Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The magnetic resonance imaging (MRI) acute stroke protocol includes T2-weighted imaging, fluid attenuated inversion recovery, gradient recalled echo, MR angiography, diffusion-weighted imaging, and perfusion-weighted imaging and can be acquired in 15–20 minutes.
Diffusion-weighted MRI is an excellent tool to detect acute cerebral infarcts and to distinguish these from chronic infarcts, in patients with stroke or transient ischemic attack.
MRI is an effective tool to select patients who are optimal candidates for acute stroke therapy.
MRI is as reliable as computed tomography (CT) in the identification of acute intracerebral hemorrhage and can therefore be used as the sole imaging modality for evaluating patients with acute stroke.
MRI is superior to CT in identifying the underlying cause of intracerebral hemorrhage.
MRA and venography are ideal imaging modalities for evaluation of cerebrovascular pathology such as arterial stenosis or occlusions, dissections, aneurysms, and venous thrombosis.
Nuclear magnetic resonance (MR) was first described in the 1930s. The first MR images were obtained in the 1970s. In the 1980s structural MR imaging (MRI) emerged as a clinically useful diagnostic modality for stroke and other neurologic disorders. In the detection of ischemic stroke lesions MRI is more sensitive than computed tomography (CT), particularly for small infarcts and in sites such as the cerebellum, brainstem, and deep white matter. In the investigation of ischemic stroke, conventional structural MRI techniques, such as T1-weighted imaging (T1WI), T2-weighted imaging (T2WI), and fluid-attenuated inversion recovery (FLAIR) imaging, reliably detect ischemic parenchymal changes beyond the first 12–24 hours after stroke onset. These methods can be combined with MR angiography (MRA) to non-invasively assess the intracranial and extracranial vasculature. However, within the critical first 3–6 hours, the period of greatest therapeutic opportunity, these methods do not adequately assess the extent and severity of ischemic changes.
The 1990s witnessed the development of diffusion-weighted imaging (DWI), which is sensitive and specific for delineating irreversibly injured ischemic brain tissue within the first 6 hours from stroke onset. , Around the same time, contrast-based MR perfusion (MRP) techniques gained popularity to delineate hemodynamic changes. , These two techniques were recognized for their potential clinical utility in the early detection and investigation of patients with stroke. , That initial optimism began to bear fruit as further technical developments, most notably echoplanar imaging (EPI), made diffusion and perfusion MRI feasible in routine clinical practice. The detection of hyperacute intraparenchymal hemorrhagic stroke by susceptibility-weighted MRI also has been established as comparable to CT. In combination with MRA, the multimodal stroke MRI examination opened the door for the detection of the site, age, extent, mechanism, and tissue viability of acute stroke lesions in a single imaging study. Stroke MRI is routinely applied as a multimodal examination to evaluate the stroke patient for arterial pathology, hemodynamic changes, hyperacute parenchymal injury, subacute and chronic infarct, and evidence of acute or chronic hemorrhage ( Fig. 48.1 ). Beyond its role as an aid in routine clinical diagnosis, applications of current MRI methodology have shown benefit as a patient selection tool for thrombolytic and interventional therapies. MRI also can be useful as a biomarker of therapeutic response in stroke trials.
In this review, we describe the applications of structural and functional MR techniques in cerebrovascular diseases. First, the general MRI principles and pulse sequences are discussed, followed by clinical applications of MRI in the evaluation of patients with transient ischemic attack (TIA), ischemic stroke, intracranial hemorrhage, and cerebrovascular pathology. Applications of MRI to specific cerebrovascular topics are also illustrated in Section IV (see Chapter 32, Chapter 33, Chapter 34, Chapter 35, Chapter 36, Chapter 37, Chapter 38, Chapter 39, Chapter 40, Chapter 41, Chapter 42, Chapter 43, Chapter 44, Chapter 45 ) of this book. A fuller description of the technical topics discussed in this chapter may be found elsewhere. ,
Routine MRI is based on the interaction of radio waves with atomic nuclei (most commonly protons or hydrogen nuclei) in tissue. Hydrogen is present in nearly all of the organs of the body. Protons have a net magnetic moment such that when they are placed in a magnetic field they align with the magnetic field and can be excited by radiofrequency (RF) pulses. Water and fat protons are the most extensively imaged nuclei. Other nuclei can be imaged, such as phosphorus, sodium, and fluorine, but these are much less abundant than hydrogen and have no current clinical application to stroke.
When undergoing an MRI study, the patient is placed in a strong magnetic field. In general, this main magnetic field is always on, so safety precautions around the MRI scanner are essential even when a scan is not being performed. The strength of the magnetic field depends on the specific scanner. In practice, a number of the current clinical MRI are performed at 1.5 tesla (1.5 T), but lower and higher field strength scanners are also in use. Over the past decade clinical 3T MRI has become commonplace. The next decade may see 7T MRI become a product for routine clinical use. In general, for brain and cerebrovascular imaging, higher field strengths give greater signal-to-noise ratio, which is advantageous for reducing scanning time and improving spatial resolution. Higher field strengths also come with challenges, including those related to susceptibility.
To acquire images, RF pulses are applied at the Larmor frequency of hydrogen, the proton’s resonant spin frequency. The energy from the RF pulses is absorbed and then released until the tissue being scanned has reemitted the energy absorbed and undergone relaxation. The echo time (TE) is the time the machine waits after the applied RF pulse to receive the RF echo from the patient. The repetition time (TR) is the time between RF pulses. The energy released occurs over a short time according to two relaxation constants, known as T1 (longitudinal relaxation constant) and T2 (transverse relaxation constant). Varying the TE and TR enables images of different contrast to be obtained, depending on which of the constants is dominant in the tissue. Spatial localization of the signal source from the tissue is achieved by the superimposition of brief gradient magnetic field pulses.
