Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The mammary gland is composed of an epithelial system of ducts and lobuloalveolar secretory units embedded in a mesenchymally derived fat pad. The growth and morphogenesis of the epithelial structures of the breast occur in various stages and are associated with concurrent hormonal changes and affected by genetic mutations. Each stage reflects the effects of systemic hormones on the glandular epithelium and also the paracrine effects of locally derived growth factors and other regulatory products produced in the stroma. Appreciating the relationship of epithelium to mesenchyme in normal growth is essential for understanding developmental abnormalities and factors that may lead to disease.
This chapter discusses the morphologic, hormonal, paracrine, and genetic changes of the breast. It also discusses the clinical correlates of the various stages of breast development, including those of the embryo, infancy and childhood, puberty, pregnancy, lactation, and menopause.
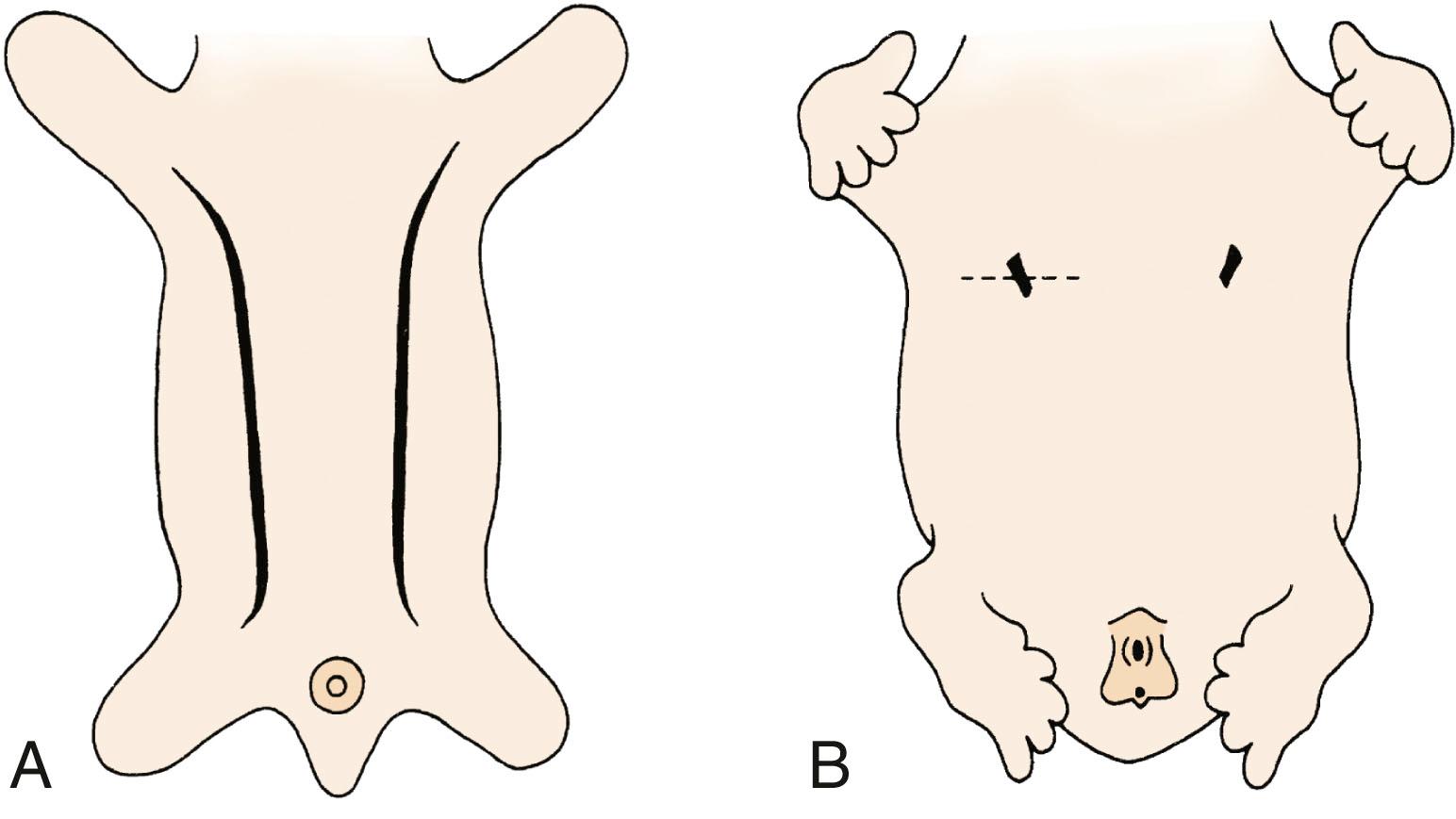
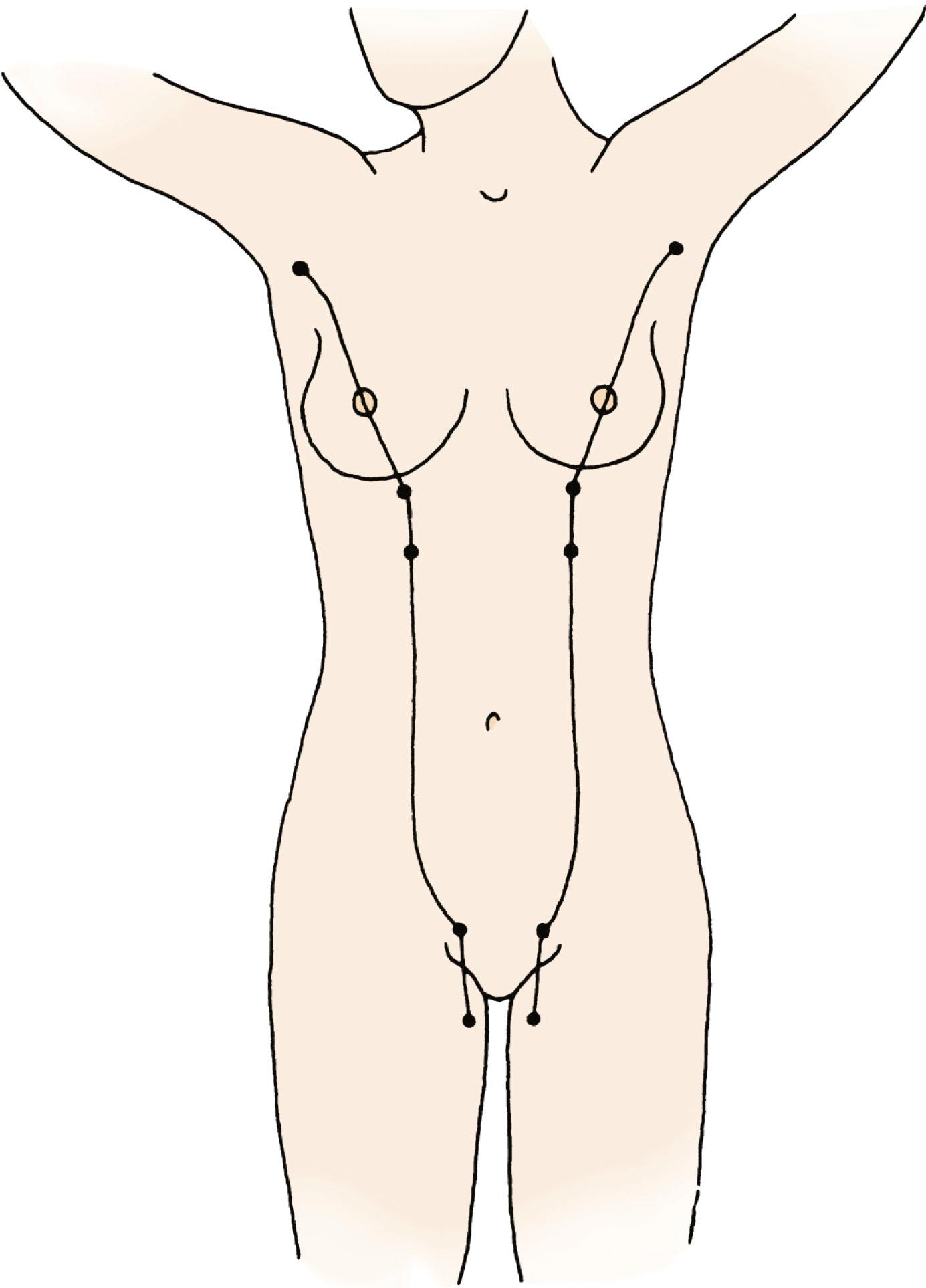
The breast of the human newborn is formed through 10 progressive fetal stages that begin in the sixth week of fetal development. During the fifth or sixth week of development, two ventral bands of thickened ectoderm, called the mammary ridges, are present in the embryo. These multilayered epithelial ridges, named the milk lines, extend from the axilla to the groin and give rise to a single pair of placodes (cluster of primitive mammary epithelial cells) in humans over the thorax ( Fig. 5.1 ). After this, the ectoderm invaginates into the surrounding mesenchyme and enters the cluster of preadipocytes that become the mammary fat pad, with subsequent epithelial budding and branching forming a rudimentary ductal tree. During the latter part of pregnancy, this fetal epithelium further canalizes and ultimately differentiates to the end-vesicle stage seen in the newborn. If the structure fails to undergo its normal regression, accessory nipples (polythelia) or accessory mammary glands (polymastia) may occur along the original mammary ridges or milk lines ( Fig. 5.2 ).
