Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Obstructive uropathy encompasses the numerous sequelae that may be observed when there is an anatomic or functional blockage of the natural flow of urine. Obstructions may occur at any level in the urinary tract, and the clinical signs and symptoms often provide information about both location and severity.
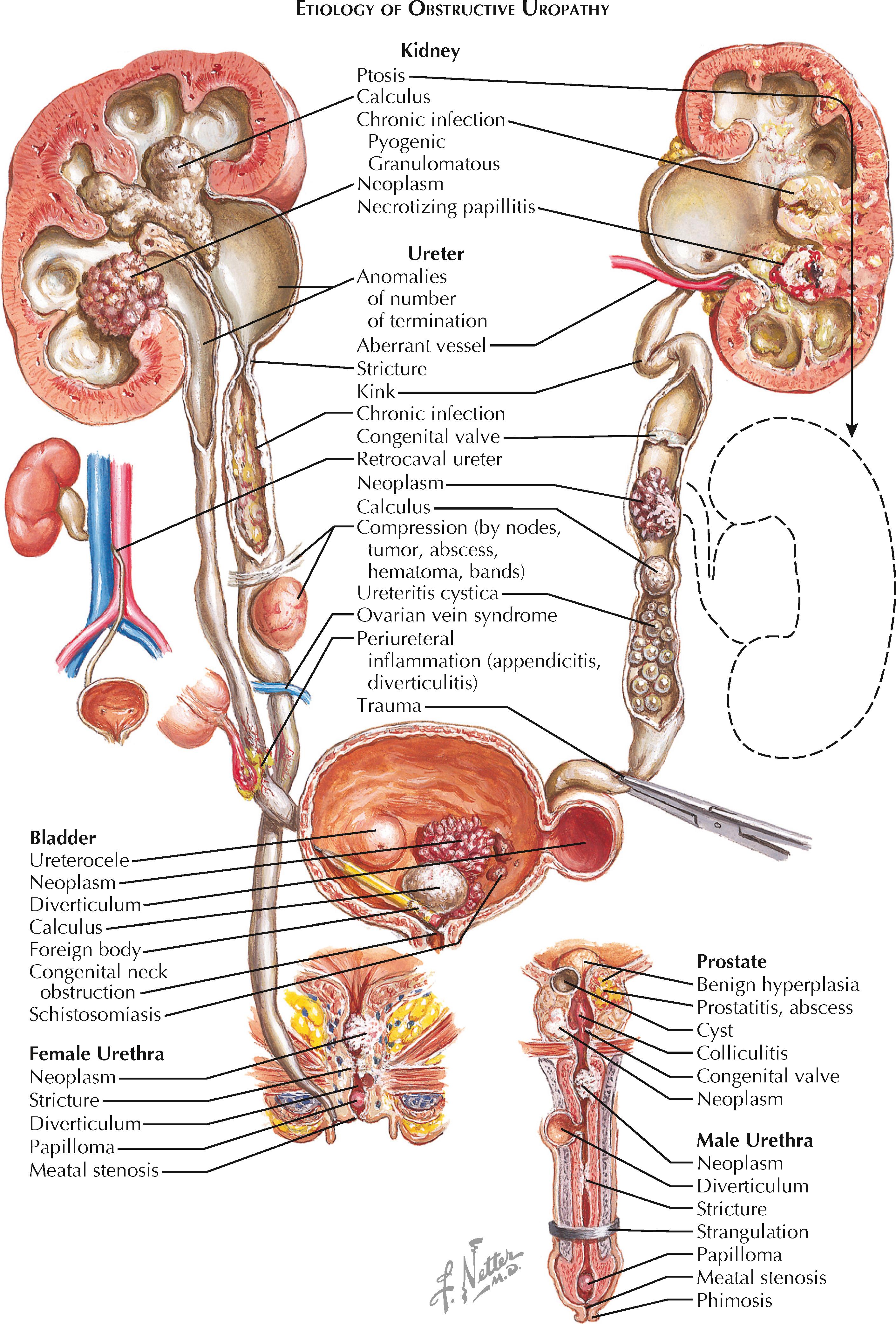
Obstructions may be classified as congenital or acquired, acute or chronic, partial or complete, and intrinsic or extrinsic. All of these characteristics must be taken into consideration when deciding on the optimal treatment plan.
Congenital obstruction may occur secondary to numerous abnormalities in normal urinary tract anatomy, including congenital urethral meatal stenosis, posterior urethral valves (in boys, see Plate 2-34 ), ureterocele (see Plate 2-26 ), ectopic ureter (see Plate 2-25 ), and ureteropelvic junction obstruction (see Plate 6-6 ). In addition, a congenital spinal dysraphism, such as myelomeningocele or sacral agenesis, may result in bladder dysfunction and functional obstruction.