In the sections below, the most commonly used MR pulse sequences are reviewed. Conventional MRI pulse sequences include T2WI, T1WI, proton density (PD) imaging, and FLAIR imaging. These are of most value in the evaluation of subacute and chronic stroke. The conventional sequences are based on two families of sequences termed spin echo (or fast spin echo) and gradient echo. In the former, the energy is refocused with the use of a series of RF pulses, whereas the latter uses a reversal of the magnetic field gradient to refocus the energy. The gradient echo sequences are most useful for MRA and hemorrhage detection. Supplementing the anatomic information obtained from conventional sequences, contemporary MR protocols also include DWI, diffusion kurtosis imaging (DKI), MRP, and MRA. These sequences allow a multimodal evaluation of the patient with acute stroke by imaging tissue injury, perfusion, and the vasculature within the short period feasible for an acute stroke evaluation.
T1WIs are based on the longitudinal relaxation of spins. They are generated primarily from sequences of short TE and short TR; the shorter the TE and TR, the more T1-weighted the image is. On T1WI, the cerebrospinal fluid (CSF) has low signal intensity, whereas fat has high signal intensity. Gray matter appears less intense (darker) than white matter. Ischemic infarcts appear hypointense on T1WI. The T1WI is not an essential part of the multimodal stroke MRI examination but is valuable in specific cases: axial fat-suppressed T1WI of neck soft tissue can identify the intramural thrombus of an arterial dissection.
T2WIs are based on the transverse relaxation of spins. They are generated from sequences of long TE and long TR. On T2WI, the CSF signal is hyperintense. Gray matter appears less intense (darker) than white matter. Ischemic lesions also appear hyperintense and may be difficult to distinguish from normal CSF spaces, a potential problem for smaller lesions.
