLT2 inhibition, controlling blood pressure, and reducing albuminuria when present. This goal is attempted while also targeting cardiovascular risk reduction. Novel methods, which require further study, involve attacking the inflammatory and fibrotic effects of the pathophysiology.
| Disease | Percentage (%) |
| Diabetes mellitus type 1 | 3.9 |
| Diabetes mellitus type 2 | 41.0 |
| Hypertension | 27.2 |
| Primary glomerulonephritis | 8.2 |
| Tubulointerstitial | 3.6 |
| Hereditary or cystic | 3.1 |
| Secondary glomerulonephritis or vasculitis | 2.1 |
| Neoplasm or plasma cell dyscrasias | 2.1 |
| Miscellaneous | 4.6 |
| Unknown | 5.2 |
The idea, then, that CKD could be generalized into one disease process is an oversimplification, because the primary processes causing kidney damage are diverse. However, the pathophysiology of progression of many of these disorders involves similar pathways, and generic treatments aimed at slowing this progression have been applied across a wide variety of kidney diseases effectively and safely. Over the last 20 years, a number of treatments have been developed and proven to delay progression to ESKD. Therefore, early recognition of CKD becomes important to help implement therapy that can alter its trajectory and reduce the associated morbidity and mortality.
The pathophysiology of CKD is complex and in large part dependent on the primary cause. After an acute or chronic insult occurs, such as in diabetic nephropathy or lupus nephritis, many common pathways are activated to perpetuate and exacerbate glomerular and tubulointerstitial injury ( Fig. 50.2 ). These harmful adaptations, occurring because of an initial injury, can be broadly categorized into those that are hemodynamically mediated or those that are nonhemodynamic.
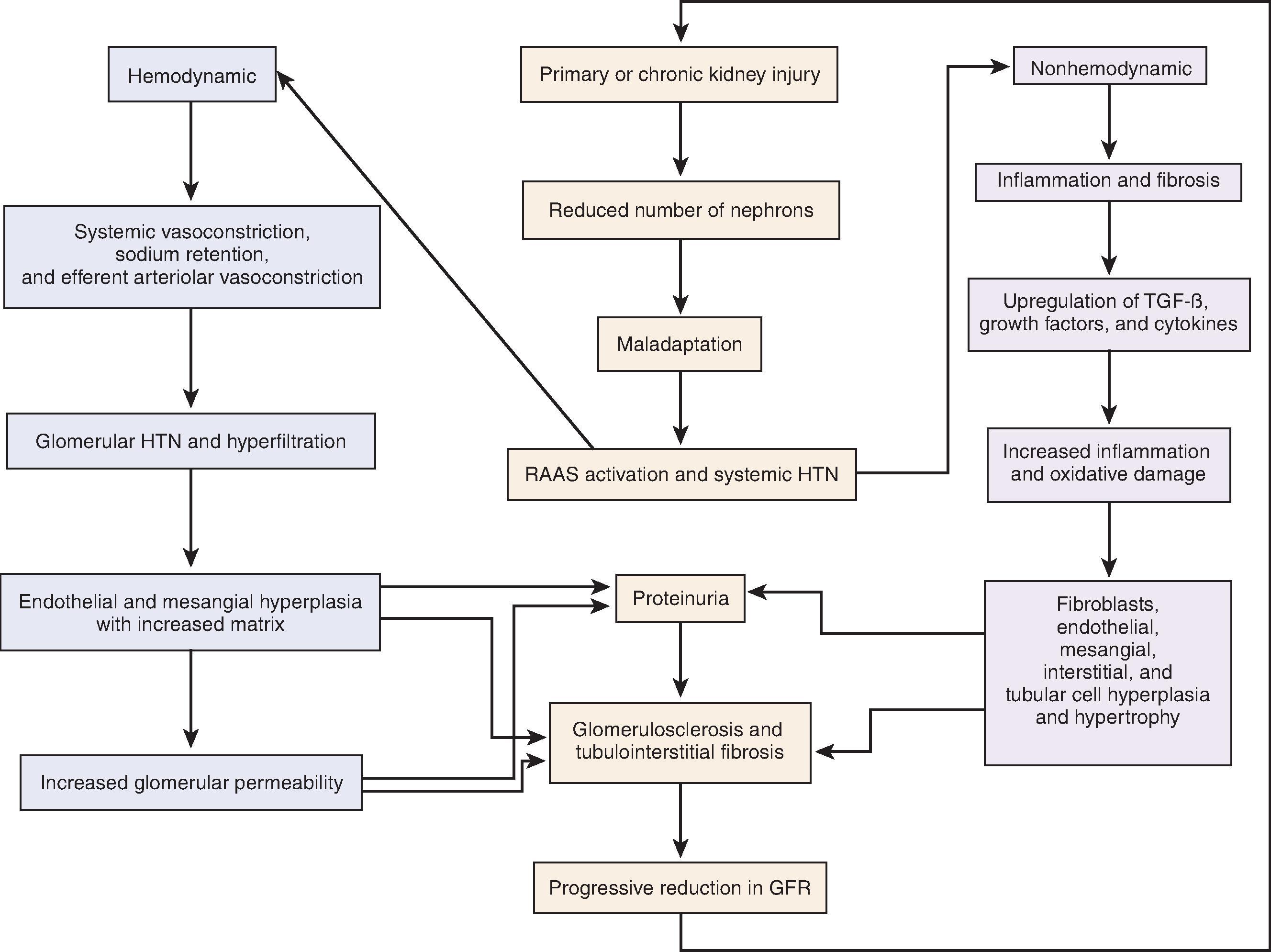
