Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Excretory function of the kidney occurs by glomerular filtration of plasma followed by selective tubular reabsorption or secretion of water and solutes to maintain homeostasis. Because glomerular filtration rate (GFR) is generally considered the best overall assessment of kidney function, this chapter focuses on GFR and its assessment, with other functions of the kidney reviewed elsewhere in the Primer .
GFR is the product of the average filtration rate of each single nephron (the filtering unit of the kidneys) multiplied by the number of nephrons in both kidneys. The normal GFR level varies considerably according to age, sex, body size, physical activity, diet, pharmacologic therapy, and physiologic states such as pregnancy. For GFR to be standardized for differences in kidney size (kidney size is proportional to body size), GFR is typically indexed for body surface area, which is computed from height and weight, and then expressed per 1.73 m 2 surface area, which was the mean body surface area of young men and women at the time indexing was first proposed. Normal average GFR values are approximately 130 and 120 mL/min/1.73 m 2 for young men and women, respectively.
Reductions in GFR can be due to a decline in the nephron number or a decline in the average single-nephron GFR resulting from physiologic or hemodynamic alterations. However, a rise in single-nephron GFR due to increased filtration pressure (e.g., increased glomerular capillary pressure) or surface area (e.g., glomerular hypertrophy) can compensate for decreases in nephron number; therefore, the level of GFR may not reflect the loss of nephrons. As a result, there may be substantial kidney damage before GFR decreases.
GFR cannot be measured directly in humans; thus, “true” GFR cannot be known with certainty. However, GFR can be assessed from clearance measurements (measured GFR [mGFR]) or serum levels of endogenous filtration markers (estimated GFR [eGFR]).
Classically, “measured” GFR is determined from the urinary clearance of an “ideal” filtration marker (inulin). Urinary clearance is calculated as the product of the urinary flow rate (V) and the urinary concentration (U x ) divided by the average plasma concentration (P x ) during the clearance period. Urinary excretion of a substance depends on filtration, tubular secretion, and tubular reabsorption. Substances that are filtered but neither secreted nor reabsorbed by the tubules are ideal filtration markers because their urinary clearance equals GFR. Alternative exogenous filtration markers include iothalamate, iohexol, ethylenediaminetetraacetic acid, and diethylenetriaminepentaacetic acid, which are often chelated to radioisotopes for ease of detection but may differ in their renal handling from inulin. Urinary clearance requires a timed urine collection for measurement of urine volume, and special care must be taken to avoid incomplete urine collections, which will limit the accuracy of the clearance calculation. Plasma clearance is an alternative method to measure GFR and has the advantage of avoiding the need for a timed urine collection but is also affected by extrarenal elimination. All these considerations mean that measured GFR may differ from true GFR.
