Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Bile secretion is one of the major functions of the liver, which serves two major purposes: (1) the excretion of hepatic metabolites—including bilirubin, cholesterol, drugs, and toxins—and (2) the facilitation of intestinal absorption of lipids and fat-soluble vitamins. More recently, through their interaction with the gut microbiome, bile acids also have been found to have important signaling functions. Through receptor activation, bile acids regulate lipid, glucose, and energy metabolism. Alterations in bile secretion also may contribute to cholelithiasis (see Chapter 33 ) and its potential complications, such as cholecystitis (see Chapter 34 ) and choledocholithiasis (see Chapters 37 and 38 ). On the other hand, obstruction of bile flow results in alterations of coagulation, the immune system, and all organ functions. This chapter will discuss the physiology of bile secretion, the pathophysiology of bile obstruction, and the management of obstructive jaundice.
The two primary roles of bile in normal physiology are the excretion of organic compounds, such as bilirubin and cholesterol, and the intestinal absorption of lipids. Bile secretion results from the active transport of solutes into the canaliculus, followed by the passive flow of water. Water constitutes approximately 85% of the volume of bile. The major organic solutes in bile are bilirubin, bile salts, phospholipids, and cholesterol. Bilirubin, the breakdown product of spent red blood cells, is conjugated with glucuronic acid by the hepatic enzyme glucuronyl transferase and is excreted actively into the adjacent canaliculus. Normally, a large enzyme reserve exists to handle excess bilirubin production, which might exist in hemolytic states.
Bile salts are steroid molecules synthesized by hepatocytes. Bile salts account for approximately 72% of the biliary lipids. The primary bile salts in humans, cholic and chenodeoxycholic acid, account for approximately 80% of those produced. The primary bile salts, which are then conjugated with either taurine or glycine, can undergo bacterial alteration in the intestine to form the secondary bile salts, deoxycholate and lithocholate. The purpose of bile salts is to solubilize lipids and facilitate their absorption. Phospholipids are synthesized in the liver in conjunction with bile salt synthesis and account for approximately 24% of biliary lipids. Lecithin is the primary phospholipid in human bile, constituting more than 95% of its total. The final major solute of bile is cholesterol, which accounts for 4% of the lipids. Cholesterol also is produced primarily by the liver with a small contribution from dietary sources.
The normal volume of bile secreted daily by the liver is 750 to 1000 mL. Bile flow depends on neurogenic, humoral, and chemical control. Vagal stimulation increases bile secretion, whereas splanchnic stimulation causes vasoconstriction with decreased hepatic blood flow and thus results in diminished bile secretion. Gastrointestinal hormones—secretin, cholecystokinin, gastrin, and glucagon—all increase bile flow, primarily by increasing water and electrolyte secretion. This action probably occurs at a site distal to the hepatocyte. Finally, the most important factor in regulating the volume of bile flow is the rate of bile salt synthesis by hepatocytes. This rate is regulated by the return of bile salts to the liver by the enterohepatic circulation.
The components of hepatic and gallbladder bile are essentially the same, but the concentration varies considerably because of the ability of the gallbladder to absorb water ( Table 8.1 ). The gallbladder absorbs water both actively via sodium-hydrogen (Na + /H + ) pumps and passively through aquaporin channels. Both chloride (Cl − ) and bicarbonate (HCO 3 − ) are absorbed by the gallbladder epithelium via the cystic fibrosis transmembrane regulator (CFTR). The secretion of hydrogen ions and the absorption of bicarbonate by the gallbladder alter the acid-base balance from basic in hepatic bile to acidic in gallbladder bile.
| CHARACTERISTICS * | HEPATIC BILE | GALLBLADDER BILE |
|---|---|---|
| Sodium | 160 | 270 |
| Potassium | 5 | 10 |
| Chloride | 90 | 15 |
| Bicarbonate | 45 | 10 |
| Calcium | 4 | 25 |
| Magnesium | 2 | 4 |
| Bilirubin | 1.5 | 15 |
| Proteins | 150 | 200 |
| Bile acids | 50 | 150 |
| Phospholipids | 8 | 40 |
| Cholesterol | 4 | 18 |
| Total solids | — | 125 |
| pH | 7.8 | 7.2 |
| Significant ranges may be seen. | ||
* All determinations are milliequivalents per liter, except for pH.