Much of the work in hemodynamic-mediated injury stems from the 5/6 nephrectomy animal model. Following unilateral nephrectomy and 2/3 removal of the contralateral kidney in rats, hypertension, proteinuria, and progressive decline in GFR ensue. Pathologic examination of the remaining tissue exhibits hyperfiltration injury, as evidenced by glomerular hypertrophy and focal segmental glomerular sclerosis (FSGS). The process occurs at a linear rate in proportion to the greater reduction in kidney mass. Micropuncture techniques reveal an increase in renal plasma flow and hyperfiltration of the remaining nephrons. Systemic and glomerular hypertension, from activation of the renin-angiotensin-aldosterone system (RAAS), causes progressive glomerular damage and proteinuria. These changes result as efferent arteriolar tone increases more than afferent tone. This net efferent vasoconstriction increases intraglomerular and filtration pressure further, perpetuating hyperfiltration injury. Animal models of other primary kidney diseases, such as that of diabetic nephropathy in the rat, reveal similar pathophysiologic changes of glomerular hypertension, hypertrophy, and hyperfiltration.
These maladaptive hemodynamic effects are mediated by the RAAS ( Figs. 50.2 and 50.3 ). With nephron loss, adaptation leads to release of renin from the juxtaglomerular apparatus because of decreased perfusion pressure and low solute delivery to the macula densa. Renin converts angiotensinogen to angiotensin I, which is converted to angiotensin II (AII) under the influence of angiotensin-converting enzyme (ACE). AII, in addition to increasing aldosterone production from the adrenal gland, is the main perpetrator of glomerular hemodynamic maladaptation. Through an increase in sympathetic activity, AII is a potent vasoconstrictor, especially predominant in the efferent arterioles. It also exhibits a role in salt and water retention, both directly through proximal tubular sodium reabsorption and indirectly through aldosterone-dependent distal sodium reabsorption. Finally, AII stimulates the posterior pituitary to release antidiuretic hormone (ADH).