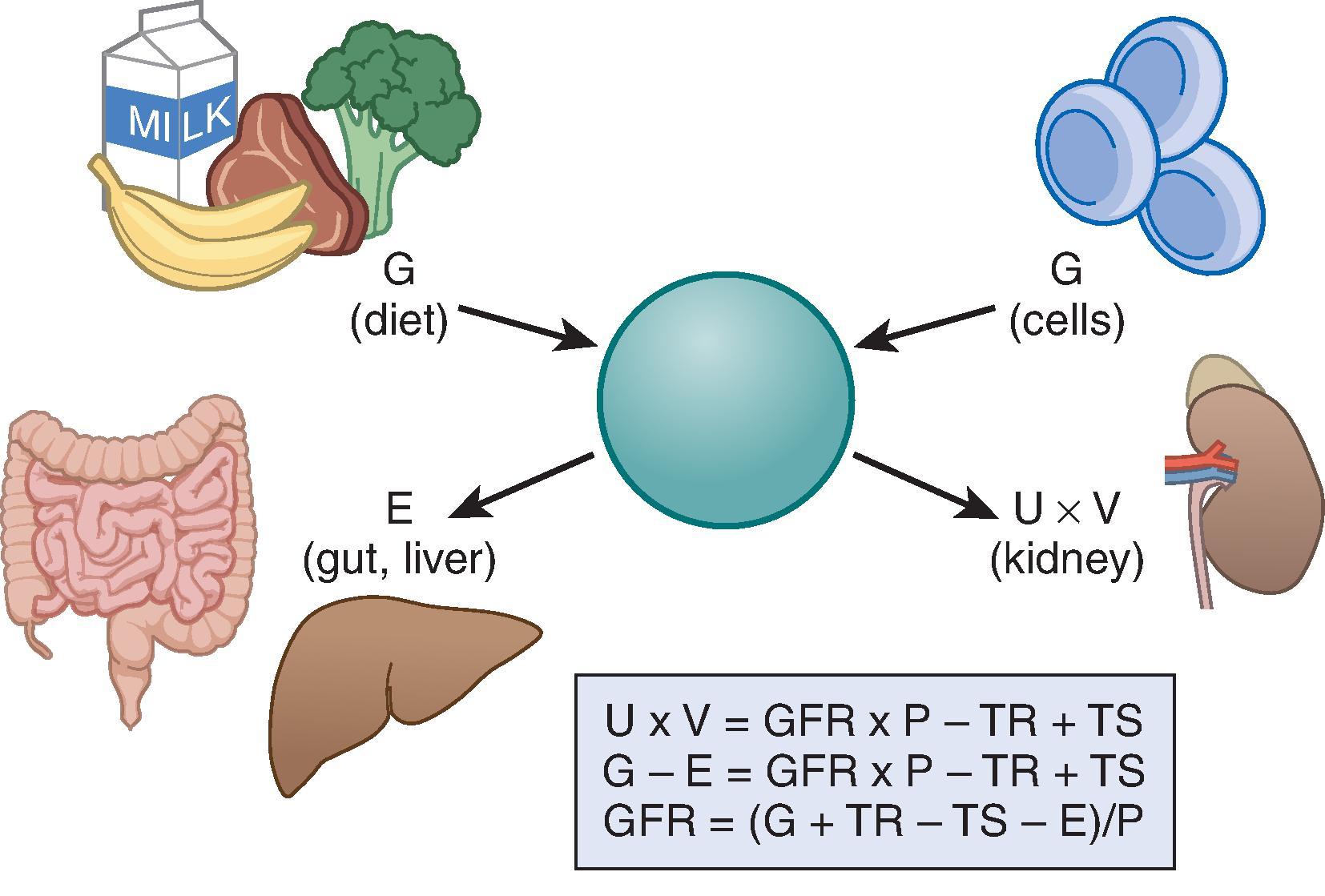
Because of the difficulties with measurement, GFR is often estimated with serum level endogenous filtration markers. For markers that are freely filtered, the plasma level is related to the reciprocal of the level of GFR, but the plasma level of many filtration markers is also influenced by generation, tubular secretion and reabsorption, and extrarenal elimination; these are collectively termed non-GFR determinants of the plasma concentration ( Fig. 3.1 ). In the steady state, a constant plasma level is maintained because generation is equal to urinary excretion and extrarenal elimination. Estimating equations incorporate demographic and clinical variables as surrogates for the non-GFR determinants and provide a more accurate estimate of GFR than the reciprocal of the plasma concentration alone. Estimated GFR may differ from measured GFR if GFR is in the nonsteady state or if there is a discrepancy between the true and average value for the relationship of the surrogate to the non-GFR determinants of the filtration marker. Other sources of error include measurement error in the endogenous filtration marker (including failure to calibrate the assay for the filtration marker to the assay used in the development of the equation) or measurement error in GFR in developing the equation. In principle, the magnitude of all these errors is likely greater at higher measured GFR, although such errors may be more clinically significant at lower measured GFR.