Acquired obstructions may occur secondary to numerous different phenomena, either intrinsic (i.e., within the ureteral lumen) or extrinsic. In the upper tract (i.e., the kidneys and ureters), the numerous causes of intrinsic obstruction include nephrolithiasis (see Plate 6-3 ), ureteral strictures (see Plate 6-7 ), tumors ( Plate 9-9 ), polyps, blood clots, and fungal balls. The numerous causes of extrinsic obstruction include retrocaval ureter (see Plate 2-19 ), retroperitoneal fibrosis, retroperitoneal hematoma, primary retroperitoneal tumor, pelvic lymphadenopathy, and pregnancy. A functional, rather than structural, obstruction may occur secondary to a nonperistaltic segment of ureter, as seen in some ureteropelvic junction (UPJ) or ureterovesical junction (UVJ) obstructions. In the lower tract (i.e., the bladder and urethra), common causes of intrinsic obstruction include urethral stricture, urethral diverticulum, foreign body, benign prostatic hyperplasia (BPH), prostate cancer, primary bladder neck dysfunction, bladder neck contracture, and bladder cancer. Meanwhile, extrinsic compression may occur secondary to local extension of cancers in adjacent organs (e.g., cervical, uterine). A functional obstruction may result from neuropathic bladder dysfunction (see Plate 8-2 ).
An obstruction has numerous effects on the urinary system, beginning with compensation and ending with symptomatic decompensation.
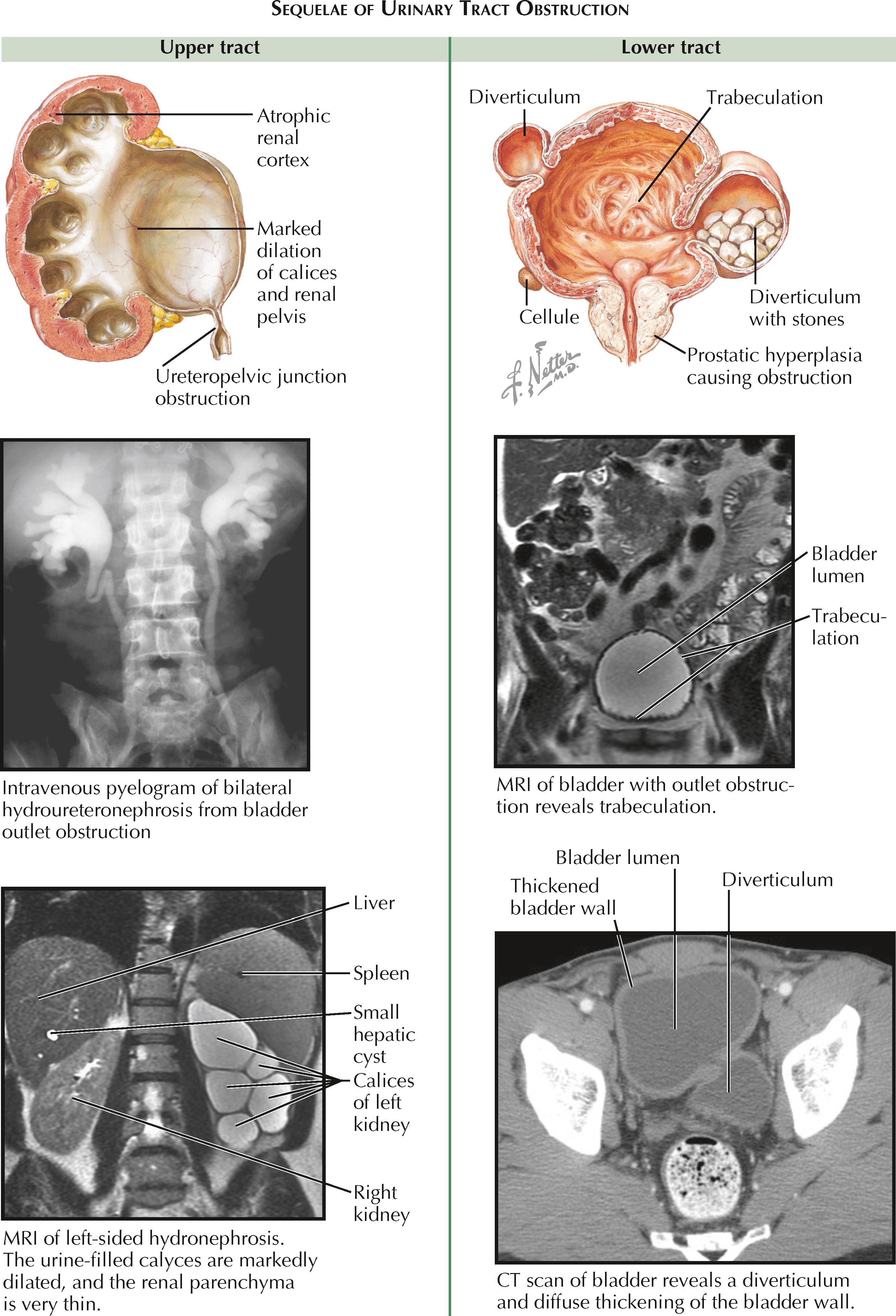
In the upper tract, compensation involves thickening of ureteral smooth muscle to increase the strength of peristaltic waves against the obstruction. In addition, there is dilation proximal to the obstruction, which is called hydronephrosis if it involves the kidney, or hydroureteronephrosis if it involves both the kidney and ureter. The degree of hydronephrosis is determined by the location, degree, and duration of the obstruction. The renal pelvis becomes dilated first, followed by dilation of the calyces. The calyces lose their normal concave shape and become blunted.