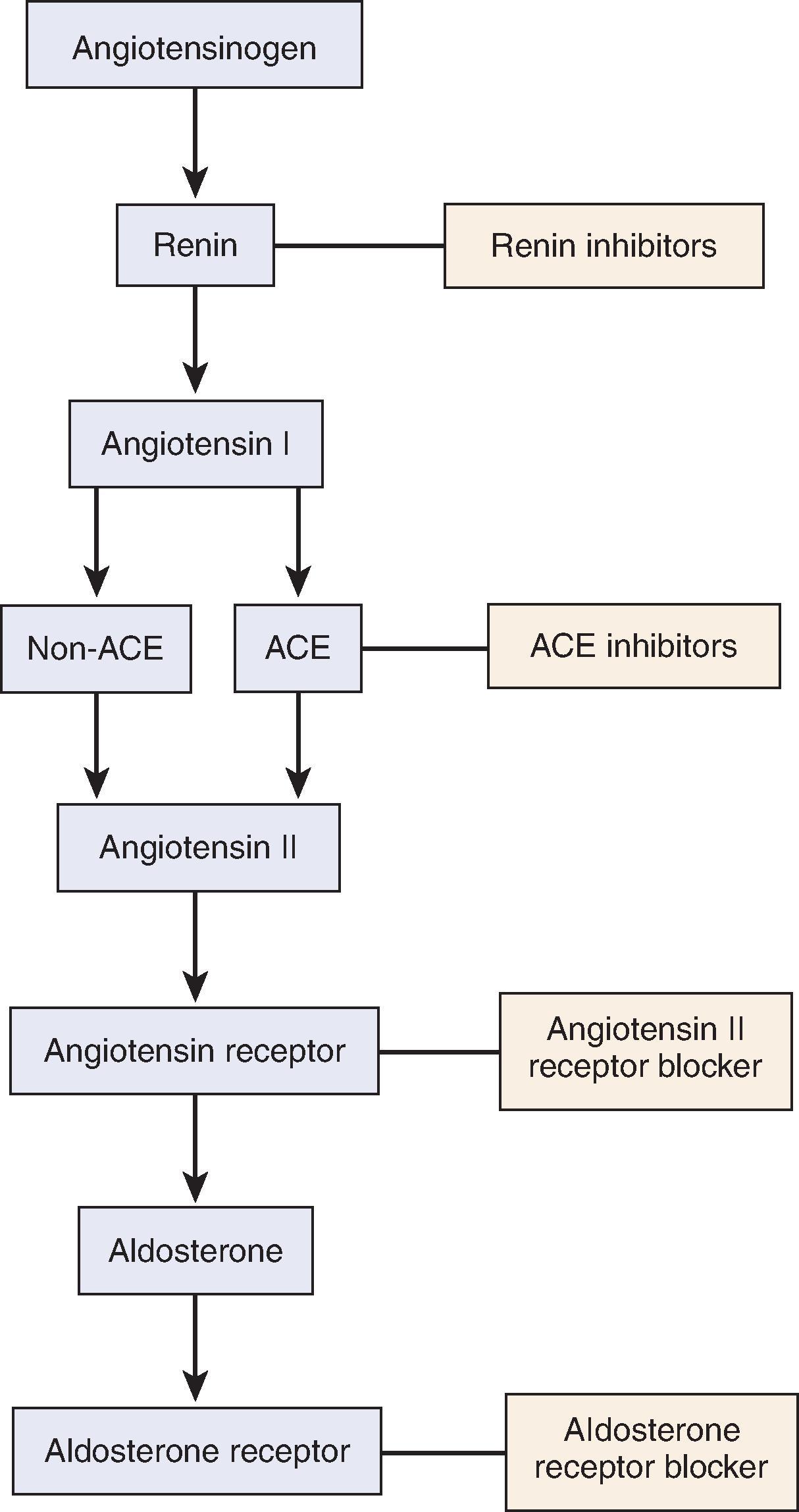
The net effect of all these mechanisms is an integral component of autoregulation, helping to maintain GFR when perfusion is decreased. However, in the setting of nephron loss through a primary kidney insult or CKD, the effect of continuous AII overactivity is perpetual maladaptation by creating systemic and, notably, glomerular hypertension. This glomerular hypertension increases the filtration fraction, increases the radius of the pores in the glomerular basement membrane (GBM) through an increase in hydrostatic pressure, and eventually results in clinical proteinuria and glomerular damage.
The best example of a human model of decreased nephron mass or number is unilateral kidney agenesis. Ashley and Mostofi originally reported 232 patients with unilateral kidney agenesis in the 1960s, and, although the pathology was not described, 16% of the patients died from kidney failure. Later, in the 1980s, autopsy series and case series confirmed the association of unilateral kidney agenesis with hypertension, proteinuria, progressive kidney disease, glomerulomegaly, and FSGS ( Fig. 50.4 ). Besides kidney agenesis, another human example is the condition known as oligomeganephronia . This is a form of congenital kidney hypoplasia in which the number of nephrons is reduced. The glomeruli hypertrophy in compensation for the reduced nephron number. The sequelae of this include hypertension, proteinuria, and FSGS related to hyperfiltration and progressive kidney failure. Other clinical human examples of disease that support this mechanism of kidney injury include obesity-related glomerulomegaly and nephropathy, dysplastic solitary kidney, or partial nephrectomy in the setting of a solitary kidney.