PD images are generated with long TR and short TE, and the CSF and fat are of similar signal intensity. One advantage of PD imaging is that lesions appear hyperintense relative to CSF, although in practice PD imaging has been supplanted by FLAIR imaging.
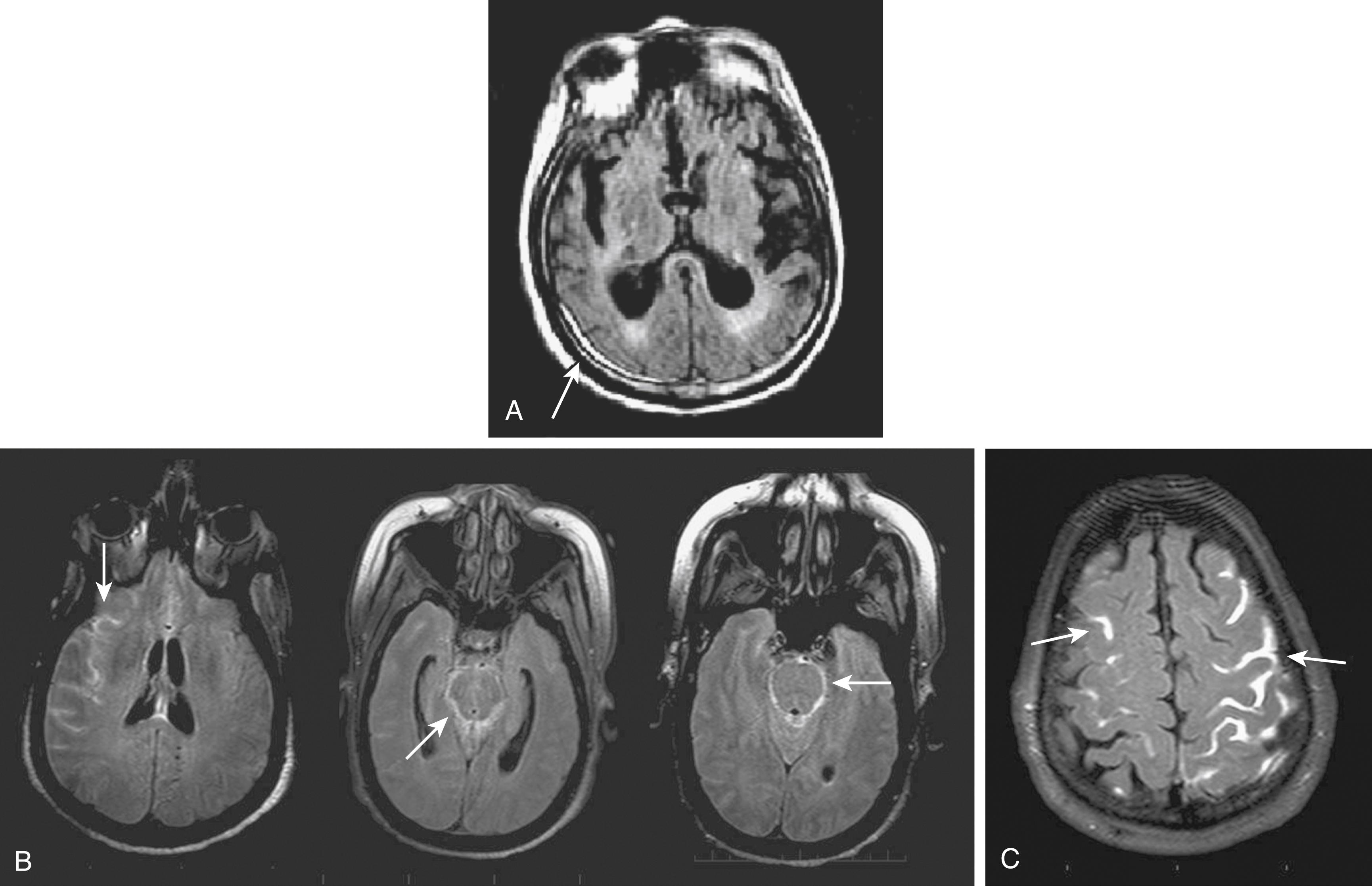
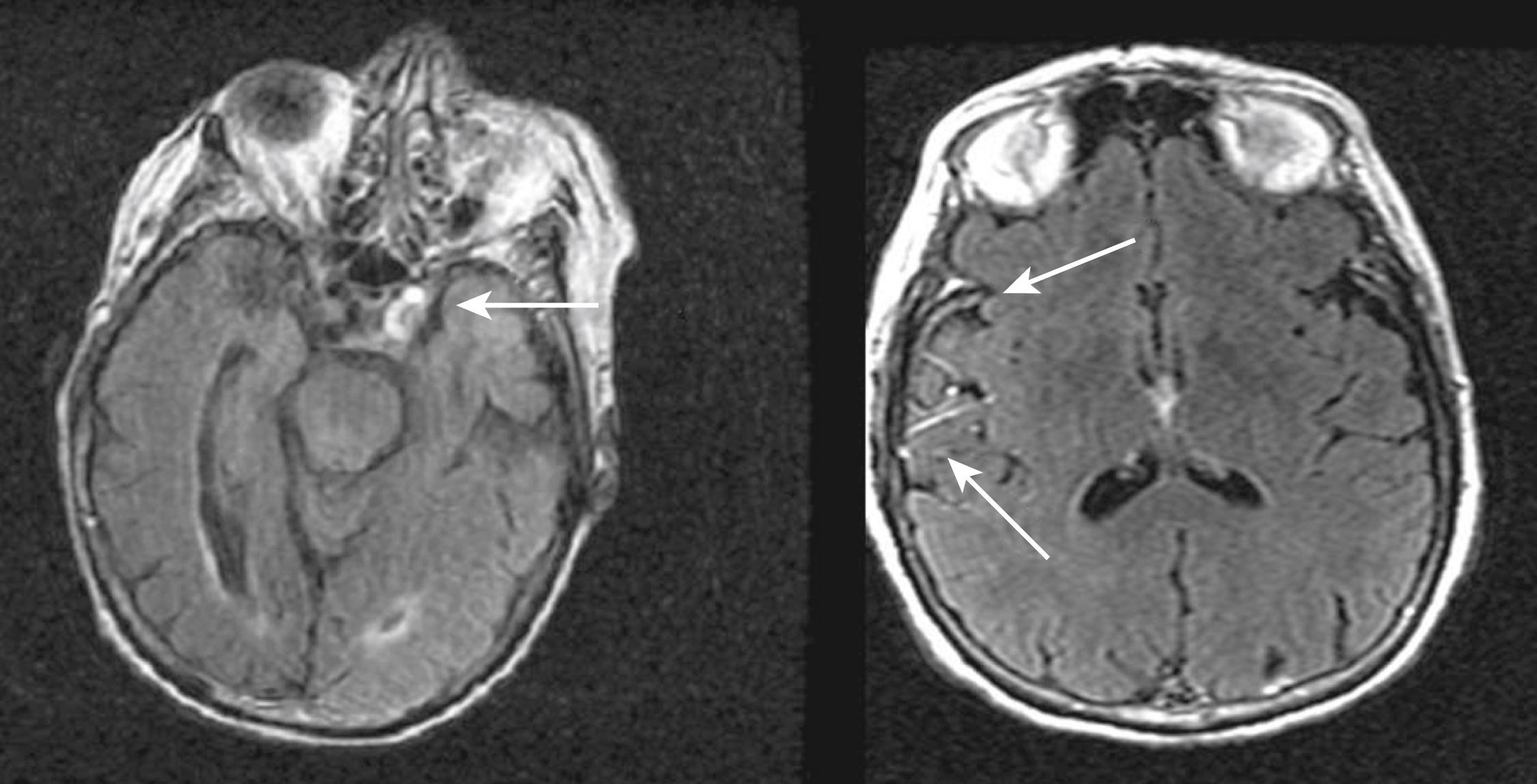
On FLAIR, an additional RF pulse (inversion pulse) is applied with the purpose of nulling signal from the normal CSF. As applied in routine practice, on T2-weighted FLAIR imaging the CSF signal is nearly fully suppressed and appears dark as in T1WI, but the lesions appear bright, as in T2WI, allowing better visualization of cortical lesions and periventricular lesions. In practice, FLAIR imaging is used in place of PD imaging and often preferred to T2WI, although FLAIR acquisition times are somewhat longer. Some radiologists prefer both FLAIR imaging and T2WI for the comprehensive head MRI examination, because of the diagnostic advantages of the latter for non-cerebrovascular pathologies. Unique features of FLAIR sequences for acute stroke imaging include hyperintensity of extra-axial blood (e.g., subarachnoid hemorrhage [SAH], subdural hematoma, Fig. 48.2A and B ) and delayed gadolinium enhancement of intrasulcal CSF, indicative of early blood-brain barrier disruption (see Fig. 48.2C ). FLAIR imaging may also depict hyperintense arterial signal indicative of very slow flow associated with acute occlusions or severe stenosis (see Figs. 48.1 and 48.3 ). A disadvantage of FLAIR imaging is its high sensitivity to pulsation artifacts of CSF that may mimic SAH, for instance in the basilar cisterns.