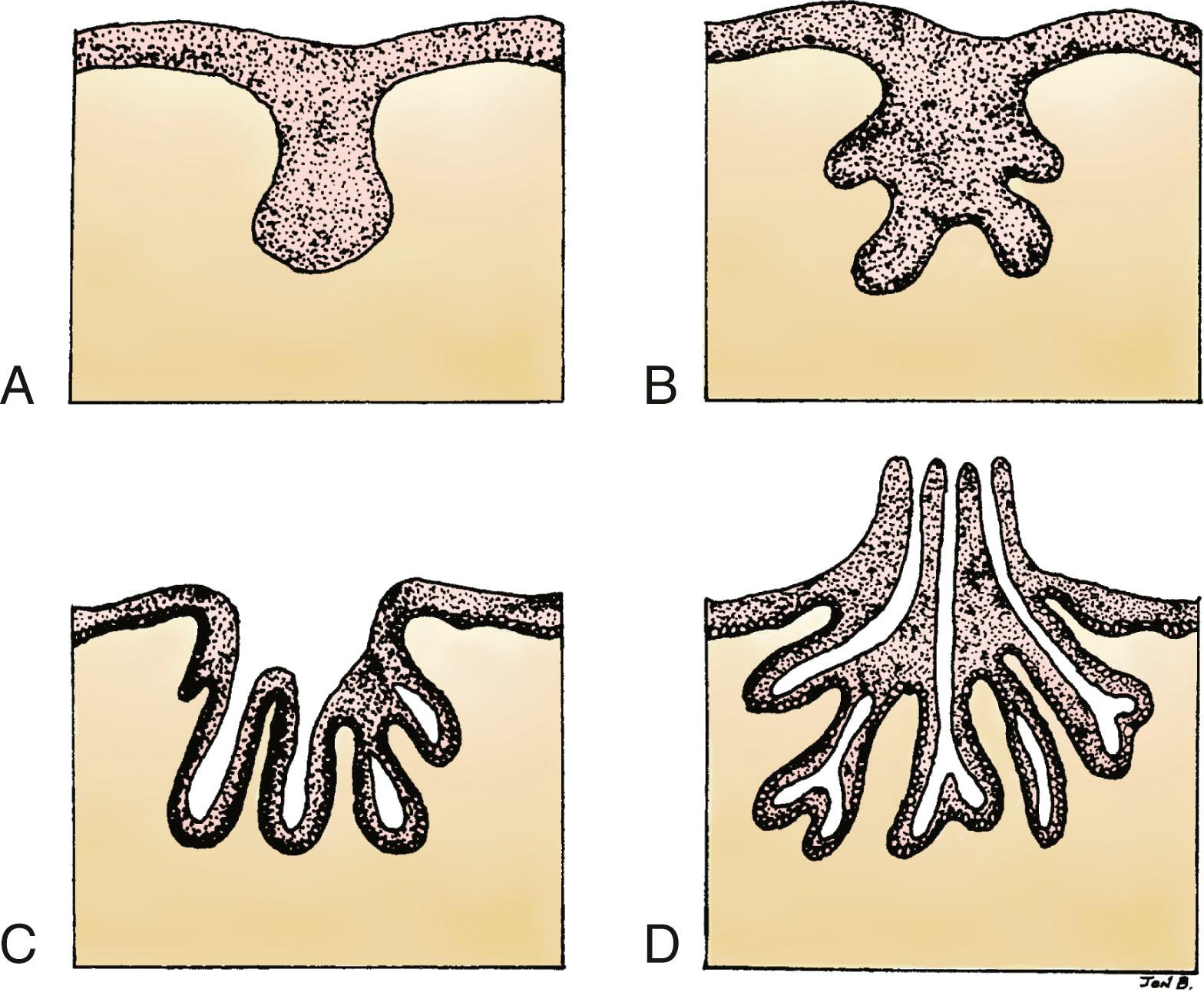
Each gland develops as the ingrowth of the ectoderm forms a primary bud of tissue in the underlying mesenchyme ( Fig. 5.3A ). Each primary bud gives rise to 15 to 20 secondary buds, or outgrowths ( Fig. 5.3B ). During the fetal period, epithelial cords develop from the secondary buds and extend into the surrounding connective tissue. By the end of prenatal life, lumens have developed in the outgrowths, forming the lactiferous ducts and their branches ( Fig. 5.3C ). At birth, the lactiferous ducts open into a shallow epithelial depression, known as the mammary pit. The pit becomes elevated and transformed into the nipple shortly after birth as a result of proliferation of the mesenchyme underlying the presumptive nipple and areola ( Fig. 5.3D ). Failure of elevation of the pit results in a congenital malformation known as inverted nipple.
In a term birth, the breast has six to eight widely patent ducts that empty at the nipple. Recent anatomic studies confirm a parallel bundle of an additional 25 smaller ducts with distinct openings at the nipple surface. All of these initial ducts contain one layer of epithelium and one layer of myoepithelial cells, terminating in a dilated blind sac. These so-called ductules are the precursors of future lobuloalveolar structures, the ultimate milk-producing units of the breast. Interestingly, despite the large number of actual ducts that drain onto the nipple, relatively few ducts contribute to the drainage of the breast. One duct and its branches have been shown to drain one-quarter of the breast and alternatively four ducts and their branches have been shown to drain less than 1% of breast volume. Similar to the development of the ductal system, the subareolar lymphatic plexus also develops from the ectoderm.
From birth until 2 years of age, there is wide individual variability in the morphologic and functional stages in the breast, with some neonates having more well-developed lobular structures and others with more secretory epithelial phenotypes. The degree of morphologic differentiation does not correlate with functional ability. The ability of the entire ductal structure to respond to secretory stimuli may even occur in the rudimentary ductal systems. Ultimately, in normal infant development, the differentiated glandular structures involute and only small ductal structures are left remaining within the stroma.
During childhood, the ductal structures and stroma grow isometrically at a rate similar to that of the rest of the body until puberty. The lymphatics grow simultaneously with the duct system, maintaining connection with the subareolar plexus. As in the fetal stage, there are no morphologic differences between the sexes.
The initial fetal stages of breast development are relatively independent of sex steroid influence. At birth, the withdrawal of maternal steroids results in secretion of neonatal prolactin (PRL) that stimulates newborn breast secretion.
It is currently accepted that epithelial ductal proliferation into the mesenchyme is modulated by local factors, which regulate the epithelial-mesenchymal interaction. Many genes have been expressed in either the epithelium or mesenchyme during mammary embryogenesis, including fibroblast growth factor 7 (FGF-7), FGF, HOX, tenascin-C, syndecan-1, hedgehog network genes, T-box family of transcription factors, androgen receptor, estrogen receptor, transforming growth factor alpha (TGF-α), TGF-β, BCL-2, and epidermal growth factor (EGF) receptor. Mammary epithelial cell line differentiation or milk line specification occurs under the influence of embryonic mammary mesenchyme. For example, NRG3 (a ligand for the receptor tyrosine kinase ERBB4/HER4) appears to be involved in milk line specification by functioning as a mesenchymal paracrine signal and may have roles in mammary epithelial lineage commitment and the promotion of placode formation. Wnt signaling emanating from the mesenchyme is also necessary for initiating mammary line specification. Formation of placodes occurs subsequently and is regulated by TBX3 (T-box transcription factor), and BMP4 (bone morphogenic protein 4) expression. In addition Lef-1, parathyroid hormone–related protein (PTHrP), and the type 1 parathyroid hormone (PTH)/PTHrP receptor (PTHR-1) are also required for mammary branching and nipple development.
