Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The authors thank Erika Woodrum and the FES Center for their assistance in preparing illustrations.
This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs through the Peer Reviewed Medical Research Program under Awards W81XWH-15-1-0607 and W81XWH-15-1-0608. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. Additionally, this study was supported by Merit Review Award # B1495-R (Capadona) and the Presidential Early Career Award for Scientist and Engineers (PECASE, Capadona) from the United States (US) Department of Veterans Affairs Rehabilitation Research and Development Service. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. Finally, funding was also received from the National Institute of Health, National Institute of Neurological Disorders and Stroke, (Grant # 1R01NS082404-01A1). None of the funding sources aided in collection, analysis, and interpretation of the data, in writing of the book chapter or in the decision to submit for publication. The authors have no conflict of interest related to this work to disclose.
This chapter outlines and discusses the history and current state of the neural recording field, specifically in the context of cortical neural prostheses. For in-depth reviews of the subject matter, the reader is referred to excellent reviews by , and . Topics covered in this chapter include:
Motivation, progress, and challenges for intracortical recording electrodes
Commonly used electrodes for intracortical recording
Summary of intracortical recording electrode failure mechanisms
The biological response to electrode implantation
Intracortical electrode technologies to evade the inflammatory response and improve long-term performance
Perspectives on the future of cortical neural recording interfaces
The development of a stable intracortical recording electrode has tremendous implications for the rapidly expanding field of neuroprosthetics and, more broadly, brain–machine interfaces (BMIs). BMIs have significant potential to reduce the burden associated with paralysis and limb loss. By bypassing damaged regions of the nervous system, BMIs offer the promise of reducing the burden of injury and enabling injured individuals to live fuller and more interactive lives ( ). Unfortunately, microelectrodes that are currently used for BMI applications demonstrate poor chronic neural recording performance and reliability. Signal-to-noise ratio degrades over several weeks to months ( ), resulting from a multifactored failure mechanism having both electromechanical (abiotic) and host tissue (biotic) root causes ( ).
In part due to the potential for BMI applications and the need for further development, on April 2, 2013, President Obama launched an initiative to “accelerate the development and application of new technologies that will enable researchers to… show how individual brain cells and complex neural circuits interact at the speed of thought.” In response to this grand challenge, the director of the National Institutes of Health (NIH) released the scientific vision of the Brain Research through Advancing Innovative Neurotechnologies (BRAIN) Working Group, which asked for $4.5 billion over the next 10–12 years to support a strategy for addressing that goal. The BRAIN Initiative looks beyond the current rehabilitative applications proposed for BMIs, to a comprehensive, mechanistic understanding of cognition, emotion, perception, and action in health and disease. The first 5 years of the NIH vision focus largely on developing new “platform” tools and technologies to advance human neuroscience and integrate them into therapeutic interventions. With notable supporters like National Science Foundation (NSF), US Food and Drug Administration (FDA), Defense Advanced Research Projects Agency (DARPA), and many private organizations, the magnitude of the potential impact of the NIH BRAIN Initiative has been compared with that of the Apollo Program and the Human Genome Project ( ).
Intracortical recording electrodes were initially developed in the 1940s to advance our basic understanding of the nervous system ( ). The first devices consisted of glass pipette and microwire electrodes and were used to interrogate the cortical electrophysiology of various animal models. Since that time, the field has achieved significant strides, both in fabrication technologies and in advancing medical applications of intracortical recording electrodes.