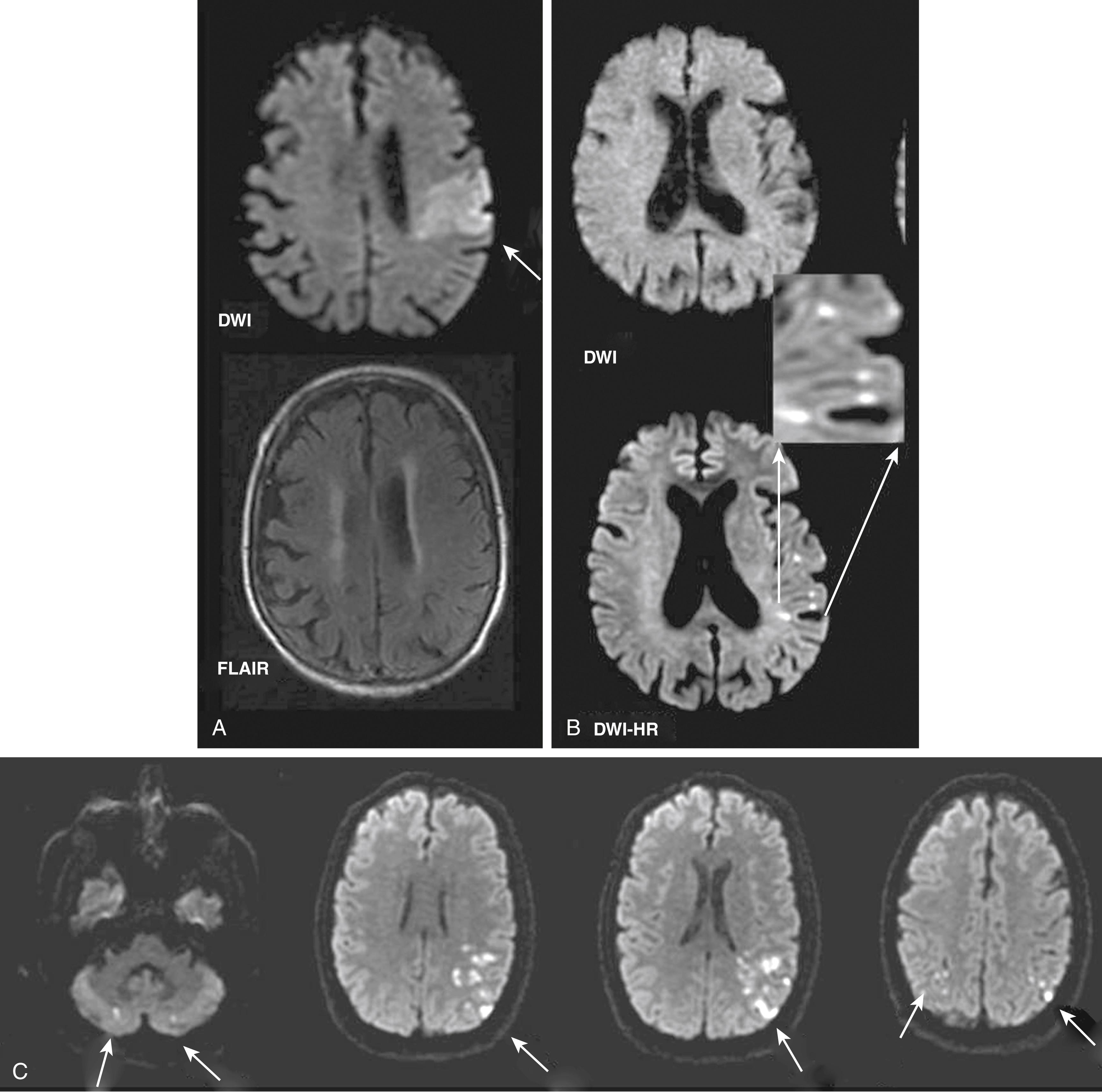
DWI has transformed the diagnosis of ischemic stroke in its earliest stage, from reliance on a mostly clinical inference about the presence, localization, and size of an ischemic lesion, to imaging confirmation of the infarct. This technique is the only brain imaging method to reliably demonstrate ischemic parenchymal impact within the first minutes to hours after onset, well before changes are detectable on CT and T2WI or FLAIR MR images ( Figs. 48.1 and 48.4 ). DWI detects the self-diffusion of water, which is the mobility of water molecules among other water molecules (brownian motion). , With use of single-shot EPI, whole-brain DWI of stroke can be obtained in a scanning time as short as 2 seconds ; in current practice, however, multiple DWI acquisitions are obtained and combined for greater signal-to-noise ratio. The typical DWI pulse sequence actually acquires two sets of images, one with and the other without diffusion weighting. An EPI T2WI sequence is set without diffusion weighting. A bipolar pair of diffusion-sensitizing magnetic field gradient pulses to the T2WI pulse sequence cause a dephasing and then rephasing of the spinning protons in water molecules. Where there has been net displacement of a water molecule (i.e., protons) between application of the two diffusion gradient pulses (on the order of tens of milliseconds), there is a net dephasing and subsequent signal loss in the resulting image. The more the water has moved, the greater the signal loss, so that signal intensity is reduced everywhere but relatively less where water movement is restricted. CSF appears very dark, normal brain appears intermediate, and ischemic brain, where parenchymal diffusion is reduced, appears relatively bright.
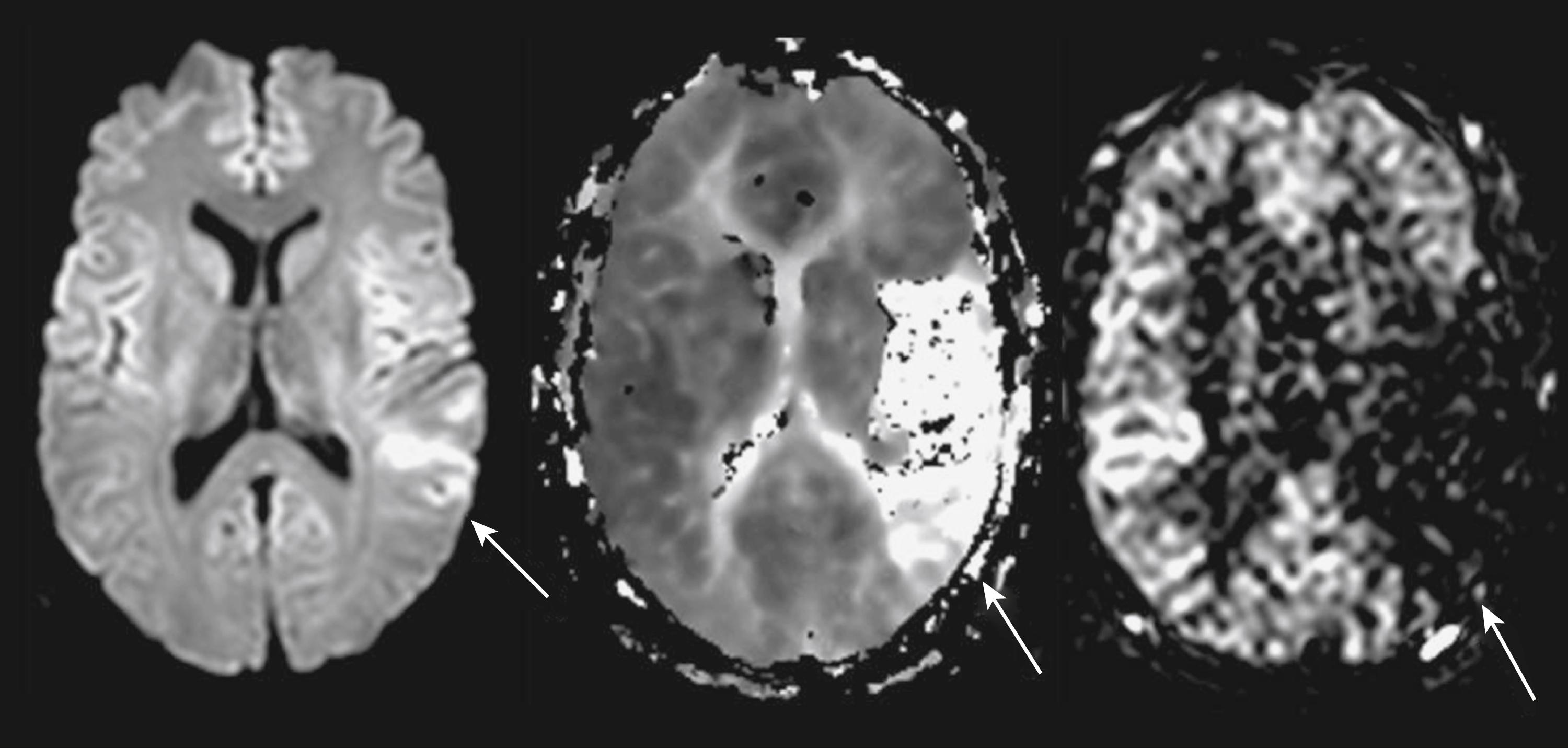
DWI is quantitative in that it both measures a physiologic parameter—the apparent diffusion coefficient (ADC) of water in mm /s—and can define the acute ischemic lesion volume, which can be used to study ischemic pathophysiology in vivo. The ADC is calculated from the reduction in signal intensity that occurs with diffusion weighting. Thus a DWI pulse sequence usually contains at least two sets of images, one without diffusion weighting, a T2WI, and one with high diffusion weighting. The strength of diffusion weighting is described by a set of pulse sequence features called the b-value, so these two sets of images may be referred to as b0, indicating the T2WI without diffusion weighting, and the b1000, referring to the most commonly used b-value in practice.
DWI measurements also contain geometric information, primarily axonal orientation, because DWI acquires its information in one direction at a time. This anisotropy results in higher signal perpendicular to fiber tracts and lower signal parallel to them. For routine stroke imaging, it is preferable to minimize anisotropy by effectively averaging the diffusion measurements across three orthogonal directions, reducing the hyperintensity not due to ischemia, a potential confounding factor for small ischemic lesion in white matter tracts. These averaged images are often referred to as isotropic DWI . Diffusion tensor imaging (DTI) , is a type of DWI that, rather than eliminate anisotropy, uses this information to determine the direction and integrity of degenerated white matter tracts. There are emerging data suggesting that DTI can be used to assess stroke recovery. ,