Creatinine is the most commonly used endogenous filtration marker in clinical practice, and cystatin C is now recommended as a confirmatory test and shows promise for wider use. In the past, urea was widely used. The concepts discussed later are relevant for children and adults; however, the specifics of the following discussion focus on estimating GFR in adults. Table 3.1 includes the two most commonly used GFR estimating equations for children, as well as recently developed equations that perform well in young adults (CKiD U25).
| Creatinine-Based Equations |
|---|
| Cockcroft-Gault Formula |
| C cr (mL/min) = (140 – age) × weight/72 × Scr × 0.85 [if female] |
| MDRD Study Equation for Use With Standardized Serum Creatinine (Four-Variable Equation) |
| GFR (mL/min/1.73 m 2 ) = 175 × S Cr −1.154 × age −0.203 × 0.742 [if female] × 1.210 [if Black] |
| 2009 CKD-EPI Equation for Use With Standardized Serum Creatinine |
| GFR (mL/min/1.73 m 2 ) = 141 × min (Scr/κ, 1)α × max(Scr/κ, 1) 1.209 × 0.993 Age × 1.018 [if female] × 1.157 [if Black] where κ is 0.7 for females and 0.9 for males, α is −0.329 for females and −0.411 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1 |
| 2021 CKD-EPI Equation for Use With Standardized Serum Creatinine |
| GFR (mL/min/1.73 m 2 ) = 142 x min (Scr/κ,1) α x max(Scr/κ,1) −1.200 x 0.9938 age x 1.012 [if female] where κ is 0.7 for females and 0.9 for males, α is -0.241 for females and -0.302 for males, min indicates the minimum of Scr/κ or 1, max indicates the maximum of Scr/κ or 1 |
| Schwartz Formula (Younger Than 18 Years of Age) |
| GFR = 0.413 × ht/Scr GFR = 40.7 × [HT/Scr] 0.640 × [30/BUN] 0.202 |
| CKiD U25 Creatinine Equation (Pediatric and Young Adults to Age 25) |
| GFR = K × height/Scr where K for males 1–11 y, 39 × 1.008 (age − 12) ; 12–17 y, 39 × 1.045 (age − 12) ; 18–25 y, 50.8; and K for females: 1–11 y, 36.1 × 1.008 (age − 12) ; 12–17 y, 39 × 1.023 (age − 12) ; 18–25 y, 41.4 |
| Cystatin C-Based Equations |
| CKiD U25 Cystatin C Equation ( Pediatric and Young Adults to Age 25 ) |
| GFR = K × 1/Scys where K for males 1–14 y, 87.2 × 1.011 (age − 15) ; 15–17 y, 87.2 × 0.960 (age − 15) ; 18–25 y, 77.1; and K for females: 1–11 y, 79.9 × 1.004 (age − 12) ; 12–17 y, 79.9 × 0.974 (age − 12) ; 18–25 y, 68.3 |
| 2012 CKD-EPI Cystatin C Equation |
| 133 × min (Scys/0.8, 1) −0.499 × max (Scys/0.8, 1) −1.328 × 0.996 Age × 0.932 [if female] where Scys is serum cystatin C, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1 |
| Creatinine-Cystatin C-Based Equations |
| 2012 CKD-EPI Creatinine-Cystatin C Equation |
| 135 × min(Scr/κ, 1)α × max (Scr/κ, 1) −0.601 × min (Scys/0.8, 1) −0.375 × max (Scys/0.8, 1) −0.711 × 0.995 Age × 0.969 [if female] × 1.08 [if black] where Scr is serum creatinine, Scys is serum cystatin C, κ is 0.7 for females and 0.9 for males, α is −0.248 for females and −0.207 for males, min indicates the minimum of Scr/κ or 1, and max indicates the maximum of Scr/κ or 1 |
| 2021 CKD-EPI Creatinine-Cystatin C Equation |
| GFR (mL/min/1.73 m 2 ) = 135 x min (Scr/k,1) α × max (Scr/k,1) −0.544 × min (Scys/0.8,1) −0.323 × max (Scys/0.8,1) −0.778 × 0.9961 age × 0.963 [if female] where k is 0.7 for females and 0.9 for males, α is -0.219 for females and -0.144 for males, min indicates the minimum of Scr/κ or 1, max indicates the maximum of Scr/κ or 1 |
| Schwartz Formula (Younger Than 18 Years of Age) |