In the human embryo, the mammary ridge first becomes apparent in the 7- to 8-mm-long embryo and atrophies before birth. It is the persistence of mammalian tissue along the milk line that results in ectopically displaced or accessory breast tissue (see Figs. 5.1 and 5.2 ). This congenital anomaly is commonly bilateral and is often unaccompanied by the areola or the nipple ( Fig. 5.4 ). A classification system to characterize accessory breast tissue was developed in 1915 that is still used: (1) the presence of a complete breast with mammary gland tissue and the nipple-areola complex, (2) the presence of gland tissue and nipple, (3) gland tissue and areola, (4) solitary gland tissue, (5) nipple-areola with fat replacement of the mammary gland tissue (pseudomamma), (6) the nipple alone (polythelia), (7) the areola alone (polythelia areolaris), and (8) the presence of a small patch of hair-bearing tissue (polythelia pilosa).
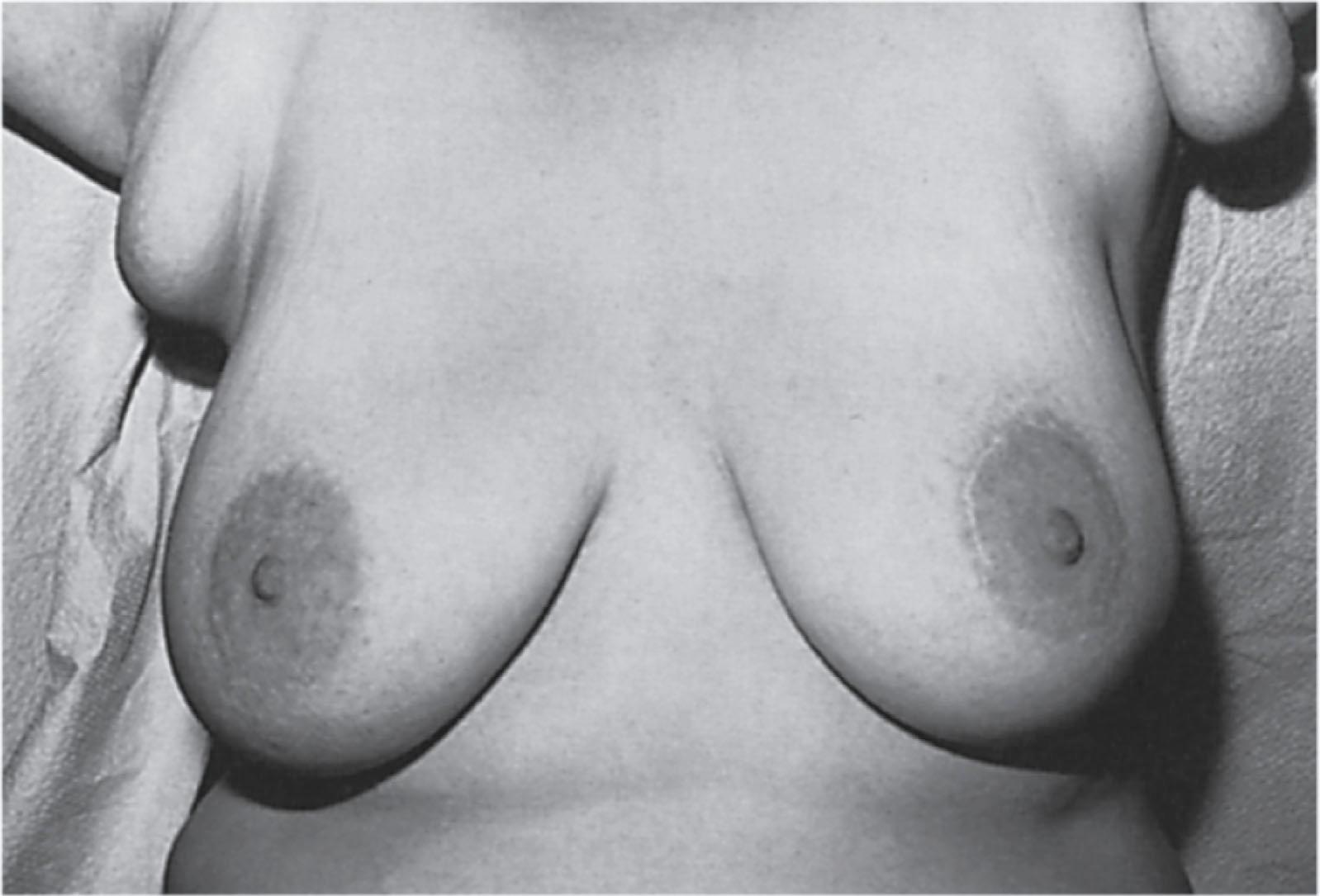
Polythelia is the presence of an additional nipple alone along the milk line. Polythelia represents the most common variant of supernumerary breast components and occurs predominantly between the breast and the umbilicus. However, glandular tissue compatible with complete or variable components of breast parenchyma can occur within the mammary ridge at sites between the axilla and the groin. The embryology, clinical presentation, diagnosis, treatment, and clinical significance of supernumerary nipples, supernumerary breasts, and ectopic breast tissue have been reviewed and important considerations concerning these common anomalies include the following :
Supernumerary nipples, supernumerary breasts, and ectopic breast tissue most commonly develop along the milk lines.
Whereas polythelia is evident at birth, supernumerary and ectopic breast tissue is evident only after hormonal stimulation that occurs at puberty or during pregnancy.
Ectopic breast tissue is subject to the same pathologic changes that occur in normally positioned breasts.
Axillary ectopic breast tissue may be confused with other malignant and benign lesions occurring in the area.
Polythelia may indicate associated conditions, most notably urologic malformations or urogenital malignancies.
The presence of supernumerary or accessory nipples ( Fig. 5.5 ) is a relatively common, minor congenital anomaly that occurs in both sexes, with an estimated frequency of 1 in 100 to 1 in 500 persons. The frequency of supernumerary nipples as 0.22% in a White European population, which is significantly lower than the incidence of 1.63% found in Black neonates. In the newborn Jewish population, the higher incidence of 2.5% for polythelia was observed. This high frequency of supernumerary nipples could possibly be related to ethnic differences but may be related to a systematic technique for examination of the newborn.