Because animal models and human congenital diseases of reduced nephron mass lead to hemodynamic maladaptation and morphologic evidence of FSGS, it is natural to speculate that a transplant donor would be at risk for this same pathophysiology. Fortunately, the development of hypertension or kidney damage in the remaining kidney in transplant donors is infrequent. This may reflect extensive screening of potential donors, resulting in a sufficiently healthy population with minimal vascular disease, such that the donor can readily compensate for a 50% reduction in kidney mass. Similar results are seen in experimental models where adult rats with unilateral nephrectomy rarely develop hypertension or kidney disease; however, when a single kidney is removed from immature rats, the glomerular lesion FSGS manifests in the remaining kidney. Therefore, hemodynamic injury may be present or clinically apparent only when the kidney is undergoing normal growth. Another explanation of this benign clinical course in patients donating a kidney is that the development of clinical pathology is directly linked to the length of time and degree of reduction of nephron mass. Indeed, there are studies demonstrating an increased risk for hypertension, proteinuria, and progressive kidney disease in patients who have more than a 50% reduction in kidney mass, such as those with bilateral partial nephrectomy for carcinoma, and a greater likelihood of progressive kidney disease with a longer duration of nephron mass reduction.
Besides the hemodynamic effects of systemic vasoconstriction, sodium retention, and efferent arteriolar vasoconstriction, activation of the RAAS leads to several nonhemodynamic maladaptive pathways (see Fig. 50.2 ), which, in turn, results in inflammation and fibrosis. AII has been demonstrated in high concentrations in virtually every compartment of the kidney in CKD, including the mesangial cells, endothelial cells, podocytes, the urinary space (Bowman capsule), and the tubulointerstitium.
Activation of the RAAS eventually results in fibrosis and a progressive decline in GFR. This fibrosis is a consequence of upregulation of several growth factors and their receptors, such as connective tissue growth factor (CTGF), epidermal growth factor (EGF), insulin-like growth factor-1 (IGF-1), platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), transforming growth factor-β (TGF-β), and monocyte chemotactic protein-1 (MCP-1). The activation of these factors by AII and aldosterone leads to cellular proliferation and hypertrophy of glomerular endothelial cells, mesangial cells, podocytes, tubulointerstitial cells, and fibroblasts. AII and TGF-β also upregulate other factors that lead to the overproduction of extracellular matrix, such as type 1 procollagen, plasminogen activator inhibitor 1, and fibronectin. In addition, excess adhesion molecules, such as integrins or vascular cellular adhesion molecule 1, allow the increased extracellular matrix and hypercellularity to accumulate and persist. This leads to cell proliferation, extracellular matrix accumulation, adhesion of these cells, and functional changes with eventual fibrosis ( Fig. 50.5 ).