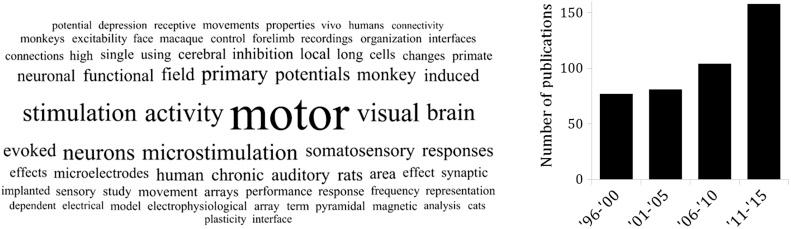
For the past two decades, hundreds of publications have been added to the literature involving “intracortical recording” (or “intracortical microelectrodes”), with over one-third being published since 2010 ( Fig. 28.1 ). With the initiation of more than 120 funded projects stemming from the BRAIN Initiative, this number is set to soar exponentially in the coming years ( ). Today, there are intracortical microelectrodes that have FDA approval (namely the Utah Array from Blackrock). Additionally, there are a broad range of advanced materials and devices with novel capabilities in the preclinical pipeline for future implementation and approval. Recent clinical trials have demonstrated tremendous success, enabling quadriplegic patients to feed themselves, not only with robotic arms ( ) but also with their own muscles with applied stimulation ( ). Very recent evidence even points to the possibility that brain–computer interfaces may be able to trigger partial lower limb recovery in paraplegic patients ( ). Wireless communication has been integrated into new generations of devices that simplifies their clinical implementation and overcomes tethering challenges ( ). The use of optogenetic techniques to allow neural recording or stimulation with light currently remains in preclinical study, but may someday soon provide a means for less invasively or more selectively interacting with neural circuits ( ). The pace of innovation and the achievement of milestones is astonishing.
While the field has made significant progress, BCI has yet to break into the mainstream. To achieve a reliable and natural brain control paradigm, neural signals must be recorded with high-quality and fine spatial resolution such that neuron populations can be triangulated, decoded, and correlated with a person’s intended movement ( ). Such applications often rely on intracortically implanted microelectrodes. Due to the moderate risk associated with implantation surgery and the limited longevity of current recording configurations, one must consider and balance risk to the patient (or animal) with the potential benefit it may serve. The Holy Grail of neural recording interfaces has yet to be perfected: a device that is minimally invasive but can still provide high-density, chronically stable signals.
A central focus of neuromodulation has been on the development of electrodes for stimulation applications . Surface chemistries and stimulation paradigms are optimized to balance charge delivery and prevent dissolution and corrosion of the electrode contacts. The host tissue response (including inflammation and encapsulation) is an important consideration, but resulting changes can often be compensated for by tuning stimulation settings (e.g., amplitude, pulse width, direction, etc.) to achieve the desired functional outcome: excitation or blockade of surrounding neurons.
For intracortical recording applications, s ome unique considerations regarding the neural electrode must be made. Researchers working in the field are challenged with a dynamically changing environment, which includes biological, material, and electrical factors, that can be roughly grouped into biotic and abiotic in origin. For a more complete discussion of failure mechanisms, the reader is referred to , and .
Biotic . In recording applications involving implanted neural electrodes, the host tissue response plays a much larger role in determining long-term recording performance and stability than it does in stimulation applications. Inflammation, the healing response, and encapsulation result in changes to the physical and electrical properties of the tissue ( ). Cellular composition and the normal pattern of neural activity surrounding the electrode are altered.
Abiotic . Electromechanical failures include the breakage of the fine electrode shanks, degradation of insulation, and corrosion of metal contacts. These failures result in changes to an electrode’s exposed conductive surface area, conductivity, and, ultimately, its ability to transduce ionic extracellular signals into electrical signals ( ).
There are a wide breadth of neural recoding electrodes available, reflecting the huge motivation in the field to produce a device capable of long-term high-quality intracortical recordings and the diversity of targeted tissue regions for a variety of applications.
There are a number of electrode designs used for cortical recordings, segmented here as: Utah arrays, Michigan-type electrodes, microwires, and encephalography (electrocorticography [ECoG] and electroencephalography [EEG]). The factors that influence the electrode selection primarily include invasiveness, spatial resolution, and relatedly quality and stability of the signal ( Fig. 28.2 ). To date, for neuroprosthetic BMI applications, the Utah and Michigan electrodes have outpaced microwires, ECoG and EEG, owing to their superior spatial resolution.
