Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The bones, joints, ligaments, and meninges that support and protect the tissues of the nervous system can give rise to numerous illnesses that affect the nervous system. These disorders sometimes border on other medical disciplines unfamiliar to the neurologist and hence can be enigmatic or difficult to diagnose. They may involve cognitive functions, disrupt cerebrospinal fluid (CSF) flow dynamics, or slowly compress and distort central and peripheral neural structures. They have a mixture of genetic, developmental, traumatic, degenerative, infectious, and inflammatory mechanisms. For the clinical neurologist, awareness greatly strengthens diagnostic skill, therefore this chapter reviews many of these disorders. Chapters of overlapping interest include Chapter 27, Chapter 33, Chapter 58, Chapter 85, Chapter 89 .
For an expanded version of this chapter that includes figures and boxes that are only available online, please visit http://www.expertconsult.com . ![]()
Heritable disorders involving the major connective tissues of the body are among the most common human genetic diseases. The focus will be on those disorders that have neurological impact ( Box 104.1 ). Many of these are being better understood at a molecular level: osteogenesis imperfecta (OI), Ehlers-Danlos syndrome (EDS), chondrodysplasias, and Marfan syndrome, to name a few.
Osteogenesis imperfecta
Hypermobile Ehlers-Danlos syndrome (type III)
Classic Ehlers-Danlos syndrome (type I)
Vascular Ehlers-Danlos syndrome
Chondrodysplasias
Achondroplasia
Pseudoachondroplasia
Stickler syndrome
Craniosynostosis (syndromic)
Marfan syndrome
MASS phenotype (mitral valve prolapse, aortic dilation, skin, and skeletal features)
Loeys-Dietz syndrome
Beals syndrome (congenital contractual arachnodactyly)
Dermal/epidermal (keratin, laminin, type VII collagen, plectin, integrin)
Epidermolysis bullosa
Basal lamina (type IV collagen, laminin, nidogen)
Alport syndrome
Classifications of heritable disorders of connective tissue can rely on pattern of inheritance, clinical features, anatomical pattern, or known molecular defects. To date, genetic or molecular classification is not always helpful clinically because there are significant genotype-to-phenotype inconsistencies, testing is not widely available, and our understanding of many disorders is incomplete. Connective tissues contain a wide range of complex macromolecules assembled in the extracellular matrix. There are at least 28 different types of collagen in bone, skin cartilage, tendons, and ligaments. Other molecules including fibrillin, elastin, proteoglycans, fibronectin, hyaluronate, osteonectin, and osteocalcin contribute to tensile strength and elasticity. Forces that control the three-dimensional organization of these components remain largely unknown. In growth and development, collagen fibrils in all these supporting tissues undergo repeated synthesis, degradation, and resynthesis. Nutrition, gravitational forces, trauma, other physical stresses, endocrine factors (such as glucocorticoids), and inflammation all modify these tissues ( ).
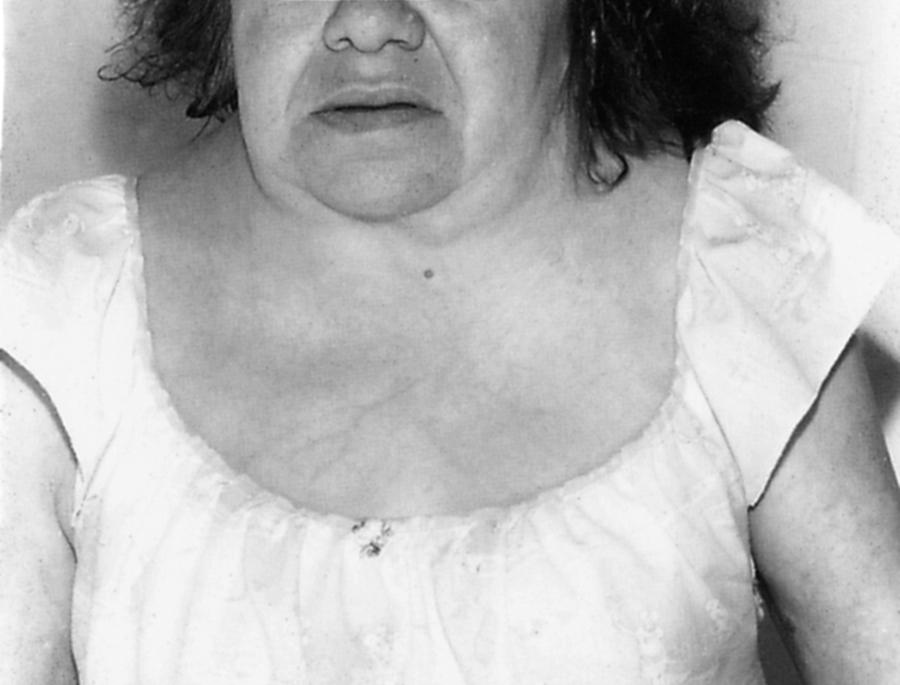
The various types of OI or “brittle bone disease” (incidence ≈1:20,000 births) are inherited, predominantly autosomal-dominant connective tissue disorders caused by gene mutations that affect type 1 collagen. Rare recessive inheritance has been reported ( ). This disorder is characterized by brittle osteopenic bones and recurrent fractures ( ). As many as nine types are known, with wide variations in severity and associated findings such as short stature, blue sclera, progressive hearing loss, poor dentition, scoliosis, and skeletal abnormalities (osteopenia, irregular ossification, multiple fractures). Laboratory studies show a molecular defect in type I procollagen in two-thirds of patients.