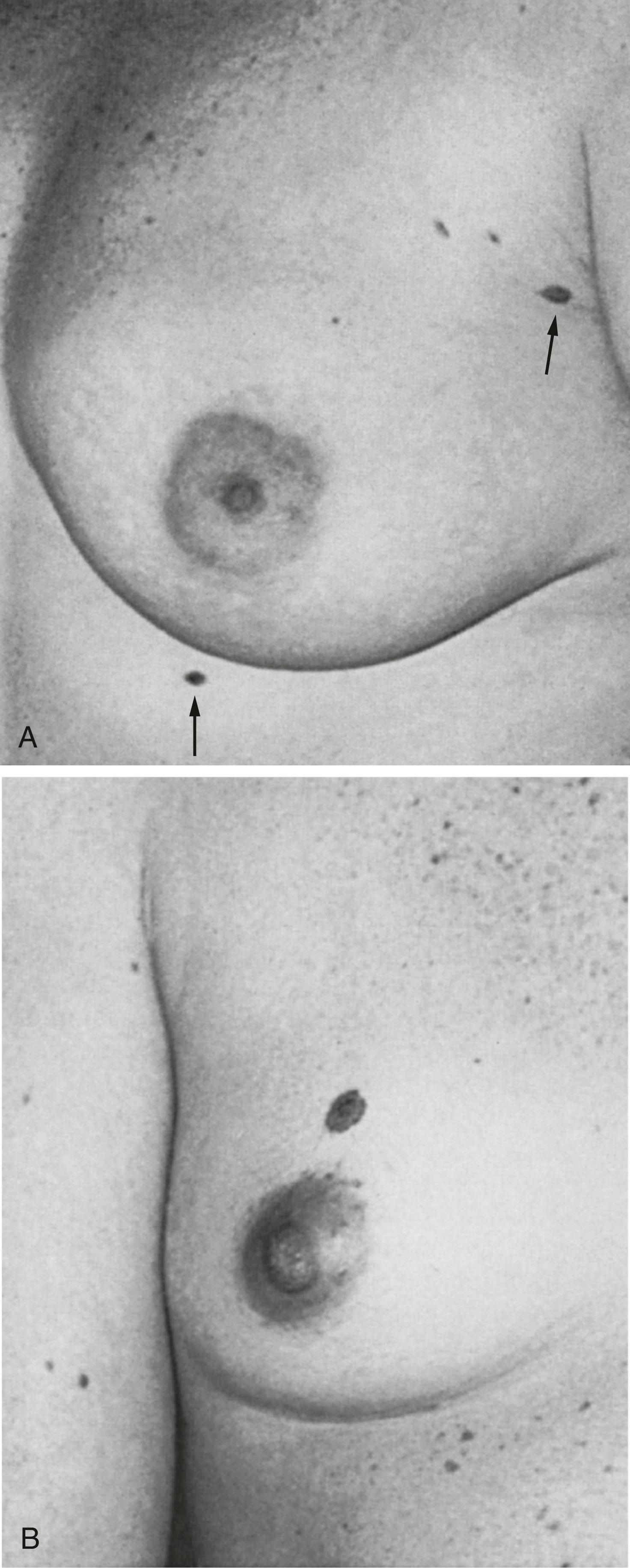
Congenital supernumerary nipples may occur in any size or configuration along the mammary milk line extending from the nipple to the symphysis pubis. As noted earlier, the supernumerary nipple anomaly may be easily overlooked in young infants in whom these ectopic lesions often appear only as a small spot with a diameter of 2 to 3 mm. Supernumerary nipples usually develop just below the normal breast in the White population, with less common occurrence in abdominal or inguinal sites. Bilateral supernumerary nipples occur in approximately half of patients with polythelia. In the ectopic sites, polythelia takes origin from the extramammary buds that are present along the ventral embryonic mammary ridges (see Fig. 5.3 ). Only a minority of persons with this clinical anomaly have more than two extra nipples.
Polythelia should be searched for in the routine physical examination of every newborn, and the presence of the condition should be reported to the parents. This is important for the following reasons :
Supernumerary breasts in females may respond to fluctuations in hormones in a physiologic manner such that pubertal enlargement, premenstrual swelling, tenderness, and lactation during pregnancy and parturition may occur.
Patients with polythelia may be subject to the same spectrum of pathologic diseases observed in normal breasts (e.g., neoplasms, fibroadenomas, papillary adenomas, cysts, or carcinomas).
The supernumerary nipples may be associated with other congenital diseases such as vertebral anomalies, cardiac arrhythmias, or renal anomalies.
In embryogenesis, polythelia occurs during the third month of gestation, when the embryonic mammary ridge fails to regress normally—an event coincident with the development of the urogenital and other organ systems. Although various malformations have been associated with polythelia ( Table 5.1 ), attention has been drawn to the high incidence of renal anomalies and malignancies in children with supernumerary nipples. The association between supernumerary nipples and occult anomalies of the urogenital system has been reported in at least two non–US pediatric populations. Studies from Hungary and Israel have reported that 23% and 40%, respectively, of children with polythelia had obstructive renal abnormalities or duplications of the excretory system. Studies in Hungarian children have shown no link between polythelia and renal anomalies. Studies have cited the prevalence of supernumerary nipples to be 4.29% among healthy newborns and 5.86% among healthy schoolchildren. Ultrasound was used to examine the urogenital system of 496 children with supernumerary nipples and 2367 control patients. The prevalence of renal anomalies was 3.74% in children with supernumerary nipples and 3.17% in the control group; 2.86% in newborns with supernumerary nipples and 1.89% in control newborns. The differences were not statistically significant. The association of polythelia with urogenital malformations continues to be controversial. Recommendations vary from no screening to the screening of all children with polythelia. The true association of these two entities likely has not been elucidated completely.
| Urinary Tract Abnormalities | Cardiac Abnormalities | Miscellaneous Abnormalities |
|---|---|---|
| Renal agenesis | Cardiac conduction disturbances, especially left bundle branch block | Pyloric stenosis |
| Renal cell carcinoma | Epilepsy | |
| Obstructive disease | Ear abnormalities | |
| Supernumerary kidney(s) | HypertensionCongenital heart anomalies | Arthrogryposis multiplex congenita |
Polythelia has also been associated with cancers of the testis and kidney. Familial as well as sporadic occurrences of polythelia with renal cancer, urogenital anomalies, and germ cell tumors have been reported. The authors suggest that this may represent a genetic or developmental link between renal adenocarcinoma and polythelia.
Intraareolar polythelia represents a nipple-areola unit within the mammary ridge such that a dichotomy of the vestigial breast and nipple-areola complex exists. Only a few cases of bilateral intraareolar polythelia have been recorded. Multiplicity of nipples is not uncommon, and they are bilateral in approximately half of patients affected. As many as 10 nipples have been recorded in a single patient. Atypical locations have been noted secondary to the displaced embryonal primordium.
The presence of supernumerary nipples may necessitate operative therapy in instances in which discharge, tumor, or cyst formation is evident. Simple elliptical excision placed in lines of cleavage or skin folds is preferred to achieve maximum cosmesis. Primary closure is usually possible and allows the surgeon to achieve a superior cosmetic result.
Polymastia, also known as accessory breasts or supernumerary breasts, results from the embryonic mammary ridge (see Fig. 5.1A ) failing to undergo normal regression (see Fig. 5.1B ). Causal factors are yet unknown. The prevalence of polymastia was 0.1% in the Collaborative Perinatal Project, although another study suggested a frequency approaching 1%. Similar to polythelia, reports on polymastia suggest an association between renal adenocarcinoma and renal malformations.
A familial occurrence of the polymastia anomaly has been observed. The association of polymastia with congenital cytogenetic syndromes, especially those involved with chromosomes 3 and 8, has been reported. Furthermore, other congenital anomalies, notably Turner syndrome (ovarian agenesis and dysgenesis with chromosomal karyotypes of 45,X, but mosaic patterns [45,X/46,XX or 45,X/46,XX/47,XXX] are seen) and Fleisher syndrome (lateral displacement of the nipples to the midclavicular lines with bilateral renal hypoplasia ) may include polymastia as a component ( Fig. 5.6 ).
A 1995 case presentation and review of the literature regarding carcinoma of ectopic breast tissue reported that of a total of 90 cases of carcinoma of ectopic breast tissue, 64 occurred in the axilla. A 20-year single-center study based out of Italy reported ectopic breast cancers occurring in 0.3% to 0.6% of all cases of breast cancer after reviewing 12,177 patients. The combined survival beyond the 4-year posttreatment period was 9.4%. No survival advantage was found for radical or modified radical mastectomy over local excision combined with axillary dissection or radiation. The researchers found that the correct preoperative diagnosis was rarely made, and they suggested that improved prognosis requires diagnostic suspicion and early biopsy of suspicious ectopic masses that occur along the embryonic milk lines. In related studies, fine-needle biopsy has been found to be useful in the diagnosis and management of ectopic breast tissue.
Ectopic axillary breast tissue is a relatively uncommon occurrence but is a relatively common variant of polymastia. The presence of accessory axillary breast tissue most commonly becomes apparent at or after puberty, with the most rapid growth observed during pregnancy. It has been suggested that axillary breast tissue may represent true ectopic tissue not contiguous with the breast but more commonly represents an enlargement of the axillary tail of Spence. Thus, to determine the presence or absence of accessory axillary breast tissue, one must distinguish between an enlargement of the axillary tail and ectopically displaced mammary tissues of the milk line. The occurrence of ectopic breast tissue outside the axilla is exceedingly rare, although a hamartoma of ectopic breast tissue in the inguinal region has been reported. The finding, confirmed with histopathologic examination, occurred in a 50-year-old woman suspected of having a chronic incarcerated hernia.