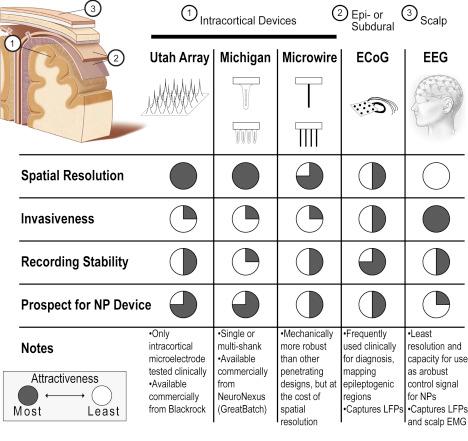
The Utah Electrode Array (UEA) consists of silicon-based microelectrode typically with a 10 × 10 array of 1.5-mm tines ( ). Developed by Normann et al. at the University of Utah in 1991, it has remained at the forefront of the intracortical recording field. It provides extremely high-resolution information, with tines spaced 400 μm apart. Several challenges prevent its wider acceptance and use in broader applications: (1) it requires an invasive procedure, involving high-speed pneumatically driven insertion, and (2) it has suffered from inconsistent performance owing to biological (neuroinflammatory) response and/or manufacturing defects ( ). Nonetheless, the UEA is the only high-density, penetrating recording electrode approved by the FDA. It has 510(k) approval for recording or stimulation experiments of less than 30 days and investigational device exemption (IDE) approval for chronic recording or stimulation studies longer than 30 days ( ). Additionally, the Utah Array has received the CE mark in Europe.
Variations of the UEA have also been explored, including a slanted-tine design for achieving various tissue depths ( ), a design shape that conforms to complex anatomical geometries ( ), ultra-high aspect ratio devices ( ), and high-density arrays ( ). Recently, a collaboration between Brown University and Blackrock Microsystems has generated a commercially available wireless version of the UEA ( ).
The majority of clinical work with UEA technology to date has focused on rehabilitative applications ( ). As part of the BrainGate clinical trials, Hochberg et al. implanted UEAs into the primary motor cortex of their volunteers with tetraplegia. Using a robotic arm, they were able to restore reach and grasp functions to the individuals by recording and decoding volitional movement intent ( ). used similar approach to achieve virtual typing for paralyzed individuals, and demonstrated reaching and grasp through brain-controlled muscle stimulation. recently demonstrated neural recording for more than 1 year with UEAs implanted in patients with amyotrophic lateral sclerosis (ALS). Other groups around the globe have also been making significant advances in the field of BCI, including from Shwartz ( ), Andersen ( ), and Nicolelis ( ).
Despite the tremendous progress that has been achieved, improving recording performance and longevity remains a principal focus ( ). For examples, in their review of DARPA-led efforts in BCIs, argue that overcoming interface failure is one of the largest remaining hurdles for BCI researchers and that without overcoming these issues, the vision of life-long BCI systems will remain just out of reach.
Whereas the Utah Arrays consist of a two-dimensional, 10 × 10 array of shanks, Michigan-style electrode arrays (MEAs) are planar devices with multiple recording contacts located along one or several silicon shanks ( ). Developed by Wise et al., first at Bell Labs in 1966 and later at the University of Michigan, these electrodes are frequently studied in preclinical BCI applications ( ). While chronic recording studies have been reported, the consistent longevity of recordings remains among the biggest hurdles ( ). To the authors’ knowledge, MEAs have yet to be studied in human clinical trials.
MEAs are sold commercially by NeuroNexus, a subsidiary of GreatBatch Inc. Recent advances have yielded on-board signal digitization (SmartProbe) and a variant that can be used as an optogenetic probe (optoelectrodes or simply “optrode”). For additional details on the development and application of MEAs, the reader is referred to a review by .
Microwires were first developed by Salcman, Bak, and Schimdt at the NIH in the early 1970s ( ). These electrodes consisted of 2- to 11-μm-diameter iridium (Ir) and platinum (Pt) alloys. In one of the first long-term demonstrations of the technology, Salcman and Schmidt recorded single neuron unit activity for up to 223 days with a microwire implanted in the motor cortex of a nonhuman primate ( ). Since then, a variety of microwire devices have been developed for BMI applications ( ). However, as with other microelectrodes, neuroinflammation appears to degrade recording performance over time ( ), most likely through the degradation of insulating materials and corrosion of metals ( ).