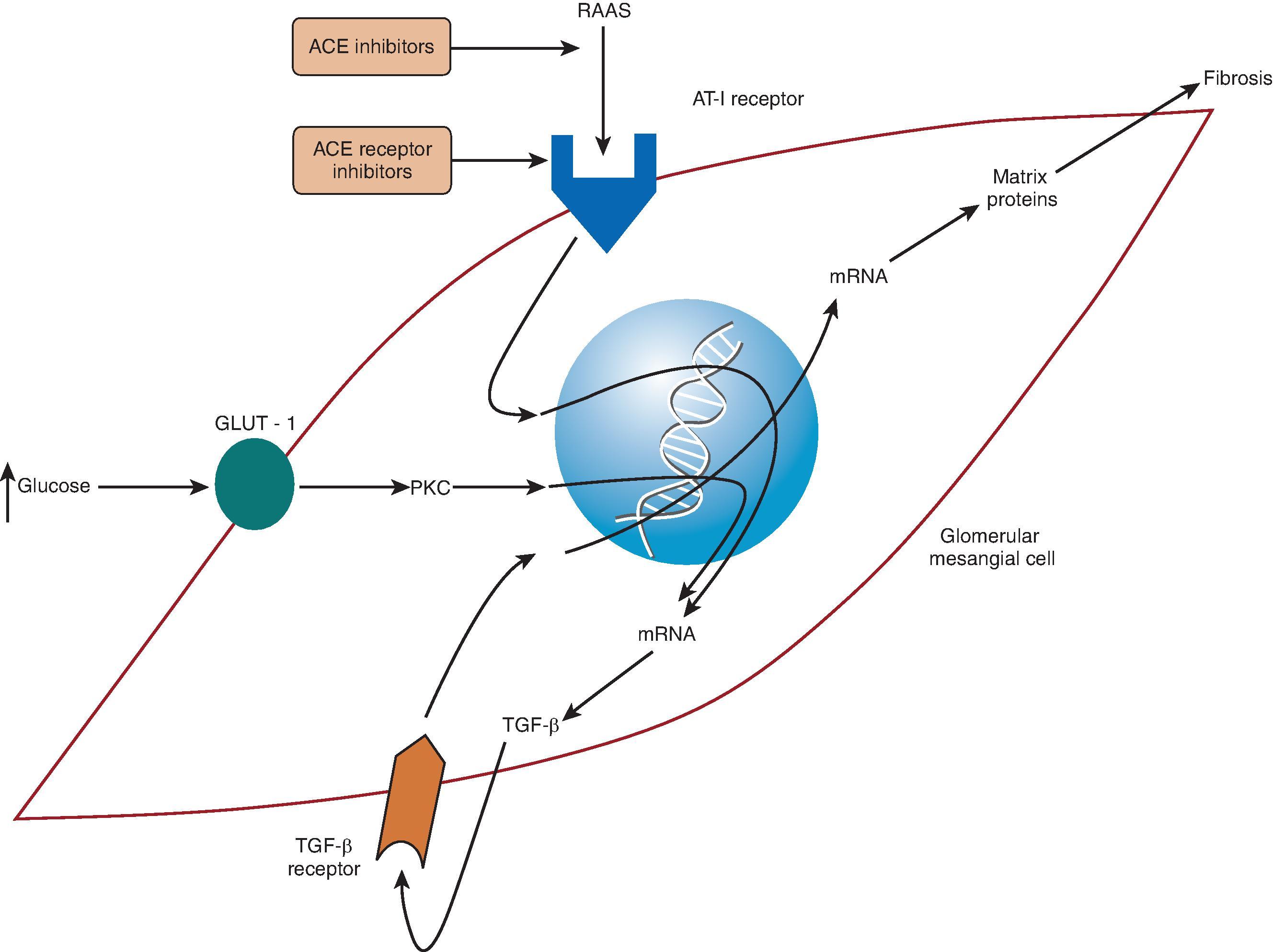
Inflammation is also a key contributor to the progression of kidney disease (see Fig. 50.2 ). This may seem obvious in diseases in which inflammation is the primary insult, such as postinfectious glomerulonephritis or severe lupus nephritis, because it is apparent by light microscopy of kidney biopsy specimens. However, inflammation is an important factor in the progression of almost all types of kidney diseases and is mediated in part by the RAAS. AII recruits T cells and macrophages by stimulating endothelin-1 (ET-1) and increases production of nuclear factor κ–light-chain enhancer of activated B cells (NF-κB); these molecules release cytokines, creating more inflammation. Increased expression of TGF-β also induces cellular recruitment. Finally, free radical oxygen species lead to additional injury, which enables further inflammation and fibrosis.
Experimental evidence also supports the idea that proteinuria itself contributes to progressive nephrosclerosis. Through hyperfiltration, the increased glomerular permeability to albumin allows reabsorption of more albumin by the proximal tubular cells. Experimental models show that when this protein becomes prevalent in the interstitium, macrophages and inflammatory mediators, such as ET-1, MCP-1, and other chemokines, are upregulated, which eventually leads to inflammation and subsequent tubulointerstitial and glomerular fibrosis.
Through primary stimulation of the RAAS, predominantly through TGF-β, a cascade of events occurs that begins with inflammation, is perpetuated by accumulation of cells and matrix, is exacerbated by adhesion and persistence of these cells and matrix, and ends with injury, glomerulosclerosis, and tubulointerstitial fibrosis (see Fig. 50.2 ). This creates a progressive course of CKD, proteinuria, GFR loss, and a vicious cycle of continuous RAAS activation.
Risk factors for progression include nonmodifiable characteristics such as older age, male sex, and Black race. One study of younger patients with CKD estimated the lifetime risk for ESKD for a 20-year-old person to be 7.8% for Black women, 7.3% for Black men, 1.8% for White women, and 2.5% for White men. Conversely, other risk factors such as hypertension, proteinuria, and recurrent acute kidney injury (AKI) are all potentially modifiable and deserve attention ( Box 50.2 ).