Decompensation occurs as the ureter lengthens and becomes tortuous, followed by replacement of normal ureteral muscle with scar tissue. As a result, the ureter progressively loses its ability to contract and transport a bolus of urine. In the kidney, pressure from the obstruction is ultimately transmitted to the renal tubules, which leads to reflex vasoconstriction and reduction of renal blood flow. The glomerular filtration rate is thus reduced in the obstructed nephrons. If bilateral, these changes may be associated with acute kidney injury. In chronic, unrelieved obstruction, there may be irreversible atrophic changes in the renal cortex resulting from chronic ischemia and inflammation.
In the lower tract, compensation involves hypertrophy of the detrusor muscle in an attempt to overcome the obstruction. Chronic hypertrophy, however, can lead to trabeculations, cellules, and diverticula. Trabeculations are interwoven bundles of hypertrophied detrusor muscle that replace the smooth surface of a normal bladder. Cellules are small pockets of mucosa that have herniated between the most superficial strands of detrusor muscle. Diverticula are more pronounced outpouchings that push through all of the detrusor muscle layers. Because there is no contractile force around the walls of diverticula, they are unable to effectively eliminate urine, which may promote formation of bladder stones.
Decompensation occurs as the bladder wall further deteriorates and becomes diffusely replaced with scar tissue. As a result, the bladder is unable to properly contract. The high pressure within the bladder lumen may overwhelm the ureterovesical junctions, causing a secondary reflux that transmits high pressure to the upper tract.
Numerous symptoms may signal the presence of a urinary tract obstruction. In the upper tract, flank pain may occur secondary to increased stretching of the renal capsule. In the case of an impacted ureteral stone, additional symptoms include hematuria, nausea, and vomiting, as well as systemic symptoms if bacteriuria or bacteremia is present. In the lower tract, outlet obstruction may cause urinary frequency and urgency, low abdominal pain (caused by bladder spasms), and penile/urethral pain in males. Over time, urinary hesitancy and a decrease in the force of the stream may occur as the bladder loses its contractile strength. Finally, complete urinary retention may occur, leading to stasis, infection, bladder stone formation, and overflow incontinence.
The most important tools for diagnosis are the history and physical examination; however, numerous imaging techniques are often used to confirm and further characterize the obstruction. Nonfunctional imaging studies include non–contrast computed tomography (CT), renal sonography, retrograde pyelogram, retrograde urethrogram, and cystography. These tests can determine the anatomic location of the blockage but cannot assess function. Functional imaging studies include contrast-enhanced CT, radionuclide studies, intravenous pyelography, and urodynamics.
More invasive tests—such as cystoscopy, ureteroscopy, and nephroscopy—allow clinicians to make diagnoses under direct vision and simultaneously perform therapeutic interventions. These tests, however, do not provide any functional information.
Acute decompression of the urinary tract may be accomplished using transient interventions, such as placement of a Foley catheter, suprapubic tube, ureteral stent, or percutaneous nephrostomy tube. Depending on the level and cause of obstruction, definitive therapy may require surgical intervention, such as a transurethral outlet surgery (e.g., urethrotomy, prostate incision or resection), ureteral surgery (e.g., incision, balloon dilation, ureteroscopy), or abdominal surgery (e.g., ureteropelvic junction obstruction repair, removal of retroperitoneal tumor).
Renal stones are common, with lifetime prevalence estimates as high as 15%. Historically, men are affected more often than women, with a ratio of 2 or 3:1, although recent evidence suggests the gender gap may be closing. Stones can form at any age, but most occur in adults between 30 and 60 years of age. The clinical and economic impact of stone disease is substantial, with an estimated $2 billion spent in the United States in 2000.
Bladder stones are also associated with significant morbidity but occur far less frequently than renal stones. Because the causes of renal and bladder stones are distinct, their associated symptoms, treatments, and prevention strategies are considered separately.
The majority of renal stones (80%) are calcium-based, most frequently calcium oxalate and less commonly calcium phosphate. Other less common stone compositions are uric acid, struvite, and cystine.
When stone-forming salts reach a urinary concentration that exceeds the point of equilibrium between dissolved and crystalline components, crystallization will occur. Although certain chemicals in the urine can delay stone formation, there is a concentration of stone-forming salts above which crystallization becomes inevitable. Thus factors that increase the propensity for stone formation do so by reducing urine volume, increasing the quantity of stone-forming salts, or decreasing the quantity of crystallization inhibitors.
The process by which crystal formation leads to stone formation remains incompletely understood. Recent evidence, however, suggests that routine cal-cium oxalate stones originate on calcium phosphate deposits, known as Randall plaques, that are located at the tips of renal papillae and act as niduses for crystal overgrowth.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here