In acute cerebral ischemia, the ischemic lesion appears hyperintense (bright) on DWI and hypointense (dark) on an ADC map (see Figs. 48.1 and 48.4 ). This appearance reflects cytotoxic edema, and a reduction in the volume and increased tortuosity of the extracellular space. As the ischemic lesion evolves through the phases of cytotoxic edema, vasogenic edema, tissue necrosis, and cavitation, the ADC normalizes and then becomes elevated in the chronic phase of stroke. This feature makes it possible to distinguish old ischemic lesions from new by calculation of the ADC value. As a rule of thumb, DWI hyperintensity without T2WI or FLAIR changes usually implies reduced ADC and can be taken as evidence of acute ischemic impact. Signal hyperintensity on DWI can persist during later stages of stroke (the “T2 shine-through” effect) and cannot by itself be evidence of acute ischemic stroke. In addition, non-ischemic pathologies may be associated with DWI hyperintensity. For these reasons, in patients with suspected ischemic stroke, DWI should always be interpreted with T2WI and FLAIR and ideally with a calculation of the ADC maps.
Brain perfusion, defined in the broadest sense as some aspect of cerebral circulation, may be studied by various MRI strategies. Two categories of MRI methods, one requiring the injection of contrast agent and the other not, have been used to study abnormal perfusion in human stroke (mainly ischemic) ( Fig. 48.5 ). The first strategy, which is the standard method in clinical practice, is dynamic susceptibility contrast (DSC) imaging and involves a bolus injection of gadolinium and the rapid acquisition of a series of susceptibility-weighted or T2∗-weighted imaging repeated every 1–2 seconds through an entire brain volume. , The intravascular passage of gadolinium in sufficiently high concentration distorts the local magnetic field owing to magnetic susceptibility effects, causing dephasing of spins in brain tissue adjacent to the blood vessels and therefore results in signal loss. The amount of signal loss over time in a series of rapidly acquired images has been shown to be proportional to cerebral blood volume (CBV) in healthy brain tissue. The time it takes for the change in signal intensity to reach a maximum is the time to peak (TTP) and is related to the time to maximum (Tmax) and mean transit time (MTT) of an idealized bolus of contrast agent. Because cerebral blood flow (CBF) in these intravascular models equals the ratio CBV/MTT, information about CBF can potentially be inferred with this technique. In patients with acute stroke, perfusion maps of the relative MTT, Tmax, CBV, and CBF can be generated, permitting visualization of perfusion defects in acute infarcts, tissue reperfusion occurring after recanalization of blood vessels, and hyperperfusion of subacute infarcts that have reperfused. Postprocessing of the MRP images occurs within minutes of image acquisition at the scanner and so the images are rapidly available to the treating physician. The optimal and accurate assessment of these and other perfusion parameters is an area of intense investigation, but in clinical practice, the MRP source images and scanner-generated perfusion maps are sufficient to determine the presence or absence of acute focal ischemia. For cases in which patient head movement during MRP acquisition renders the maps inadequate for diagnosis, assessment of the individual source MRP images can be useful, because these EPI source images are virtually unaffected by patient motion.