The gallbladder mucosa also absorbs calcium (Ca +2 ) and magnesium (Mg +2 ). Nevertheless, calcium absorption is not as efficient as the absorption of sodium and water, which leads to a significantly greater relative increase in the concentration of calcium in the gallbladder. Similarly, the concentration of bilirubin, which is not actively absorbed by the gallbladder, may be as high as 10-fold. Thus precipitation of calcium bilirubinate crystals, the major component of pigment gallstones, is much more likely to occur within the gallbladder. In addition, the biliary lipids, bile salts, phospholipids, and cholesterol all become more concentrated in the gallbladder. While gallbladder bile becomes concentrated, several changes occur in the capacity of bile to solubilize cholesterol. The solubility in the micellar fraction is increased, but the stability of the phospholipid-cholesterol vesicles is greatly decreased. Because cholesterol crystal precipitation occurs preferentially by vesicular, rather than micellar, mechanisms, the net effect of concentrating bile is an increased tendency to form cholesterol crystals.
Bile is secreted from the hepatocyte into canaliculi, which drain their contents into small bile ducts. Secretion of bile salts is the major osmotic force for the generation of bile flow. Bile acids are formed at a rate of 500 to 600 mg per day. The bulk of the bile salt pool is maintained in the gallbladder, followed by the liver, the small intestine, and the extrahepatic bile ducts. Bile acids are synthesized from cholesterol via two main pathways: a classic pathway leads to the formation of cholic acid, and an alternative pathway results in the synthesis of chenodeoxycholic acid. The classic pathway is the predominant mode of bile acid synthesis in humans. As a result, 60% to 70% of the bile acid pool consists of cholic acid and its metabolite deoxycholic acid, with chenodeoxycholic acid occurring less commonly in human bile. ,
In plasma, bile acids circulate bound to either albumin or lipoproteins. In the space of Disse within the liver, bile salt uptake into the hepatocytes is very efficient. This process is mediated by sodium-dependent and sodium-independent mechanisms. The sodium-dependent pathway accounts for more than 80% of taurocholate uptake but less than 50% of cholate uptake. In recent years, a number of transport proteins have been identified that play a key role in this process ( Fig. 8.1 ). The bile salt transporter is termed the sodium-taurocholate cotransporting polypeptide (NTCP) and is exclusively expressed in the liver and located in the basolateral membrane of the hepatocyte. Sodium-independent hepatic uptake of bile acids is mediated primarily by a family of transporters termed the organic anion transporting polypeptides (OATPs). In contrast to NTCP, these transporters have a broader substrate affinity and transport a variety of organic anions, including the bile salts. OATP-C is the major sodium-independent bile salt uptake system, but OATP-A also takes up bile acids, and OATP-8 mediates taurocholate uptake.