Potential neurological complications of OI include communicating hydrocephalus, basilar invagination, macrocephaly, kyphoscoliosis, skull fractures, subdural hematomas, and epilepsy. The basilar invagination can lead to brainstem compression ( ). Spinal cord compression, syringomyelia, Chiari I malformation, Dandy-Walker cysts, leptomeningeal cysts, microcephalus, or central nervous system (CNS) tumors are rarer associations. Progressive, variably conductive and sensorineural hearing loss usually begins in the second decade and affects more than half of patients by age 30 years. Joint laxity, indistinguishable from that of EDS, can result in permanent dislocations. Rarely, OI is complicated by cervical artery dissection resulting from fragility of large blood vessels ( ). Aortic regurgitation, floppy mitral valves, and mitral incompetence can lead to cerebrovascular complications. For unknown reasons, some patients develop a hypermetabolic state with elevated serum thyroxine levels, hyperthermia, and excessive sweating. Wide phenotypic variation is characteristic and appears to be related to the abundance of over 900 unique mutations in the type I procollagen COL1A1 and COL1A2 genes, combined with undefined chance events during embryonic and fetal development ( ).
Treatment is symptomatic and tailored to the severity of symptoms, though women require special attention during pregnancy and after menopause when the risk of fractures increases. More severely affected children require more comprehensive physical therapy and orthopedic management. For severe hearing loss, stapedectomy or cochlear implant may prove helpful. Neurological complications relating to potential brainstem and spinal compression require appropriate attention. Oral or intravenous bisphosphonates are commonly prescribed to individuals with OI. Although studies demonstrate increased bone mineral density as a result, it is unclear whether bisphosphonates improve clinical status ( ).
The EDSs are a rare group of disorders with an overall frequency of 1:5000. They are a clinically and genetically heterogeneous group of inherited connective tissue disorders characterized by joint hypermobility, skin hyperextensibility and tissue fragility. The Villefranche nosology in 1998 identified 6 EDS subtypes based on clinical features, mode of inheritance, and biochemical and genetic findings ( ). In 2017 the EDS Consortium revised the classification to now include 13 subtypes. However, given the vast genetic heterogeneity and phenotypic variability of the subtypes and the clinical overlap between the subtypes, a definitive diagnosis of all EDS subtypes (except for the hypermobile subtype) relies on identification of the causative genetic variant ( ).