Albuminuria
Hypertension
Episodes of acute kidney injury
Underlying cause of kidney disease (e.g., diabetic nephropathy)
Obesity
Hyperlipidemia
Smoking
High-protein diet
Metabolic acidosis
Hyperphosphatemia
Hyperuricemia
Hyperglycemia
Elevated plasma soluble urokinase receptor (suPAR)
APOL1 alleles
Black or Native American race
Male sex
Older age
Family history of diabetes mellitus, chronic kidney disease, or end-stage kidney disease
Low birth weight
With increased activity of the RAAS resulting in vasoconstriction and sodium retention, hypertension perpetuates the cycle of progressive CKD. In patients who have diabetic nephropathy from type 2 diabetes mellitus (DM), elevated blood pressure is the most common cause of CKD and a clear risk factor for a progressive decline in GFR, and, notably, treating this hypertension reduces CKD progression. The effect of therapy is even more pronounced in this patient population when treatment is with RAAS blockade ( Fig. 50.6 ). Early in the 1980s, Mogensen established that in patients with diabetic nephropathy, treating elevated blood pressure (mean 162/103 mm Hg) to an achieved level of 144/95 mm Hg reduced the rate of GFR loss from 1.23 mL/min per month to 0.49 mL/min per month. Since that time, other observational studies and well-designed clinical trials have demonstrated that hypertension is clearly a risk factor for ESKD in diabetic nephropathy, and that blood pressure reduction, especially with RAAS blockade, attenuates this risk.
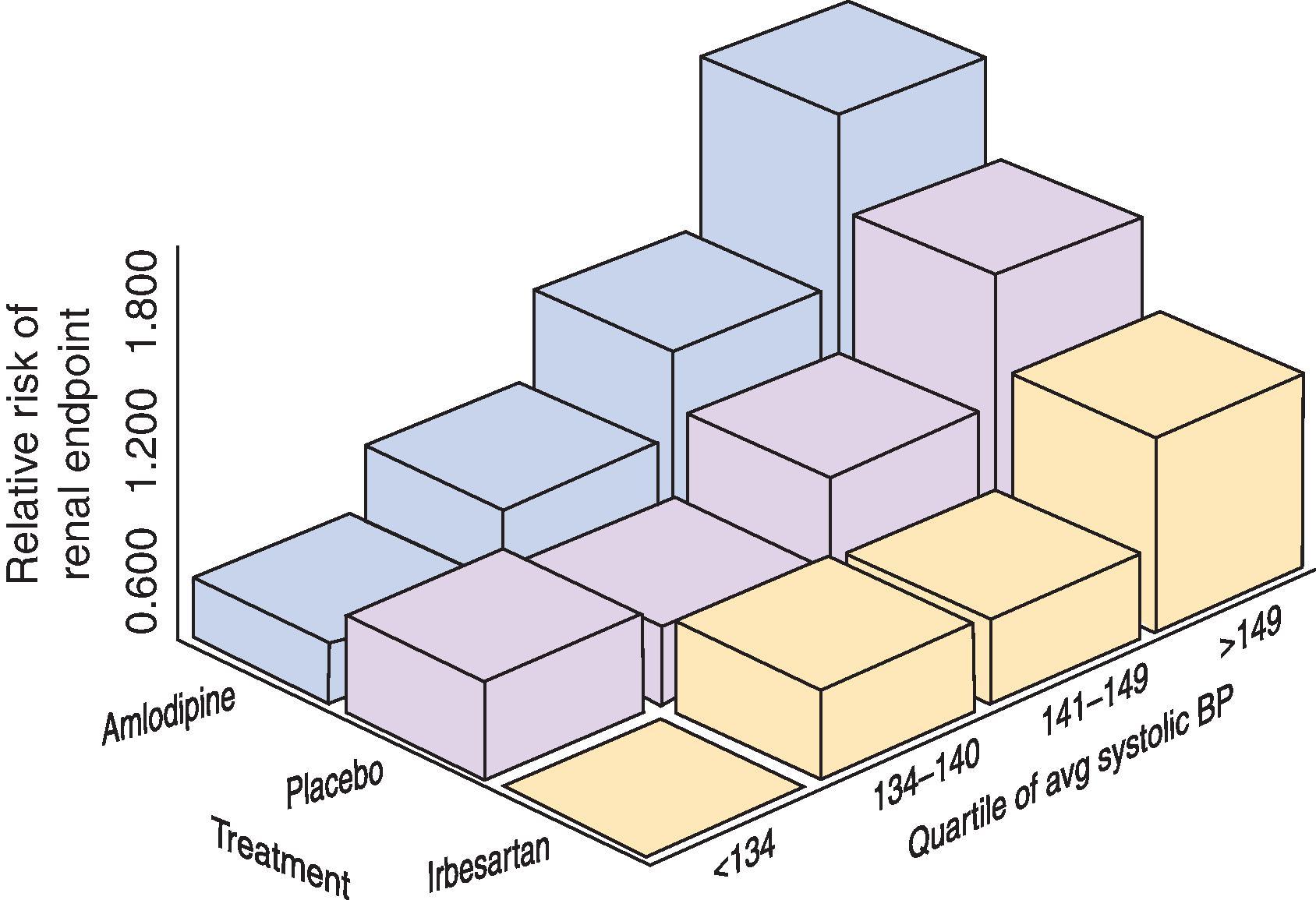
Hypertension is also an established risk factor for progression of nondiabetic kidney disease. The Multiple Risk Factor Intervention Trial (MRFIT), which used various interventions to treat patients with multiple cardiovascular risk factors, such as smoking, hypertension, obesity, and hyperlipidemia, demonstrated that elevated blood pressure was an independent risk factor for the development of kidney failure. Other studies of nondiabetic kidney disease, such as the African American Study of Kidney Disease (AASK), reveal a similar pattern, indicating that patients with nondiabetic CKD benefited from a lower achieved blood pressure, especially if proteinuria was present. Similarly, observational studies suggest slower progression in patients with other causes of CKD, such as polycystic kidney disease, when blood pressure is controlled.