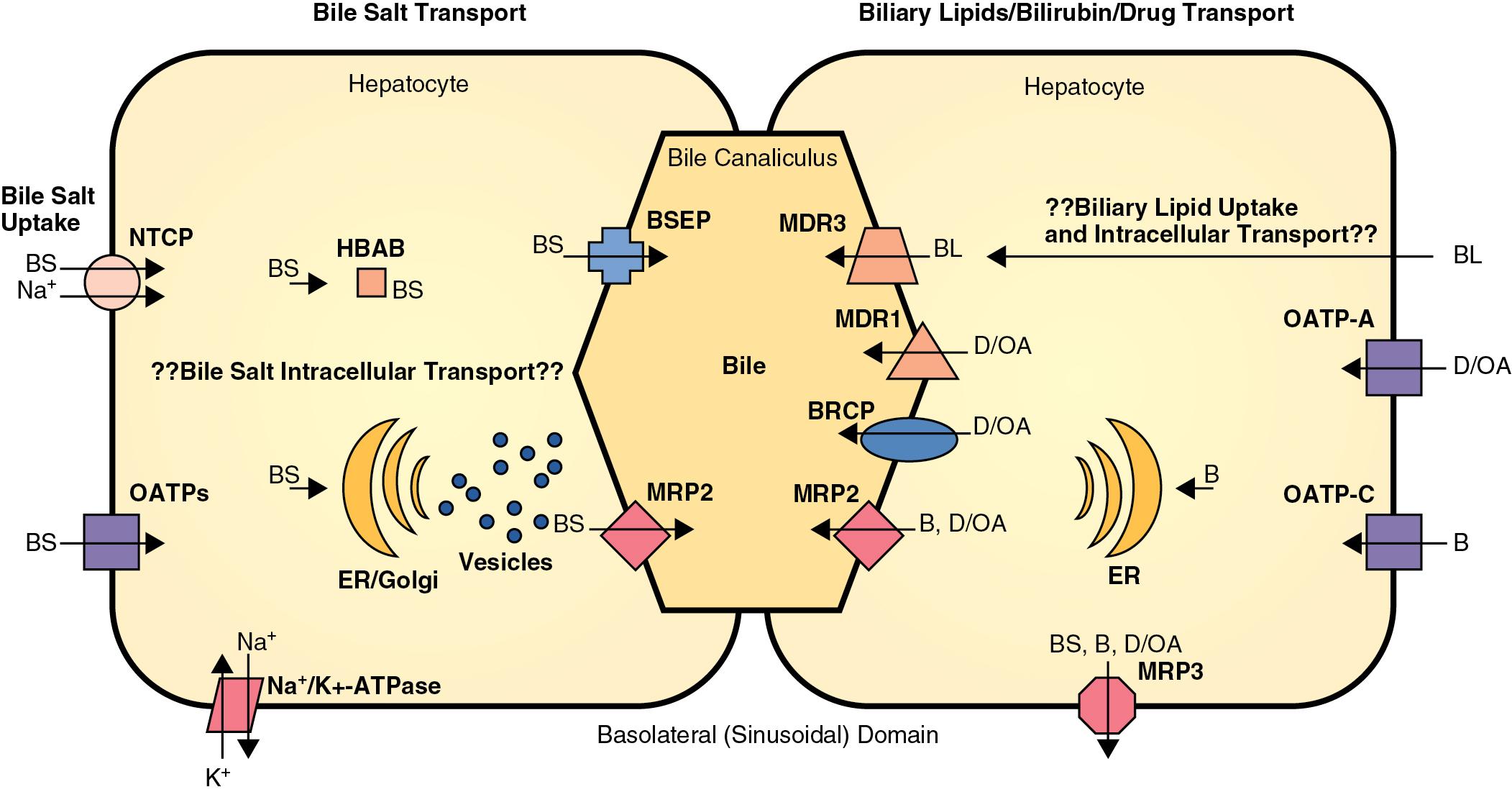
Intracellular bile acid transport occurs within a matter of seconds. Two mechanisms may be responsible for bile acid transcellular movement: One involves transfer of bile acids from the basolateral membrane to the canalicular membrane via bile acid–binding proteins ; the other moves cellular bile salts through vesicular transport. In contrast, the transport of bile salts across the canalicular membrane of hepatocytes represents the rate-limiting step in the overall secretion of bile salts from the blood into bile.
Bile salt concentrations are 1,000-fold greater within the canaliculi than in the hepatocytes. This gradient necessitates an active transport mechanism, which is an adenosine triphosphate (ATP)-dependent process. The ATP-binding cassette transporter ABCB 11 (formerly known as the bile salt export pump [BSEP]) plays a key role in this process. The ABC transporters mediate the transport of metabolites, peptides, fatty acids, cholesterol, and lipids in the liver, intestines, pancreas, lungs, kidneys, brain, and in macrophages. Although ABCB 11 is the major transporter for monovalent bile salts into the canaliculus, MDR-related protein-2 (MRP2), a member of the multidrug-resistant protein family, also transports sulfated and glucuronidated bile salts into the canaliculus. MRP2 also mediates the export of multiple other organic anions, including conjugated bilirubin, leukotrienes, glutathione disulfide, chemotherapeutic agents, uricosurics, antibiotics, toxins, and heavy metals.