Of importance is that many of these subtypes can include neurological features that may lead a patient to a neurologist for evaluation before a diagnosis of EDS has been established. Neurological features include a number of neuromuscular symptoms such as weakness, hypotonia, myalgia, and paresthesia ( ). Uncommon neuromuscular disorders include axonal polyneuropathy and compression neuropathies. In addition, skeletal disorders include cranial cervical instability and scoliosis. Postural orthostatic hypotension (POTS) may occur. Any of the above symptoms/signs coupled with joint hypermobility, a feature present to a greater or lesser degree in most subtypes, and skin changes as noted above, should raise the suspicion for EDS. The Beighton joint flexibility score is a useful screening tool for the degree of joint mobility ( Table 104.1 )
| Thumb to forearm | □ Right | □ Left | |
| Bending little fingers back to 90 degrees or more | □ Right | □ Left | |
| Elbows hyperextend to 10 degrees or more (i.e., bend backwards 10 degrees or more in the wrong direction) | □ Right | □ Left | |
| Knees hyperextend to 10 degrees or more (i.e., bend backwards 10 degrees or more in the wrong direction) | □ Right | □ Left | |
| Palms flat to floor with legs straight | □ Able | or | □ By history |
| Total score | |||
The differential diagnosis includes Marfan syndrome and OI. If EDS is suspected, referral to a clinical geneticist is essential.
Chondrodysplasias, also referred to as skeletal dysplasias , are heritable skeletal disorders characterized by dwarfism and abnormal body proportions. These are the most common cause of abnormally short stature. This category also includes some patients with craniosynostosis (see later discussion) who have cranial and facial malformations associated with ocular change and cleft palate but normal stature and body proportions; this is more common in the more severe chondrodysplasias. Many patients develop premature degenerative joint disease. Mild chondrodysplasias in adults may be difficult to differentiate from primary generalized osteoarthritis.
They encompass over 400 disorders, which vary from mild distortions of cartilaginous structures and the eye to severe malformations that are fatal in early life ( ). A number are unique and identified by eponyms based on isolated case reports. Among the features of these syndromes are high forehead, hypoplastic facies, cleft palate, short extremities (with gross distortions of the epiphyses, metaphyses, and joint surfaces), cataracts, degeneration of the vitreous, and retinal detachment. We will highlight those types most prone to neurological complications.
Achondroplasia is an autosomal dominant disorder of endochondral bone formation. A specific mutation of a fibroblast growth factor receptor gene ( FGFR3 ) is present in over 90% of patients. It is the most common skeletal dysplasia in humans. Approximately three-fourths of cases occur because of spontaneous new mutations. The syndrome is the most common cause of short-limbed dwarfism accompanied by macrocephaly and dysplasias of the metaphyses of long bones. The mutant genotype has complete penetrance. The diagnosis can be confirmed by pathognomonic radiographic changes and/or by deoxyribonucleic acid (DNA) testing. Neurological complications associated with achondroplasia are common ( Box 104.2 ). Young achondroplastic children should be observed for complications such as hydrocephalus, compression at the foramen magnum, thoracolumbar kyphosis, and sleep apnea. Neurological complications of spinal stenosis tend to occur later in life.
Macrocrania, with or without hydrocephalus
Foramen magnum abnormalities with cervicomedullary compression
Respiratory disturbances, including sleep apnea and sudden infant death syndrome
Syringomyelia, diastematomyelia
Spinal stenosis with spinal cord or nerve root compression
Infantile hypotonia
Cortical atrophy
Atlantoaxial subluxation
Psychomotor delay (most have normal intelligence)
Pseudoachondroplasia, another autosomal dominant cause of short stature, is due to gene mutations for the cartilage oligomeric matrix protein (COMP), a protein that interacts with both collagens and proteoglycans in cartilage. Patients have leg deformities, short digits, vertebral anomalies, and proclivity to develop severe osteoarthritis. However, neurological complications are less common than in true achondroplasia. Nonetheless, patients are at risk for atlantoaxial (AA) dislocation. Children who carry both the achondroplasia and pseudoachondroplasia mutations have been reported, with early onset of neurological complications from lumbar or foramen magnum stenosis.
Marfan syndrome is an autosomal dominant disorder of fibrous connective tissue with variable penetrance and variation of phenotypic expression; its most prominent features affect the skeleton, heart, great vessels, and eye. More than 90% of patients have a mutation in the fibrillin-1 gene. Fibrillin-1 is an important matrix component of both elastic and nonelastic tissues. The classic patient is tall with inordinately long limbs and digits. Other skeletal changes are anterior chest deformity, scoliosis, thoracic lordosis, high arched palate with crowded teeth, and some ligamentous laxity. Ocular problems include myopia, flat corneas, and ectopia lentis. The major life-threatening problem is aortic aneurysm or dissection; aortic and mitral valves can also be affected. Case reports link Marfan syndrome to intracranial vascular abnormalities such as arterial dissections, giant aneurysms, or hemifacial spasm associated with vascular compression of the facial nerve. However, aneurysms or dissections are rare in patients with Marfan syndrome, and their highest risk of stroke is from cardiogenic emboli, especially if they have prosthetic heart valves or atrial fibrillation ( ). Patients with Marfan syndrome can have sleep apnea, possibly secondary to their skeletal deformities.