Because of the recognized toxicity of gadolinium-based contrast agents in patients with renal failure, which is not uncommon in older stroke patients, there has been increased interest in MPR methods that do not require contrast agent administration. The second MRP strategy involves arterial spin labeling (ASL) methods, which use RF inversion pulses to magnetically label spins in the arterial supply to brain regions, using arterial water as an endogenous diffusible tracer. It can be applied either as pulsed, , as continuous labeling, or as a combination of both. In ischemic stroke, ASL appears to give comparable diagnostic information to that from the gadolinium bolus tracking methods (see Fig. 48.5 ), although the reduced signal-to-noise ratio requires averaging of multiple acquisitions, requiring longer acquisition times, and thus is more vulnerable to motion artifacts. However, the more straightforward quantitative measurement of tissue perfusion with ASL , is an advantage of this methodology over the bolus tracking method. Innovations have now permitted multiple brain slices to be imaged with ASL, and ASL perfusion methods are available for routine imaging on most MRI scanners.
In cerebrovascular diagnosis, MRA with contrast-enhanced (CE) or time-of-flight (TOF) methods are the standard approaches. In CE-MRA, a rapid MR acquisition is timed to a bolus injection of contrast agent over a large field of view, permitting routine imaging of the vasculature from the aortic arch through to the branches of the circle of Willis (see Fig. 48.1 ). The vascular anatomy is outlined by the blood containing the contrast agent. In TOF MRA, no contrast agent injection is required, and the vascular signal depends on direction and velocity of blood flowing into the plane of imaging. The magnetization of protons in stationary tissue occurs through saturation by repeated low-flip-angle RF pulses, whereas protons in the vessels flowing into the tissue remain unsaturated and appear relatively bright. The data are then postprocessed for an angiographic reconstruction. In practice, inspection of the source images is often necessary to evaluate subtle or ambiguous findings, and most scanners permit scrolling through a reformatted slab of source MRA images for this purpose (see Fig. 48.1 ). For imaging of the arteries of the circle of Willis, 3D rather than 2D TOF MRA is the most common method of MRA in clinical practice, since it gives superior spatial resolution and is less prone to signal loss from turbulent flow at sites of stenoses. Limitations of TOF MRA are its tendency to overestimate the degree of stenosis (particularly when there is slow or turbulent flow or calcifications) and its insensitivity to collateral sources of flow. Its advantages include greater spatial resolution than CE-MRA for the intracranial circulation and its application to the imaging of the cerebral venous system (MR venography [MRV]). TOF is an alternative for patients unable to receive gadolinium-based contrast agents. Notwithstanding its tendency to overestimate the degree of stenosis, MRA rivals CT angiography and conventional angiography for the detection of arterial stenoses, occlusions, and dissections. The sensitivity of MRA to detect aneurysm after SAH is estimated to be 69%–100%, with a specificity of 75%–100%. The sensitivity for smaller aneurysms is less.
Susceptibility-weighted imaging (SWI) refers to a family of MRI sequences in which the tissue contrast is based on magnetic susceptibility differences between different tissue types. Magnetic susceptibility is the property of matter that distorts an applied magnetic field. Although often a source of artifacts at the interface of differing tissue types or in the presence of metal, this principle can be used to make pulse sequences sensitive to hemorrhage, to functional changes in blood oxygenation, and to hemodynamic parameters. The conventional gradient recalled echo (GRE) pulse sequence (commonly called T2∗-weighted images) is sensitive to the susceptibility effects of paramagnetic molecules such as gadolinium-containing contrast agents as well as deoxyhemoglobin and other hemoglobin breakdown products that are present during all stages of intracranial hemorrhage. Single-shot EPI has intrinsic susceptibility weighting, and EPI using a GRE technique, as in MRP, is the most sensitive of all to susceptibility effects in routine clinical practice. A specific variation of GRE, termed simply SWI, measures phase differences and may be more sensitive than conventional GRE in the detection of acute hemorrhage, chronic microbleeds, and cerebral veins.
MR spectroscopy (MRS) allows the non-invasive in vivo assessment of brain chemistry by measuring resonances from important metabolites. Clinical studies of MRS have been performed predominantly on 1 H nuclei (proton MRS), for which there is a relatively favorable signal-to-noise ratio compared with MRS of other nuclei. Although data collection from single voxels (single voxel spectroscopy) is more straightforward, differences between tissue compartments following stroke are more easily appreciated with chemical shift imaging (CSI). In CSI, spectra from multiple voxels within a grid corresponding to one or more slices are displayed. Typically, metabolite peaks are presented as a spectrum in the “frequency domain” on a scale expressed in parts per million (ppm), which conventionally runs from right to left. CSI data can also be displayed as a color-coded “map” overlying a structural image, but it should be stressed that analysis of individual spectra is mandatory. Although a large number of metabolites can be detected, particularly in those studies using a short TE, stroke studies have focused on metabolites with large peaks including N -acetyl- l -aspartate (NAA) at 2.01 ppm, lactate at 1.33 ppm, and, to a lesser extent, choline at 3.22 ppm and creatine at 3.03 ppm. Values of metabolite peaks can be expressed in relative terms, for example, as a ratio to another metabolite within the same voxel, or to the same metabolite in a voxel in the contralateral hemisphere. Absolute concentrations may be derived by using the signal from water as an internal reference or, less commonly, by using a reference solution placed in the scanner external to the patient. MRS is time-consuming and therefore has limited clinical applications for acute stroke imaging.
The objective of multimodal MRI of acute stroke, also referred to as the stroke MRI examination, is to obtain diagnostic information about the acute parenchymal injury, subacute or chronic infarct, arterial pathology, tissue perfusion, and presence of hemorrhage. In this section the clinical application of multimodal MRI in the diagnostic workup of patients with TIAs, ischemic stroke, intracranial hemorrhage, and vascular pathology is discussed. Finally, we describe the utility of MRI as a tool to guide acute stroke therapy.