The discovery of accessory axillary breast tissue usually occurs during the first pregnancy because of the secondary changes initiated with hormonal stimulation by ovarian estradiol and placental estriol. The symptomatic axillary breast tissue becomes painfully enlarged and, on rare occasion, may develop galactoceles with milk secretion via contiguous skin pores. Although these anomalies may not become evident until the first pregnancy, once the lesions are recognized, they continue to recur with subsequent pregnancies and may undergo cyclical changes during menstruation. Often, the clinician identifies the lesion as excess axillary fat, although lymphadenitis, lymphoma, metastatic carcinoma, and hidradenitis suppurativa are common misdiagnoses. After identification of the hormonal dependency with pregnancy or menstruation, the clinician can often establish the diagnosis, especially if a history of lactation during the puerperium is confirmed.
Management consists of reassuring the patient of its common benignity and its embryologic origin. However, accessory axillary tissue may be misdiagnosed as the symptomatic alterations inherent with pathologic changes of breast tissue. Treatment of symptomatic accessory breast tissue during the puerperium and pregnancy involves conservative management for most clinical presentations. The presence of dense, nodular masses suggestive of malignant transformation necessitates aggressive approaches to rule out carcinoma. As this hormonally dependent accessory breast tissue rapidly regresses when lactation ceases, the patient can be reassured but should be admonished that enlargement and painful, lactating accessory tissue may recur with subsequent pregnancy. Elliptically placed incisions in skin folds of the axilla allow complete dissection and removal of the breast tissue beneath the skin and over the underlying fascia. The cosmetically oriented resections of the accessory tissue are usually curative, although the lesion may recur if excision is incomplete.
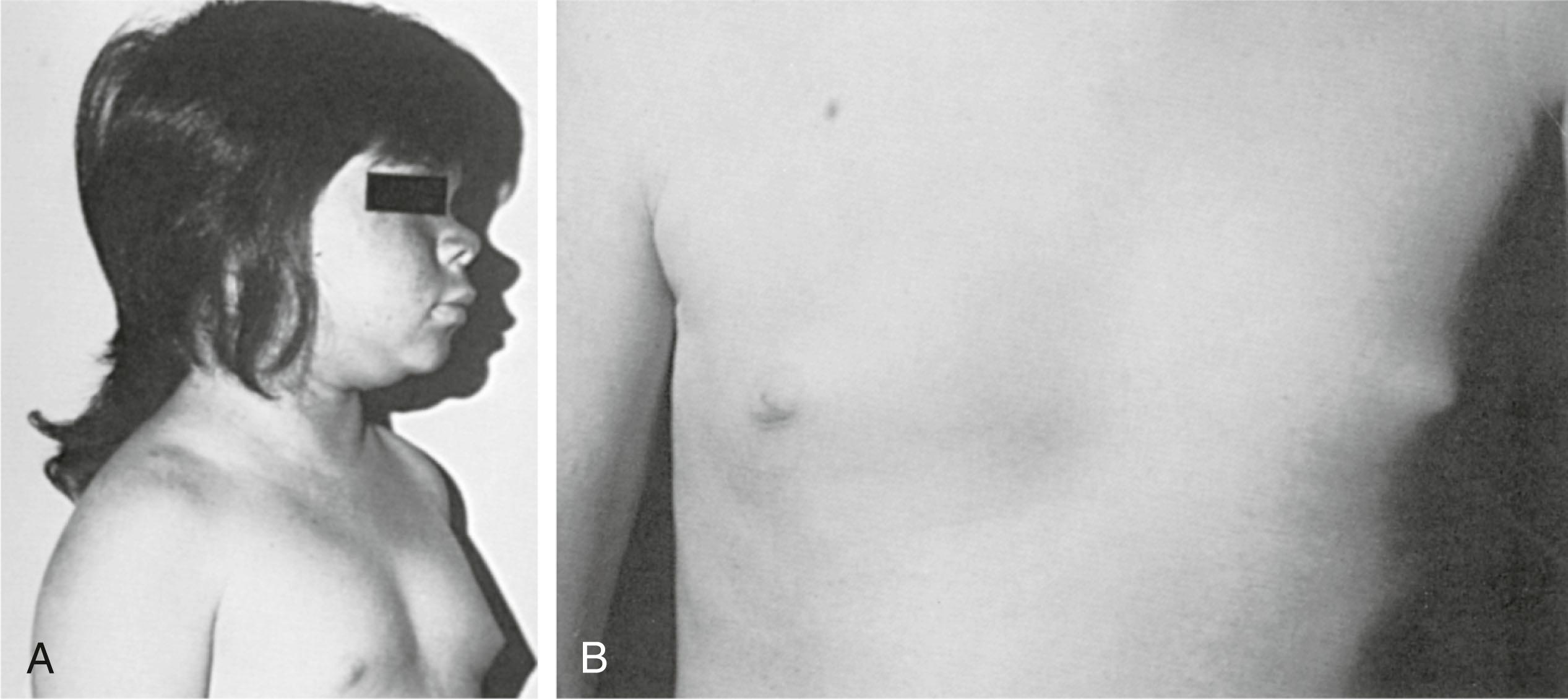
The congenital absence of one or both breasts (amastia) is a rare clinical anomaly. Unilateral absence of the breast ( Fig. 5.7 ) is more common than bilateral amastia, and such subjects are most commonly female. This rare physical defect occurs as a result of complete failure of the development of the mammary ridge at about the sixth week in utero. Most often, abnormalities are not associated with bilateral absence of nipple and breast tissue. However, association with cleft palate; hypertelorism and saddle nose; and anomalies of the pectoral muscle, ulna, hand, foot, palate, ears, genitourinary tract, and habitus have been observed. Occasionally, several members of a family may be affected. At least four reports document the transmission of this anomaly with pedigree penetrance consistent with dominant inheritance. For example, mutations in TBX3 result in autosomal dominant ulnar-mammary syndrome , characterized by malformation of upper limb structures, apocrine/mammary hypoplasia and/or dysfunction, dental abnormalities, and genital abnormalities. Apocrine abnormalities may be as significant as complete absence of breasts (amastia).
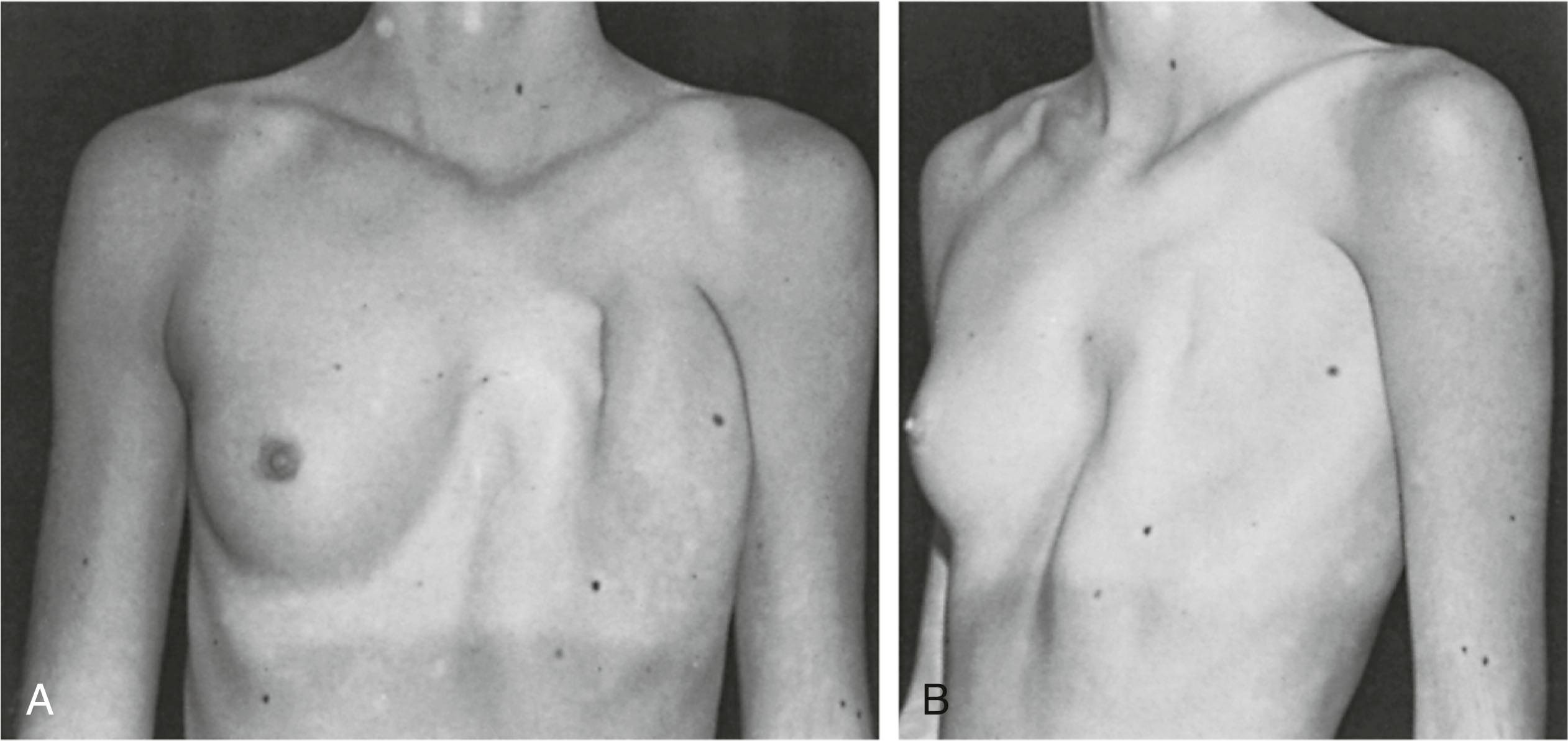
Poland syndrome is characterized by unilateral congenital absence of the pectoralis major and minor muscles, associated absence of the external oblique, and partial absence of the serratus anterior. Additionally, hypoplasia or complete absence of the breast or nipple, costal cartilage and rib defects, hypoplasia of subcutaneous tissue of the chest wall, and brachysyndactyly may also be observed. Poland syndrome is rare with incidence estimated to be 1:20,000 to 1:50,000. The etiology of this malformation is unknown but believed to be related to improper development of the subclavian axis with a resulting impedance of blood flow to the affected structures.