ECoG is frequently used clinically to map epileptogenic regions of the brain and facilitate the surgical excision of operable focal lesions. ECoG arrays are placed temporarily intraoperatively and removed immediately or shortly after lesion resection surgery.
Many groups have investigated the use of ECoG arrays placed subdurally or epidurally to collect neural signals for use in BMI applications ( ). ECoG arrays pick up LFP signals and are reported to have a spatial resolution of about 4 mm ( ). Even with this limitation, applications involving two- and three-dimensional arm movements ( ) that predict arm movement with 3 degrees of freedom ( df ) ( ), and determine trajectory and kinetics for use in an FES system ( ) have been developed, with individual reports of chronic recordings up to 7 years ( ). While ECoG arrays have the distinct benefit of being placed superficial to the meninges, and thus potentially less invasive than implanted MEAs, most applications have focused on gross motor rather than fine motor tasks that may require a higher spatial resolution.
Due to noninvasiveness contacts placed on the scalp, EEG benefits from sampling a wider number of areas around the skull. However, its spatial resolution is relatively coarse at about 3 cm ( ). To date, the best performance of an EEG BCI system in control of extrinsic operations is 3 df , which was achieved only after months of intensive training ( ). Other BMI investigations include those by surveying the EEG response to various motor task ( ) and decoding movement intent in a sitting-to-standing task in healthy volunteers ( ). Interestingly, as one group has recently shown, EEG may be used in a complimentary fashion to other sources of neural control ( ): electrooculography in conjunction with EEG to improve hand-grasp task of an exoskeleton.
Optical microelectrodes (or “optrodes”) are also under development by a number of groups, primarily for use in optogenetic research ( ). Notably, developed a micro-ECoG array that was capable of recording large-scale cortical activity spanning the S1 somatosensory and M1 motor regions in a nonhuman primate. With recent advances in optogentic techniques and novel implantable probe designs, such devices are improving at a rapid rate.
Failure of microelectrode recordings is characterized by a decrease in signal-to-noise ratio, leading to inability to accurately discriminate neuronal firing from background ( ). There are a number of underlying failure mechanisms that have been described, but, by definition, all lead to either electrical or neural circuit disruption. Failure modes for microelectrode recordings include:
Degradation of insulation (e.g., dissolution, peeling of polymer coatings) ( )
Corrosion of electrical contacts (e.g., oxidation, pitting of electrode contacts and connectors) ( )
Local neurodegeneration (e.g., neuronal loss, dysfunction) ( )
Device migration (e.g., movement of microelectrode away from neuron population of interest) ( )
Direct mechanical damage (e.g., electrode, lead fractures), ( )
As suggested in Fig. 28.3 , the primary mechanisms are influenced by a number of secondary and tertiary factors, namely:
Secondary (neuroinflammation, tissue strain, chemical environment, material fatigue)
Tertiary (acute implantation injury, chronic micromotion and exposure, and manufacturing defects)
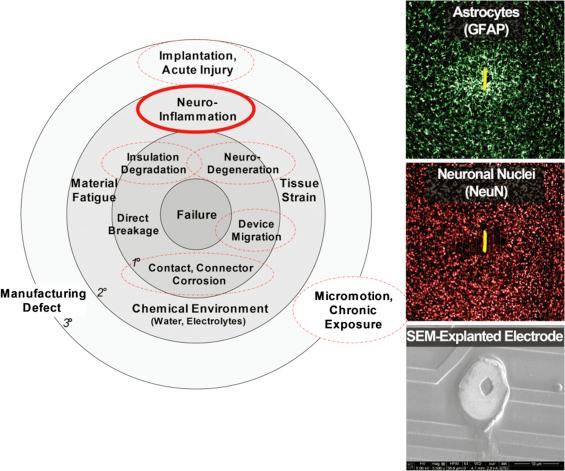
These factors are not mutually exclusive and may contribute synergistically to failure. In particular, it seems that neuroinflammation may underpin a number of interrelated failure mechanisms ( ). Neuroinflammation releases a number of cytotoxic substances (leading to neurodegeneration), altering the microenvironment by lowering pH and releasing reactive oxidative species (leading to corrosion and degradation of insulation), and depositing scar tissue (electrically and physically isolating the device from neurons).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here