Recent studies suggest that bile acids are signaling molecules that regulate lipid, glucose, and energy metabolism. This function of bile acids is mediated primarily by the nuclear receptor farnesoid X receptor (FXR) and the G-protein–coupled receptor TGR5. Bile acids in the small and large intestine regulate the gut microbiome, incretin secretion, and the production of fibroblast growth factors 15 and 19 (FGF15/FGF19). These FGFs, in turn, modulate lipid, glucose, and energy metabolism and may play a role in the rapid improvement in glycemic control after gastric bypass surgery. In addition, FXR and TGR5 receptors exist in other tissues, such as the heart and the kidneys, and, therefore, may help to explain the dysfunction that occurs in these organs with biliary obstruction.
Compared with bile salts, the biliary lipids, phospholipids and cholesterol play a secondary role in the formation of bile. Phospholipids and cholesterol are formed primarily from low-density lipoproteins circulating in plasma and from de novo synthesis by hepatocytes. Less is known about the secretion of biliary lipids compared with bile salt secretion; however, biliary lipid secretion is crucial for cholesterol disposal, intestinal absorption of dietary lipids, and cytoprotection against bile acid–induced hepatocyte and cholangiocyte injury.
Phospholipid secretion involves the delivery of phospholipids to the inner leaflet of the canalicular plasma membrane. In humans, the MDR3 transporter translocates phospholipids from the inner to the outer leaflet of the canalicular membrane. Progressive familial intrahepatic cholestatis type 3 develops in humans with an MDR3 deficiency. These patients have no phosphatidylcholine in bile and therefore do not form mixed micelles with bile salts. As a result, toxic bile salts injure the biliary epithelium, resulting in neonatal cholestasis, cholestasis of pregnancy, and cirrhosis in adults.
Less is known about the role of transporter proteins in cholesterol secretion, but the ABC transporters ABCG5 and ABCG8 have been demonstrated to be involved in the elimination of plant steroids. Cholesterol is highly nonpolar and insoluble in water and, therefore, also is insoluble in bile. The key to maintaining cholesterol in solution is the formation of micelles, a bile salt–phospholipid–cholesterol complex. Bile salts are amphipathic compounds that contain both a hydrophilic and hydrophobic portion. In aqueous solutions, bile salts are oriented with the hydrophilic portion outward. Phospholipids are incorporated into the micellar structure, allowing cholesterol to be added to the hydrophobic central portion of the micelle. In this way, cholesterol can be maintained in solution in an aqueous medium.