Clinical manifestations of Poland syndrome are extremely variable, and rarely are all features recognized in a single individual. At least two reports confirm a variant of the disorder associated with large melanotic spots. Because breasts and melanocytes both originate from the ectoderm, abnormalities of breast hypoplasia and hyperpigmentation probably develop from within this germinal layer. Patients often do not request treatment of the pigmented abnormalities, and standard methods used in the therapy of hyperpigmentation often yield unsatisfactory results. Such hyperpigmented areas appear to have no neoplastic risk.
Poland syndrome is invariably unilateral, with a higher incidence in female than male patients. When a chest wall defect (ribs, cartilage, or both) is evident, there is usually a deep concavity on expiration and lung herniation with inspiration (see Fig. 5.7 ). The right side is more commonly affected than the left. The most common defect, breast hypoplasia, is readily recognized, and the rudimentary breast tissue is usually higher on the involved side and medially displaced from its normal anatomic position.
Although the cause is unclear, this syndrome is seldom familial. Despite hypoplastic breast tissue, several cases of breast cancer have been documented on the side affected by Poland syndrome. Standard techniques for sentinel lymph node biopsy can be used in these patients even though they have altered anatomy. Leukemia has been associated with the syndrome, as have other rare congenital anomalies. Similar defects have been noted with exposure to drugs, such as thalidomide.
Treatment of patients with Poland syndrome varies with the number of anomalies and their physical expression. With the presentation of one or two typical characteristics of Poland syndrome, patients’ general complaint is with their appearance. These patients are not functionally embarrassed by their lack of anterior chest wall muscle mass or the small size of their breast. Only in extreme cases, as with total absence of the costal cartilage or segments of the anterior ribs, are patients physically impaired and emotionally disturbed by their deformity. Indications for operative intervention include progression of chest depression, lack of protection of the heart and lung, and paradoxical chest wall movement. Surgery may also be needed for cosmetic reconstruction of the breast or chest wall musculature.
Surgical procedures to correct the deformities of the chest wall have been documented and include (1) subperiosteal grafts from adjacent ribs with free flaps of latissimus dorsi or external oblique, (2) autologous split-rib grafts, (3) split-rib grafts with periosteum that has been detached posteriorly and rotated from the anterior aspect of the defective rib to the sternum, (4) heterologous bone grafts, (5) metallic mesh implants followed by rib grafts from the opposite chest wall, and (6) customized silicone breast and chest wall prostheses to reconstruct both structures in difficult cases. In the 1970s, the use of split-rib grafts from the opposite chest wall that are placed across the defect and reinforced with Teflon felt was popularized. Another technique used autologous tissue of the latissimus dorsi myocutaneous flap to augment the hypoplastic breast and to contour the anterior chest wall while simultaneously augmenting the involved hypoplastic breast. When this procedure was initially attempted using free latissimus dorsi flaps, it was unsuccessful because the transplanted muscle atrophied as a result of the omission of the neurovascular pedicle from the transplant, emphasizing the value of preservation of the pedicle when employing this technique. In 1950 the use of a latissimus dorsi muscle flap transferred through the axilla for anterior chest wall reconstruction with preservation of the neurovascular bundle was described. This, too, was abandoned.
The value of a single-stage reconstruction has since been emphasized. The high success rate and the reliability of this technique, which uses the latissimus dorsi myocutaneous flap, represents remarkable advance over the aforementioned methods. Latissimus dorsi myocutaneous flaps continue to be the most commonly used flap for breast and chest wall reconstruction in patients with Poland disease. This procedure is now being performed with an endoscopic approach for flap harvest and reconstruction. Additionally, tissue expanders followed by replacement with permanent prosthetic implants is a technique commonly employed for these patients.
Computed tomography (CT) provides useful information in planning reconstructive surgery in patients with Poland syndrome. A three-dimensional CT scan may be used as an adjunct for planning chest wall and breast reconstruction in Poland syndrome. Follow-up with three-dimensional magnetic resonance imaging (MRI) reformation is valuable to demonstrate the results of the implant reconstruction. The authors suggest that these imaging techniques can be used to accurately portray the three-dimensional tissue deficit and assist in the selection of muscle transposition flaps and reconstructive technique.
As previously described, PRL stimulates newborn breast secretion. This secretion, called witch’s milk , contains water, fat, and debris; it occurs in 80% to 90% of infants, regardless of sex. The secretion dissipates within 3 to 4 weeks as the influence of maternal sex hormones and PRL decreases. It should not be mechanically expressed, which could predispose to staphylococcal infection and result in breast bud destruction. The secretion may sequester at the nipple epithelium and resemble a pearl. This is called a lactocele and will resolve with time.
Premature thelarche is breast development before 8 years of age without concomitant signs of puberty. Usually bilateral, it is most commonly seen within the first 2 years of life. It is believed to be the result of the persistence or increase in the breast tissue present at birth, and it resolves within 3 to 5 years with no adverse sequelae. It has been suggested that although the initial stimulation of the infant breast may be secondary to maternal influences, the persistence of breast tissue may be related to infant hormones that may support breast growth. Persistent elevations of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol in infants support this hypothesis ( Fig. 5.8 ). With the increasing prevalence of premature puberty, there is concern for environmental endocrine disruptors contributing to this phenomenon, although no statistically significant relationship for this has been established at this time.