The full multimodal MR sequences listed in Table 48.1 can be acquired within 15–20 minutes of scanning. All patients must be screened by MRI personnel for safety related to metal or electronic devices. Updated online resources relating to MRI safety are available (e.g., www.mrisafety.com ). Approximately 10%–15% of patients suspected of acute stroke are unable to undergo MRI because of contraindications. In 2006, a link between gadolinium-based contrast agents and nephrogenic systemic fibrosis (NSF), a potentially debilitating fibrosing disease of the skin and viscera occurring in patients with chronic kidney disease, was recognized. Readers are referred to European and North American guidelines for specific management recommendations. , In general, caution is recommended when the glomerular filtration rate (GFR) is between 30 and 60 mL/min/1.73 m 2 in the patient with chronic kidney disease, but local hospital policies may differ. Gadolinium should not be used when the GFR is less than 30 mL/min/1.73 m 2 or if the patient is dialysis dependent. Estimated GFR based on serum creatinine is used to screen patients undergoing MRI for this risk.
| Sequence | Primary Diagnostic Use in Cerebrovascular Disease |
|---|---|
| Diffusion-weighted imaging | Hyperacute acute ischemic lesions |
| Distinguish old lesions from new by apparent diffusion coefficient imaging | |
| T2-weighted imaging | Subacute and chronic ischemic lesions |
| Rule out non-cerebrovascular pathology | |
| Fluid-attenuated inversion recovery | Subacute and chronic ischemic lesions |
| Rule out non-cerebrovascular pathology | |
| Hyperintense vessel sign | |
| Blood–brain barrier breakdown | |
| Gradient recalled echo | Acute intracranial hemorrhage |
| Hemorrhagic transformation | |
| Microbleeds | |
| Intravascular thrombus | |
| MR angiography | Acute arterial occlusion |
| Other arterial lesions: stenosis, dissection, aneurysm | |
| MR venogram for sinus and cerebral venous thrombosis | |
| Perfusion-weighted imaging | Focal hemodynamic defect |
| Diffusion–perfusion mismatch as marker of ischemic penumbra |
Conventional MRI (e.g., T2WI) is more sensitive than CT in identifying both new and preexisting ischemic lesions in patients with TIAs (employing the classic definition of TIA as a focal neurologic deficit of vascular etiology that resolves within 24 hours). One of the earliest studies reported that 77% of patients had focal ischemic changes on MRI compared with 32% on CT. In subsequent studies, the percentage of TIA patients with at least one infarct on conventional MRI varies from 46% to 81%, but the majority of lesions do not correlate with symptomatology. , The percentage of patients with an infarct on MRI in a location that could have accounted for the deficits observed during the TIA varies from 31% to 39% on conventional MRI. , With conventional MRI it is difficult to determine what proportion of these appropriately localized infarcts occurred at the time of the index TIA and what proportion existed prior to the presenting event. CE MRI studies can help determine the acuity of the lesion. In series of TIA patients who underwent CE MRI, contrast enhancement of an infarct was observed in 11%–39%. ,
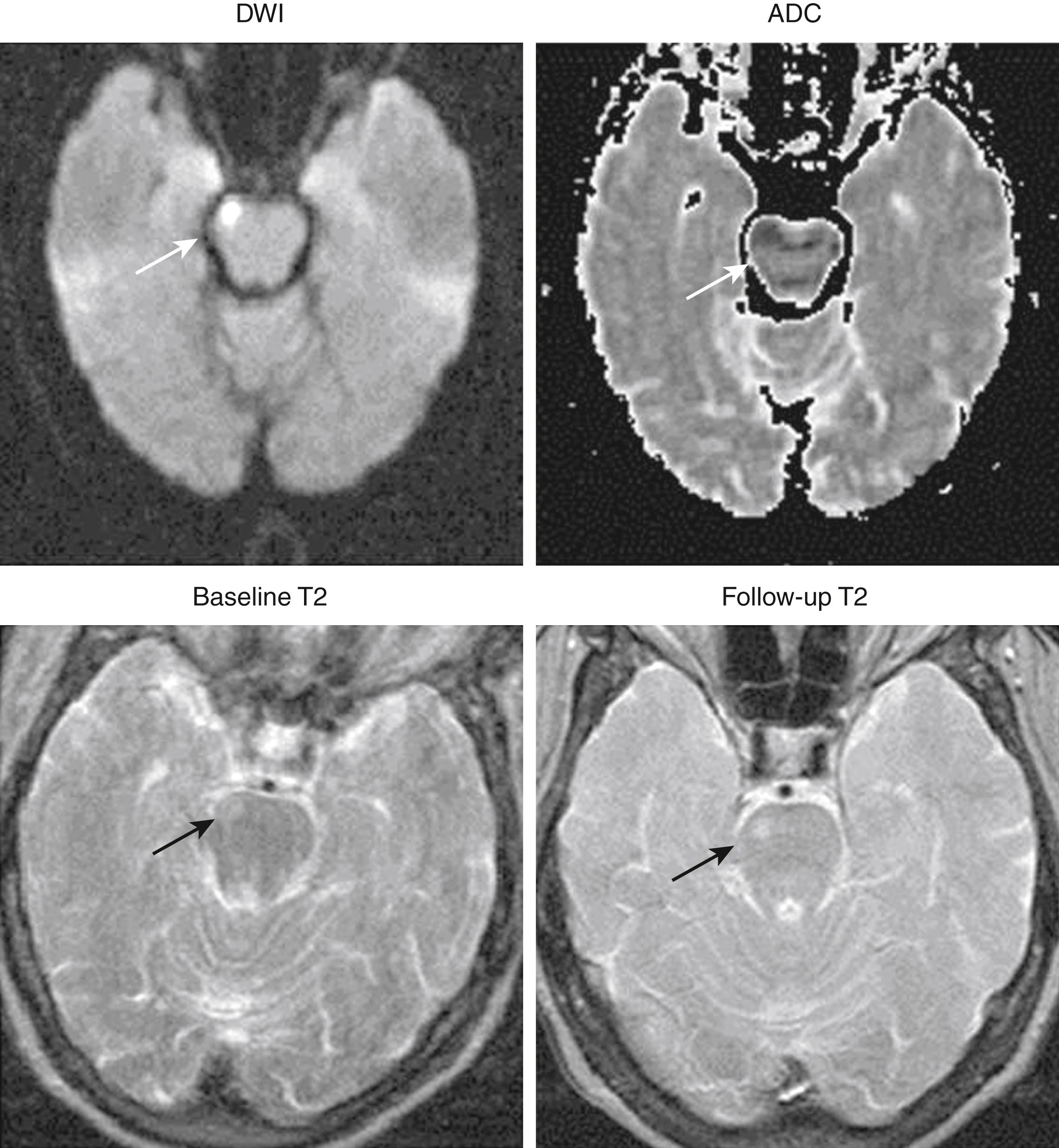
Newer MRI techniques, including diffusion weighting and perfusion, have revolutionized the MRI assessment of patients with TIA ( Fig. 48.6 ). The high sensitivity and specificity of DWI for detection of acute infarcts has been particularly useful in the workup of TIA patients. In a pooled analysis of 19 studies, the aggregate rate of DWI positivity was 39%, with frequency ranging from 25% to 67%. In studies that also obtained a follow-up MRI, 56%–80% of patients demonstrated a subsequent infarct in the region corresponding to the original DWI abnormality ( Fig. 48.7 ). , Several studies have demonstrated that DWI positivity is associated with specific clinical characteristics, including longer symptom duration, motor deficits, aphasia, and large-vessel occlusion demonstrated on MRA. , In a recent prospective cohort study, DWI positivity was 13.5% among 1028 patients presenting with “low-risk” focal neurologic symptoms or signs. Nonmotor or language symptoms of any duration, or presentations with motor weakness or language deficits lasting no longer than 5 minutes, were included. Therefore, an MRI with DWI should be strongly considered in virtually all patients with suspected TIAs, even for patients with “low risk” features. A study exploring the characteristics of “DWI-negative” patients with TIA found that patients with brainstem location ischemia or lacunar syndromes were most likely to have a negative initial DWI result and a positive follow-up imaging result.