Albuminuria is another well-established risk factor for progression of CKD. In patients with overt diabetic nephropathy, the degree of baseline albuminuria is directly correlated with a more rapid decline in GFR ( Fig. 50.7 ). This is also true in nondiabetic kidney diseases, such as IgA nephropathy or lupus nephritis. In the predominantly nondiabetic patient population recruited for the Modification of Diet in Renal Disease (MDRD) study, those with albuminuria had the highest risk for progressive kidney disease. The Ramipril Efficacy in Nephropathy (REIN) study similarly noted that, in nondiabetic CKD, the baseline level of albuminuria was the strongest predictor of kidney failure, independent of the baseline GFR. Even in patients with earlier stages of CKD, with an estimated GFR of greater than 60 mL/min/1.73 m 2 , those who demonstrate albuminuria by ≥2 + on dipstick or greater than 300 mg albumin/g creatinine are over 3 times more likely to double the serum creatinine over time compared with those with lower levels (30 to 300 mg albumin/g creatinine). This trend is also true when comparing patients who have 30 to 300 mg albumin/g with those who have less than 30 mg/g.
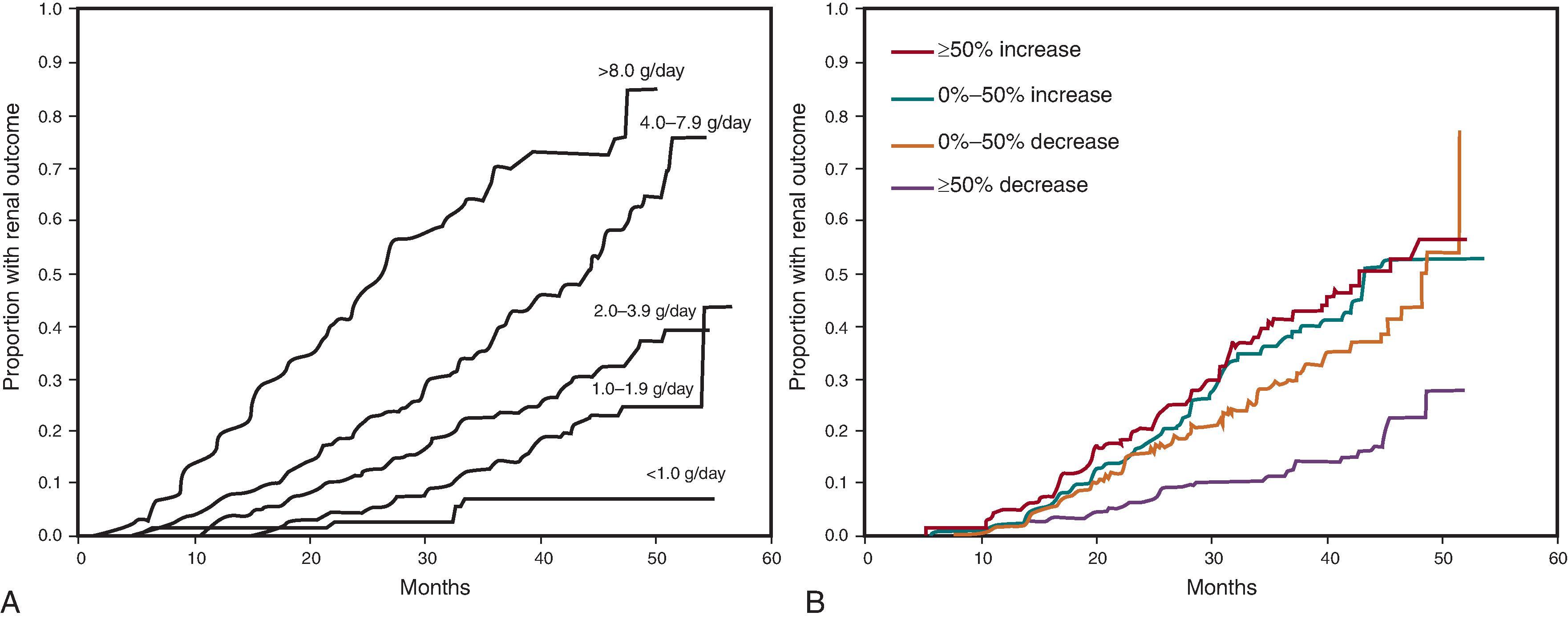
Despite these robust data, controversy exists as to whether albuminuria is truly a pathogenic risk factor or just a marker of kidney disease severity. If albuminuria were a simple manifestation of advanced disease, such as a cough in the setting of pneumonia, then treating the symptom would have minimal to no effect on improving the disease outcome. However, targeting and reducing albuminuria is highly effective at improving kidney outcomes, both in diabetic (see Fig. 50.7 ) and nondiabetic kidney diseases.