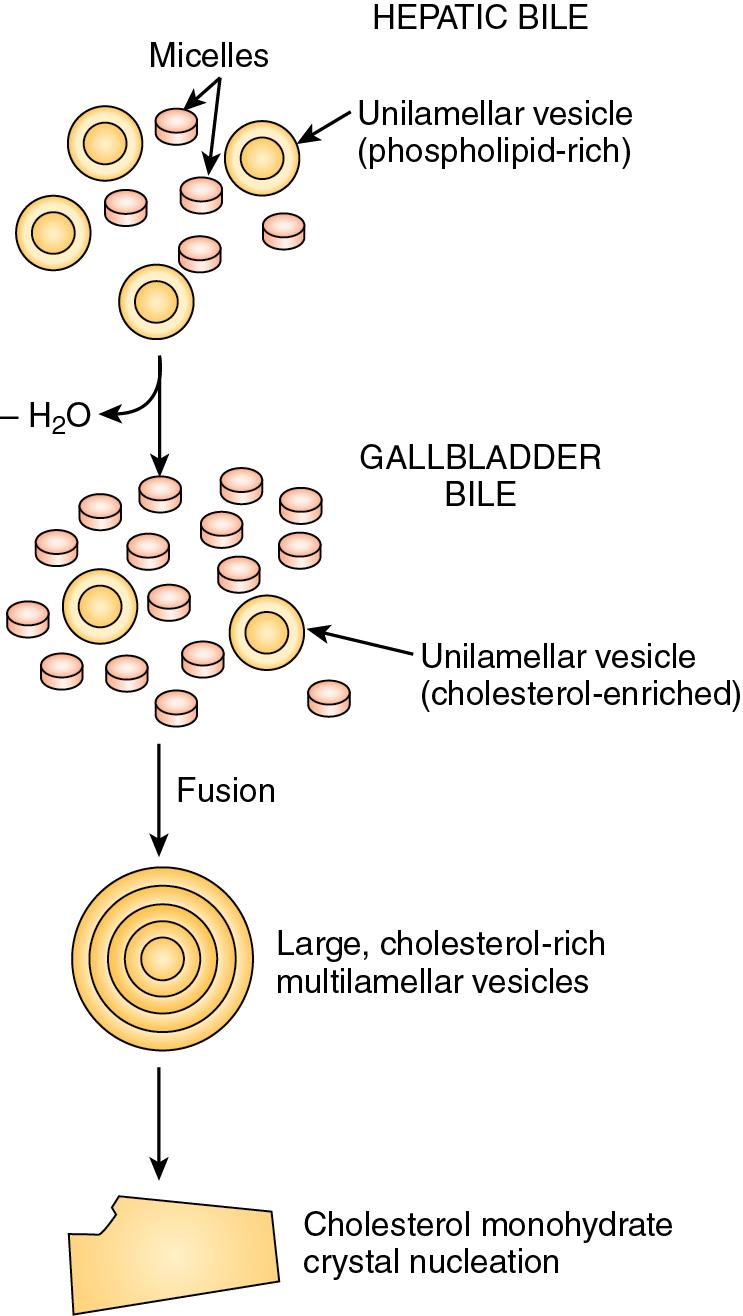
The concept of mixed micelles as the only cholesterol carrier has been challenged by the demonstration that much of the biliary cholesterol exists in a vesicular form. Structurally, these vesicles are made up of lipid bilayers of cholesterol and phospholipids. In their simplest and smallest form, the vesicles are unilamellar, but an aggregation may take place, leading to multilamellar vesicles. Present theory suggests that in states of excess cholesterol production, these large vesicles also may exceed their capability to transport cholesterol, and crystal precipitation may occur ( Fig. 8.2 ).
Heme is released at the time of degradation of senescent erythrocytes by the reticuloendothelial system. Heme is the source of approximately 80% to 85% of the bilirubin that is produced daily. The remaining 15% to 20% is derived largely from the breakdown of hepatic hemoproteins. Both enzymatic and nonenzymatic pathways for the formation of bilirubin have been proposed. Although both may be important physiologically, the microsomal enzyme heme oxygenase—found in high concentration throughout the liver, spleen, and bone marrow—plays a major role in the initial conversion of heme to biliverdin, which is then reduced to bilirubin by the cytosolic enzyme biliverdin reductase before being released into the circulation. In this “unconjugated” form, bilirubin has a very low solubility and is bound avidly to plasma proteins, primarily albumin, before uptake and further processing by the liver. The liver is the sole organ capable of removing the albumin-bilirubin complex from the circulation and esterifying the potentially toxic bilirubin to water-soluble, nontoxic, monoconjugated and deconjugated derivatives.
In the sinusoidal membrane of the hepatocyte, bilirubin is taken up by OATP-C, a membrane transporter belonging to the OATP family. OATP-C is involved with the uptake of both conjugated and unconjugated bilirubin, but unconjugated bilirubin also can cross hepatic sinusoidal membranes by a diffusion process. In the hepatocyte, bilirubin binds to a driver of glutathione- S -transferase and is catalyzed by bilirubin uridine-5′-diphosphate glycosyltransferase to form bilirubin glucuronides. Mutations in the gene encoding bilirubin UDP-glycosyltransferase are associated with the unconjugated hyperbilirubin syndromes, Crigler-Najjar and Gilbert syndromes.