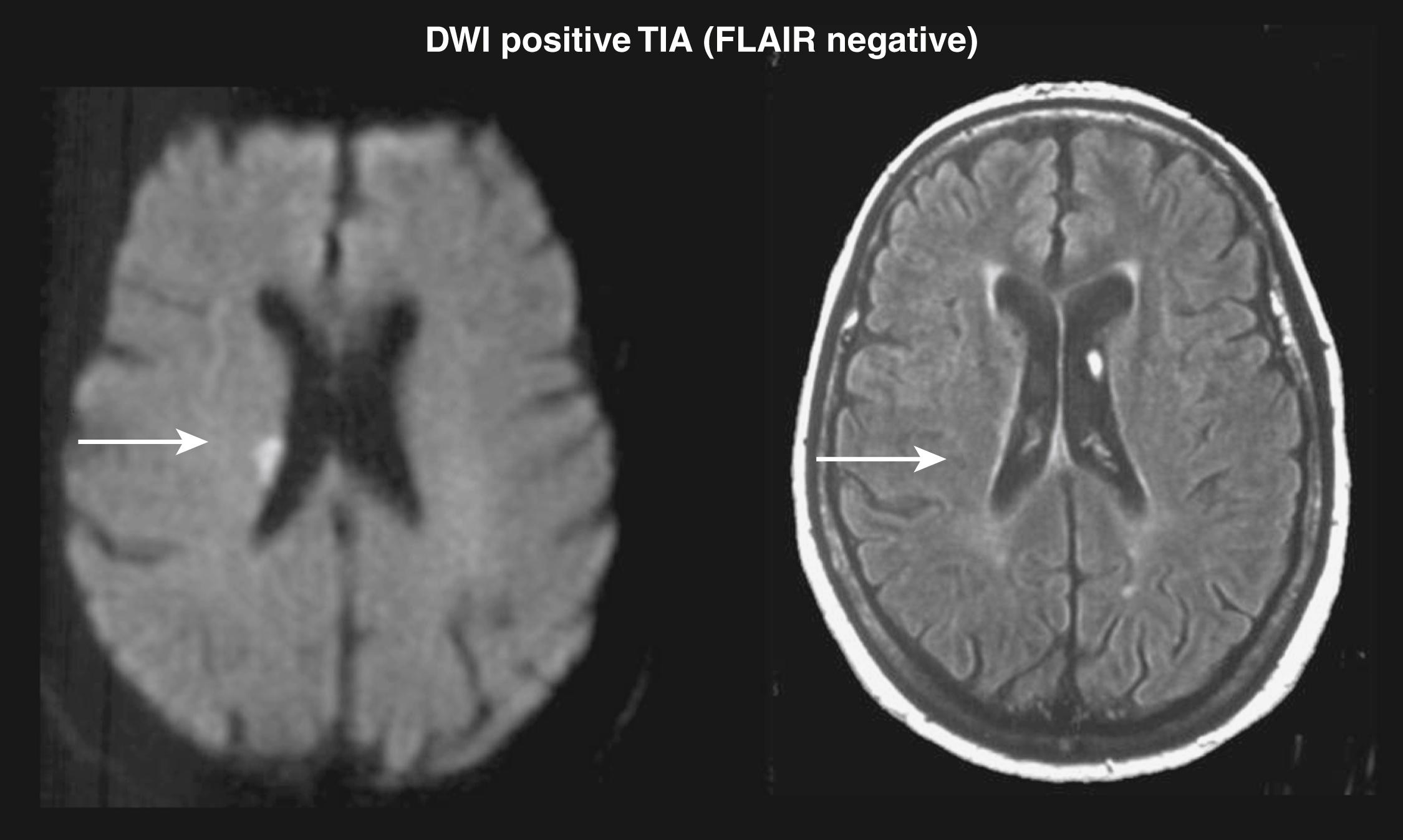
Identification of DWI-positive lesions in TIA patients is often clinically relevant. For patients who have multiple lesions on T2WI, DWI helps to clarify whether the lesions were related to a recent ischemic event in 31%, and the DWI image alters the physician’s opinion regarding vascular localization and probable TIA mechanism in also approximately one-third of patients. It has also been shown that patients with TIA who have abnormalities on DWI scans have a higher risk of recurrent ischemic events than those without such abnormalities. , , Patients with multiple acute DWI lesions appear to be at particularly high risk. The presence of a DWI lesion alone or in combination with clinical variables such as the ABCD score (age, blood pressure, type of symptoms, and symptom duration) is a strong predictor of 7-day and 3-month stroke risk. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here