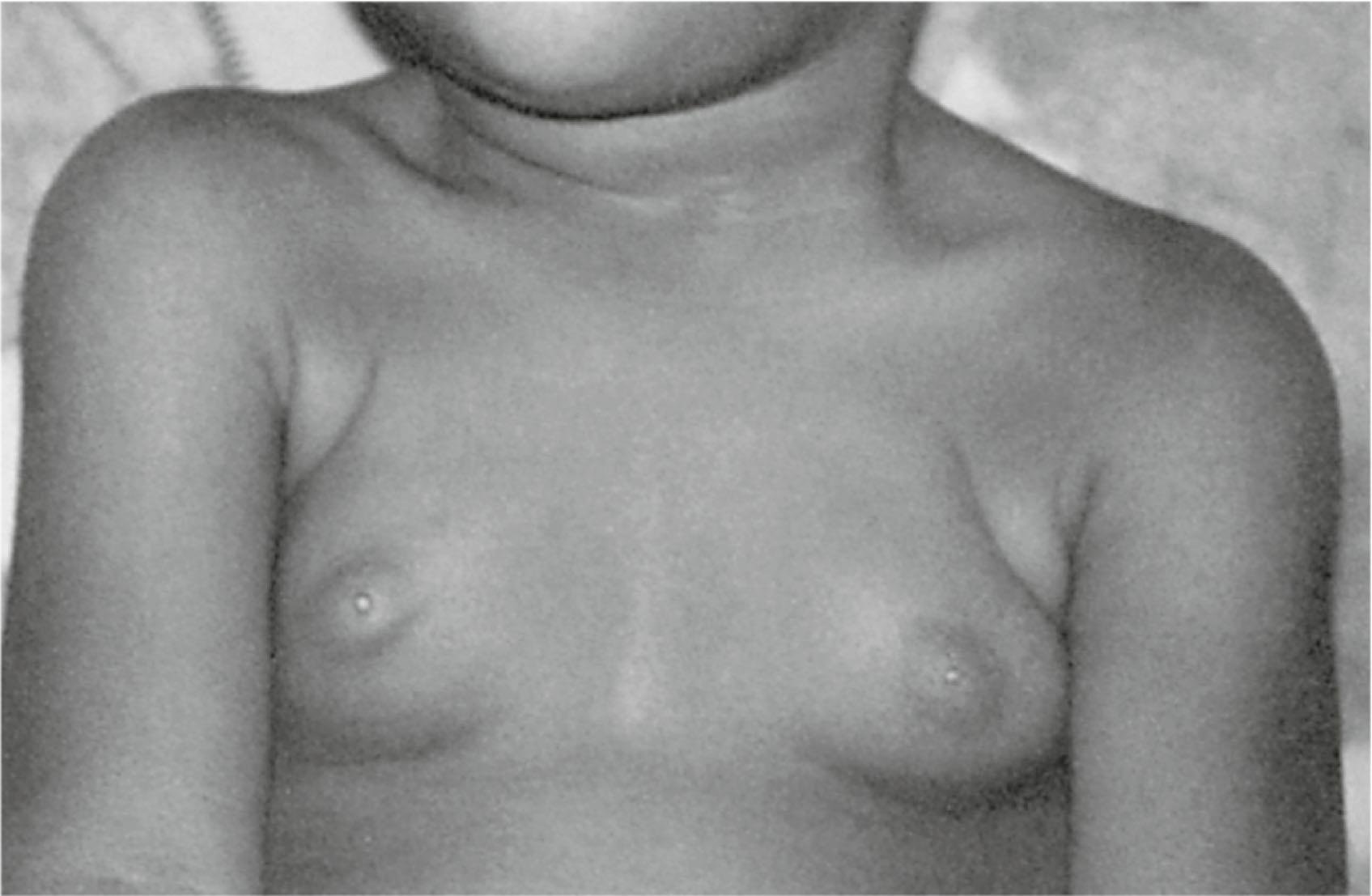
A second period of premature thelarche may occur after 6 years of age. The reason for breast bud formation is unclear; because it can occur before the rise in estrogen levels, it is unlikely that estrogen is the key signal. Serum androgen levels, free estrogen level, altered FSH secretion, and altered serum insulin-like growth factor-I (IGF-I)-to-IGF-binding protein-3 ratios have been implicated as potential factors. The breast tissue may persist or regress, but puberty occurs at the usual time and progresses normally. If assessment of bone age reveals no evidence of precocious puberty, no further evaluation is needed.
Breast development before 8 years of age that is accompanied by other signs of puberty defines precocious puberty. Altered or premature gonadotropin-releasing hormone (GnRH) secretion may cause central precocious puberty ( Fig. 5.9 ). Although more commonly idiopathic, central precocious puberty can be caused by cerebral infections and granulomatous conditions. Certain tumors, in particular hypothalamic hamartomas, can contain GnRH and disrupt the inhibition of GnRH, or release TGF-α (a stimulant of GnRH), resulting in precocious puberty. Peripheral precocious puberty may result from the effects of estrogen from food, ovarian cysts, constitutionally activated ovaries in McCune-Albright syndrome (a triad of café-au-lait spots, long-bone fibrous dysplasia, and precocious puberty), or in primary hypothyroidism ( Fig. 5.10 ). In addition to a history and physical examination, serum gonadotropin and sex steroid levels should be obtained in the workup of precocious puberty. High levels of both gonadotropins and sex steroids indicate central precocious puberty. MRI or CT imaging of the head can be performed to rule out a central lesion before treatment with GnRH analogs. Suppressed (low) levels of gonadotropins and high levels of sex steroids are consistent with peripheral precocious puberty.
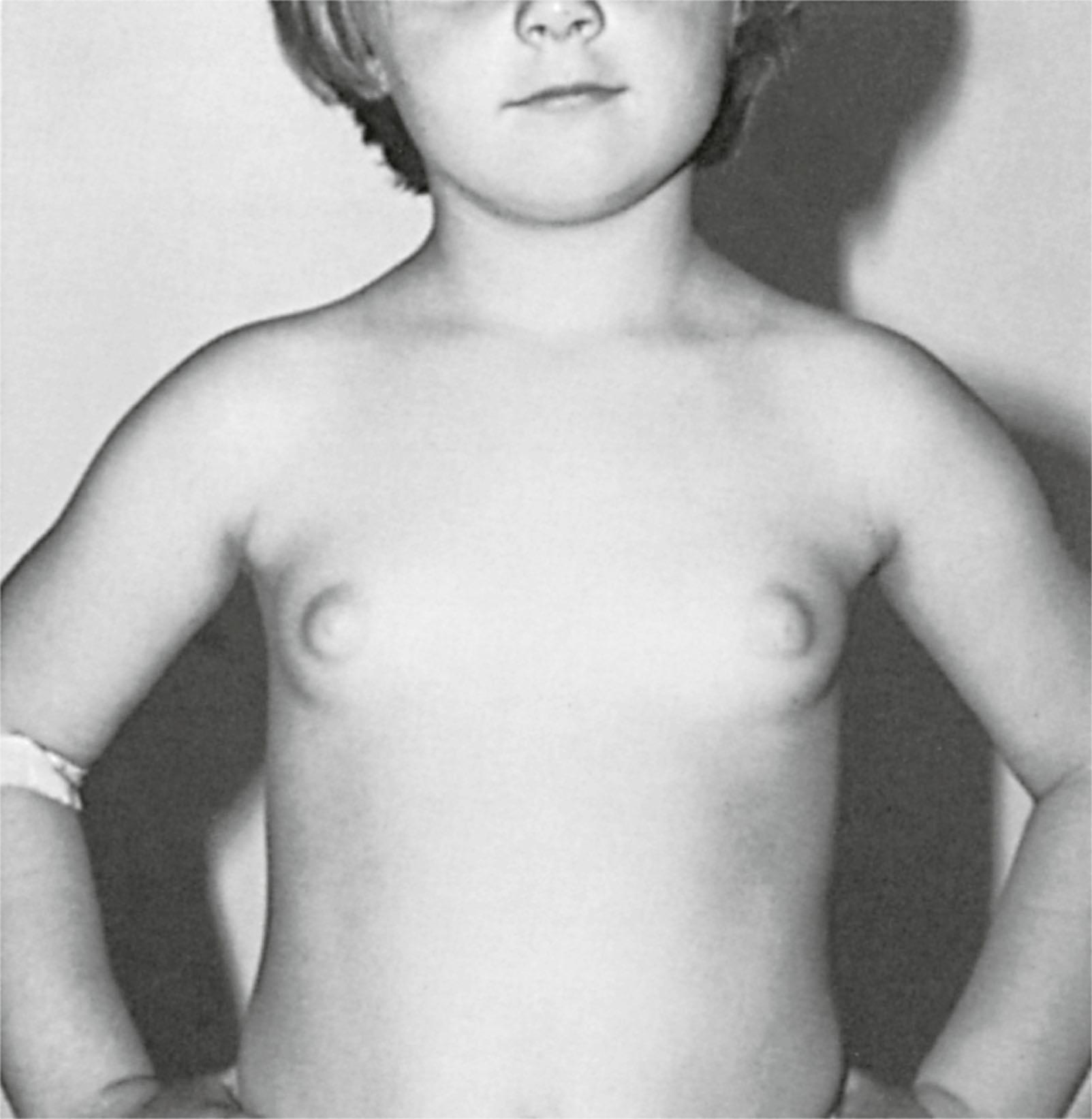
Exposure to toxins early in life may increase the mammary glands’ susceptibility to future carcinogenic exposure as well as have direct carcinogenic effects. For example, diethylstilbestrol (DES), a synthetic estrogen used to prevent miscarriage from the 1940s through 1970s, has been associated with breast cancer in women who were directly exposed, as well as their daughters.