The idea that recurrent or episodic AKI leads to progressive chronic kidney dysfunction is based on the pathophysiology of the disease (see Fig. 50.2 ). Multiple studies have now shown that in patients with preexisting CKD, AKI is a risk factor for the development of ESKD. The degree of preexisting CKD, severity of AKI, advanced age, presence of DM, and low serum albumin amplify this risk. In a retrospective study by Ishani et al., the hazard ratio of developing ESKD for older adult patients (>67 years) who had CKD without AKI was 8.4, whereas for those patients who had CKD and AKI, the hazard ratio for progressing to ESKD was 41.2.
Knowledge that AKI affects CKD is important so that future therapies can be developed and evaluated in an attempt to retard this progression. It is also relevant as minimizing episodes of iatrogenic AKI in CKD patients can often be achieved. It is sensible to avoid, if possible, situations that may cause AKI, such as iatrogenic hypotension or nephrotoxic injury from polypharmacy, iodinated contrast exposure, atheroemboli, and nonsteroidal antiinflammatory agents in vulnerable patients.
Many other factors have been associated with a progressive decline in GFR (see Box 50.2 ). In later stages of CKD, the kidney loses the ability to handle the daily dietary acid load, and metabolic acidosis can occur. Its manifestations include accelerated bone disease, sarcopenia, and faster CKD progression. Multiple randomized, controlled trials and a recent meta-analysis suggest that treatment of metabolic acidosis may slow CKD progression, and new therapies to treat acidosis are on the horizon. Finally, the underlying kidney disease affects the rate of progression, as glomerular diseases and polycystic kidney diseases tend to progress faster than most tubulointerstitial diseases. Evidence exists for the APOL1 genetic mutations as not only a cause of CKD, but also a factor for progression. Elevated levels of the soluble urokinase-type plasminogen activator receptor (suPAR) have also been linked to the development and progression of CKD. Although evidence establishing hyperlipidemia, tobacco dependence, or obesity as risk factors is not as robust as that pointing to hypertension or proteinuria, these associations do exist and targeting these risk factors when present is prudent.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here