| 39.1 × (HT/Scr) 0.516 × (1.8/cysC) 0.294 × (30/BUN) 0.169 × (HT/1.4) 0.188 × 1.099 [if male] |
Creatinine, an end product of muscle catabolism, has a molecular mass of 113 Da. It is derived by the metabolism of phosphocreatine in muscle and distributed throughout extracellular fluid. Generation can be increased by creatine intake in meat or dietary supplements. Advantages of creatinine are that it is freely filtered and is easily measured at low cost. The main disadvantage of creatinine is the large number of factors other than GFR that may affect its serum level (termed non-GFR determinants ), meaning that a given serum creatinine level may correspond to a wide range of true GFRs (see Fig. 3.1 and Table 3.2 ). The effect of tubular secretion and extrarenal elimination on the serum level of creatinine is greater in patients with reduced GFR. Clinically, it can be difficult to distinguish a rise in serum creatinine due to inhibition of creatinine secretion or extrarenal elimination from a decline in GFR, but these causes should be suspected if the serum level of other filtration markers remains unchanged.
| Primary Use | eGFRcr | eGFRcys |
| Initial Test for Assessment of GFR | Confirmatory Test for Assessment of GFR | |
| Nonsteady state (AKI) | Change in eGFR lags behind the change in mGFR (eGFR overestimates mGFR when mGFR is declining and underestimates mGFR when mGFR is rising) | Change in eGFR lags behind the change in mGFR (eGFR overestimates mGFR when mGFR is declining and underestimates mGFR when mGFR is rising) |
| Non-GFR determinants a | Directly measured in clinical studies | Hypothesized from clinical observations and epidemiologic studies |
| Factors affecting generation | Decreased by large muscle mass, high-protein diet, ingestion of cooked meat and creatine supplementsIncreased by small muscle mass, limb amputation, muscle-wasting diseases | Decreased in hyperthyroidism, glucocorticoid excess, and possibly obesity, inflammation, and smokingIncreased in hypothyroidism |
| Factors affecting tubular reabsorption or secretion | Decreased by drug-induced inhibition of secretion (trimethoprim, cimetidine, fenofibrate) | NA |
| Factors affecting extrarenal elimination | Decreased by inhibition of gut creatininase by antibioticsIncreased by dialysis, large losses of extracellular fluid (drainage of pleural fluid or ascites) | Increased by large losses of extracellular fluid (drainage of pleural fluid or ascites) |
| Range | Less precise at higher GFR, due to higher biological variability in non-GFR determinants relative to GFR and larger measurement error in Scr and GFR | Less precise at higher GFR, due to higher biological variability in non-GFR determinants relative to GFR, and larger measurement error in Scys and GFR |
| Interference with assays | Spectral interferences (bilirubin, some drugs)Chemical interferences (glucose, ketones, bilirubin, some drugs) | NA |
a Errors in eGFRcrcys related to non-GFR determinants of creatinine or cystatin are hypothesized to be less than for eGFRcr and eGFRcys alone. Effects of factors affecting non-GFR determinants refer to effects on eGFR. AKI , acute kidney injury; eGFRcr, glomerular filtration rate estimates based on serum creatinine; eGFRcys, glomerular filtration rate estimates based on cystatin C; GFR , glomerular filtration rate; mGFR, measured glomerular filtration rate; Scr, serum creatinine; Scys, serum cystatin C.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here