Dural ectasia , dilation of the caudal thecal sac, occurs in 90% of patients with Marfan syndrome and is a major diagnostic criterion. Ectasia varies in severity, increases with age, and can cause lumbar radiculopathy. Leakage from ectatic dura can cause intracranial hypotension. Case reports link Marfan syndrome to Chiari I malformation (CM-I). It is not clear whether these patients had classic CM-I or tonsillar herniation secondary to CSF hypotension. Patients can have axonal neuropathy or mild myopathy.
Many patients with Marfan-like features have neither Marfan syndrome nor any other currently identified mutations. These patients are sometimes labeled as having MASS syndrome (mitral valve, aorta, skeletal, and skin involvement) and vary widely in severity of manifestations ( ). A few examples of Marfan-like syndromes of neurological import are:
Homocystinuria, a group of autosomal recessive disorders that can cause marfanoid skeletal changes, myopia, and lens ectopia. It is important to distinguish homocystinuria from Marfan syndrome, since the former can cause mental retardation and hypercoagulability leading to strokes and because it can be treated with vitamin B 6 , folate, and vitamin B 12 .
Loeys-Dietz aneurysm syndrome is similar to Marfan syndrome in skeletal and aortic manifestations but does not have lens ectopia and is often associated with craniosynostosis. Neurological manifestations can include Chiari I malformation, hydrocephalus, and developmental delay. It is usually due to mutations in either TGFB2 or the closely related TGFB1 genes.
Shprintzen-Goldberg syndrome is also characterized by marfanoid features, normal lenses, and craniosynostosis. Neurological issues can include hypotonia, Chiari I malformation, developmental delay, mental retardation, and obstructive sleep apnea.
Beals syndrome (congenital contractural arachnodactyly [CCA]) can cause marfanoid features, joint contracture, and some features of OI. Like Marfan syndrome, it is autosomal dominant, but the mutated gene is for fibrillin-2 rather than fibrillin-1.
Craniospinal malformations result from abnormalities of the bones of the skull and spinal column, connecting ligaments, or other soft tissues and may cause hydrocephalus, brain deformation, spinal cord compression, and syringobulbia or syringomyelia ( ). Many of these are congenital. Magnetic resonance imaging (MRI) and computed tomography (CT) scanning have improved the detection, understanding, and treatment of these anomalies.
Craniosynostosis is among the most common and relatively benign abnormalities, affecting about 1 in 2500 live births. It is caused by premature closure of one or more of the sutures of the skull bones. For babies who have abnormal skull shapes, CT scans can distinguish craniosynostosis from results of fetal head position, birth trauma, or positional plagiocephaly—flattened or misshapen areas on the head that may develop due to sleeping position. Fig. 104.1 illustrates the various types of craniosynostosis.