Bilirubin glucuronides are excreted into the bile canaliculus primarily via MRP2, which also plays a role in the transport of glucuroniductal bile salts and a wide spectrum of organic anions, including the antibiotic ceftriaxone. MRP3, which is expressed in the basolateral membrane of hepatocytes and cholangiocytes, also participates in the transport of bilirubin monoglucuronide. In addition, MRP3 may prevent intracellular accumulation of conjugated bilirubin, bile salts, and other organic anions in cholestatic situations.
The bile ducts, gallbladder, and sphincter of Oddi act in concert to modify, store, and regulate the flow of bile. Bile flow is primarily driven by bile salt secretion. During its passage through the bile ductules, canalicular bile is modified by the absorption and secretion of electrolytes and water. Bicarbonate secretion by the bile ducts plays an important role in bile salt–independent bile flow. The gastrointestinal hormone secretin increases bile flow primarily by increasing the active secretion of chloride-rich fluid by the bile ducts. Bile duct secretion also is stimulated by other hormones, such as cholecystokinin and gastrin. The bile duct epithelium is capable of water and electrolyte absorption, which may be of primary importance in the storage of bile during fasting in patients who have previously undergone cholecystectomy. The main functions of the gallbladder are to concentrate and store hepatic bile during the fasting state and deliver bile into the duodenum in response to a meal. The usual capacity of the human gallbladder is about 40 to 50 mL. Only a small fraction of the bile produced each day would be stored, were it not for the gallbladder’s remarkable absorptive capacity.
The enterohepatic circulation provides an important negative feedback system on bile salt synthesis. Should the recirculation be interrupted by resection of the terminal ileum or by primary ileal disease, abnormally large losses of bile salts occur. This situation increases bile salt production to maintain a normal bile salt pool. Similarly, if bile salts are lost through an external biliary fistula, increased bile salt synthesis is necessary. Except for those unusual circumstances in which excessive losses occur, however, bile salt synthesis matches losses, maintaining a constant bile salt pool size. During fasting, approximately 90% of the bile acid pool is sequestered in the gallbladder.
Bile salts are synthesized and conjugated in the liver; secreted into bile; stored temporarily in the gallbladder; passed from the gallbladder into the duodenum; absorbed throughout the small intestine, especially in the ileum; and returned to the liver via the portal vein. This cycling of bile acids between the liver and the intestine is referred to as the enterohepatic circulation ( Fig. 8.3 ). The total amount of bile acids in the enterohepatic circulation is defined as the circulating bile pool. In this highly efficient system, nearly 95% of bile salts are reabsorbed. Thus, of the total bile salt pool of 2 to 4 g, which recycles through the enterohepatic cycle 6 to 10 times daily, only about 600 mg is actually excreted into the colon. Bacterial action in the colon on the two primary bile salts, cholate and chenodeoxycholate, results in the formation of the secondary bile salts, deoxycholate and lithocholate. In fact, the bile acid signature of an individual is very dependent on gut microbial modification. , Bacterial enzymes modify primary bile acids through deconjugation, dehydrogenation, dehydroxylation, and sulfation reactions. In turn, bile acids restrict bacterial proliferation and overgrowth. However, the physiology of bile salts, biliary lipids, bilirubin, bile flow, and the enterohepatic circulation is dramatically altered when the bile ducts become obstructed.

The evaluation and management of the patient with biliary obstruction is a common problem facing the general surgeon. Over the past 40 years, significant advances have been made in our understanding of the pathophysiology, diagnosis, and management of the jaundiced patient. Similarly, advances have been made in perioperative and operative management that have resulted in improved survival of the jaundiced patient. Obstructive jaundice affects multiple organ systems, including hepatic, renal, cardiovascular, hematologic, and immune systems. This section will review the causes, pathophysiology, and management of biliary obstruction.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here