Thelarche , the beginning of adult breast development, marks the onset of puberty in the majority of White women and occurs at a mean age of 10 years; in Black women, it occurs at 8.9 years and is usually preceded by the appearance of pubic hair. Changes in the breast contour and events in nipple development characterize the milestones in the staging system detailed by Marshall and Tanner ( Fig. 5.11 ). However, these outward changes in the breast do not necessarily correlate with underlying structural events occurring with the new hormonal milieu of puberty.
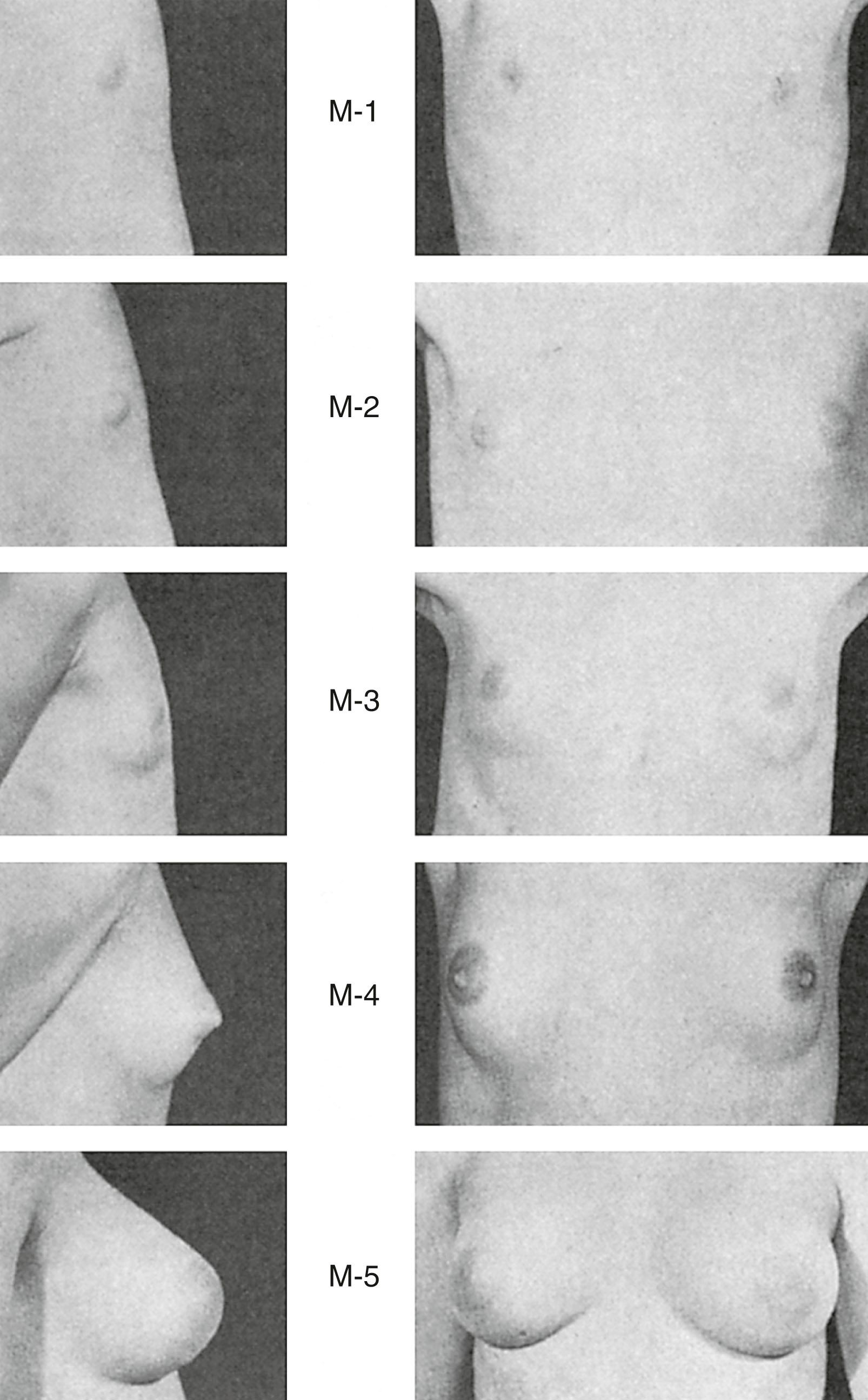
The immature ductal system before puberty is believed to undergo a sequential progression to a mature lobuloalveolar system during adolescent development ( Fig. 5.12 ). In the ductal growth phase , ducts elongate, ductal epithelium thickens, and periductal connective tissue increases forming an extensive mammary tree. Lateral branches lead to terminal ducts ending with terminal ductal lobular units (TDLUs). These units comprise numerous acini (ductules) embedded in fibroblastic stroma. Mammary gland stroma includes vascular supply, lymphatic drainage, immune barrier, adipose-rich fat pad, and an extracellular matrix working together to promote mammary epithelial cell growth, differentiation, and regression during branching morphogenesis.

In early puberty, the TDLU is termed a virginal lobule or lobule type 1 (Lob 1). Lob 1 is the predominant lobule found at this stage of development. Under the cyclic influence of ovarian hormones, some of the Lob 1 will undergo further division and differentiate into a lobule type 2 (Lob 2). In Lob 2 the alveolar buds become smaller but four times more numerous than those in Lob 1; these buds are termed ductules or alveoli . Lob 2 are present in moderate numbers during the late teens but then decline after the mid-20s. Ultimately, the greatest number of lobules will be found in the upper outer quadrant.
In the mature breast, the subareolar lymphatic plexus (Sappey’s plexus) contains communications with both deep and superficial intramammary lymphatics and provides a high volume of lymphatic outflow to regional lymph nodes. In lymphatic studies, there appears to be a consistent channel that originates from the subareolar plexus and extends to the regional lymph nodes, termed the sentinel lymphatic channel.
The pattern of release of GnRH from the hypothalamus initiates and regulates the secretion of FSH and LH seen with puberty. The initial immaturity of the hypothalamic-pituitary axis results in anovulatory cycles for the first 1 to 2 years after menses begins, subjecting the breast and the endometrium to the effects of unopposed estrogen. It is during this period of unopposed estrogen stimulation, considered an “estrogen window,” that the ductal growth phase occurs (see Fig. 5.11 ).
The major hormonal influence on the breast at the onset of puberty is estrogen. A potent mammogen, estrogen primarily stimulates ductal growth but also increases fat deposition and contributes to later phases of development. Impaired ductal growth has been demonstrated in both mice lacking the functional gene for the estrogen receptor and mice treated with tamoxifen, an estrogen receptor modulator. The estrogen receptor ERα is thought to be the key mediator of estrogen effects and has only been documented in luminal epithelium in humans. Despite its influential role, estrogen is unable to work independently. Lyon’s classic experiments using oophorectomized, adrenalectomized, and hypophysectomized rodents demonstrated that a minimum combination of estrogen, growth hormone (GH), and corticoids is necessary to induce ductal growth.
The effects of GH may be mediated by enhancing stromal secretion of IGF-I. IGF-I synergizes with estrogen to increase elongation and growth at the terminal end bud (TEB) in a paracrine manner. In some reviews, glucocorticoids contribute to the maximal growth of ducts, but extensive ductal growth can occur in its absence. Progesterone does not appear to be necessary in early ductal growth but appears to be essential for lobuloalveolar development. The ratio of the progesterone receptor (PR) isoforms PGR-A and PGR-B may play a critical role in modulating the effect of progesterone. PRL, GH, estrogen, and glucocorticoids, in addition to progesterone, have been found to be necessary for full lobuloalveolar development.
PRL-deficient knockout mice with adequate progesterone levels exhibited incomplete lobule formation. PRL also works indirectly by facilitating progesterone’s actions on the breast. PRL increases progesterone secretion from the corpus luteum by inhibiting progesterone’s degradation enzyme, 20α-hydroxysteroid dehydrogenase. In addition, PRL upregulates the PR in the mammary epithelium. Estrogen contributes to lobuloalveolar development by upregulating PRs. Insulin can bind to IGF-I receptors and may contribute to ductal or lobuloalveolar development, but it is not essential.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here