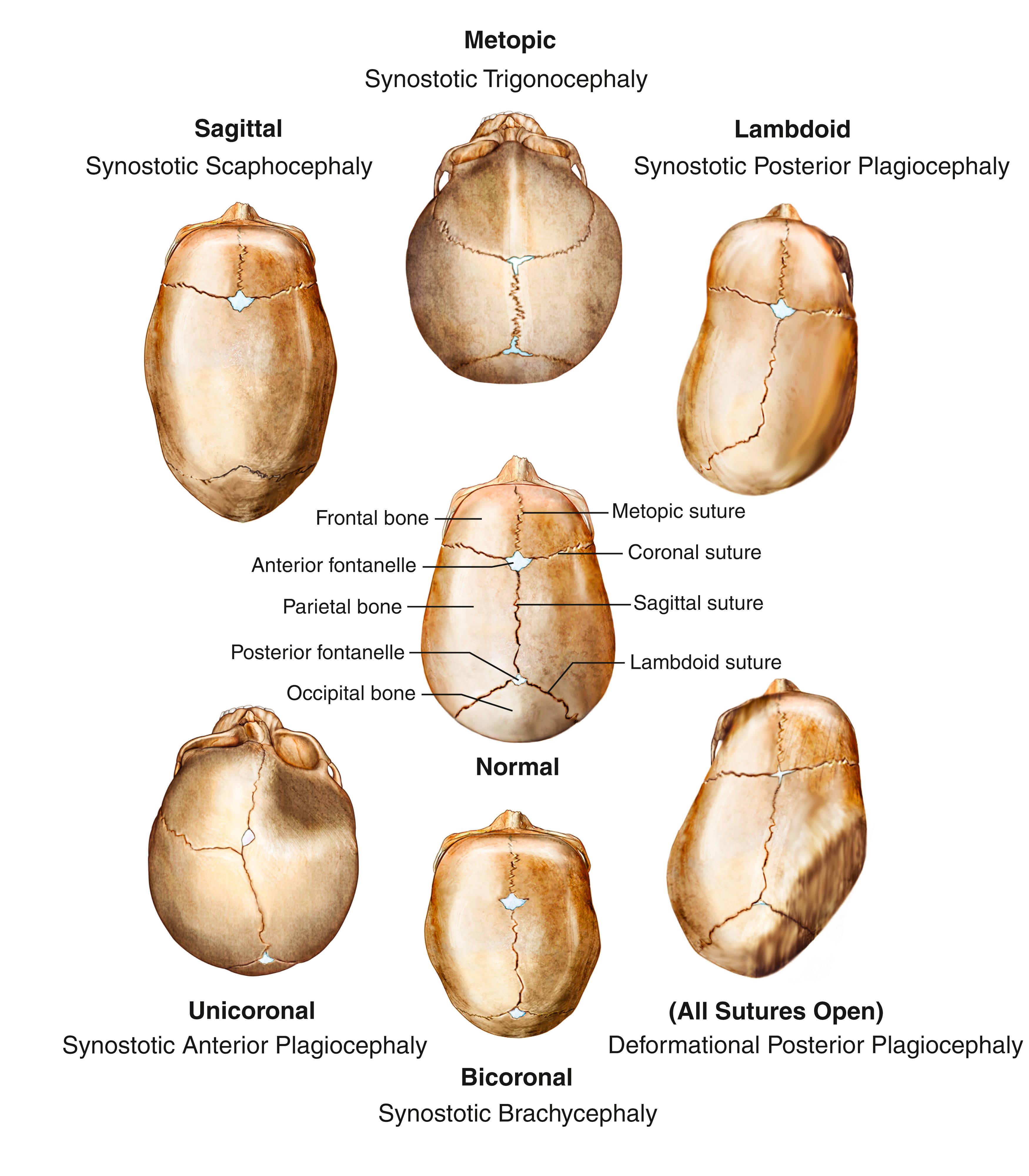
Craniosynostosis often occurs alone, but about 20% of cases are associated with syndromes affecting other parts of the body; the most common of these are Crouzon and Apert syndromes. However, there are over 150 syndromes associated with craniosynostosis, with considerable overlap of symptoms between them. Clinical evaluation by a geneticist may be necessary to determine the most appropriate diagnosis. Most patients with syndromic craniosynostosis appear to have a mutation in the related FGFR2 gene. Genetic testing may be necessary to confirm the diagnosis of a specific syndrome. A family history of abnormal head shape can sometimes be found with genetic syndromes, though many syndromes are caused by de novo mutations.
Occipitalization or assimilation of the atlas refers to congenital partial or complete fusion of the atlas to the occiput ( eFig. 104.2 ) ![]() . The anterior arch of the atlas may fuse to the lower end of the clivus, or the posterior arch of the atlas may fuse to the occiput. The anomaly is often asymptomatic until early adult life but may become symptomatic sooner after trauma. Unilateral occipitalization of the atlas is one cause of torticollis in young children. The loss of movement between the occiput and atlas increases the stresses at the AA joint, predisposing it to gradual degeneration or traumatic dislocation. Patients with occipitalization of the atlas may have associated anomalies such as the Klippel-Feil anomaly, basilar impression, or Chiari malformation ( ).
. The anterior arch of the atlas may fuse to the lower end of the clivus, or the posterior arch of the atlas may fuse to the occiput. The anomaly is often asymptomatic until early adult life but may become symptomatic sooner after trauma. Unilateral occipitalization of the atlas is one cause of torticollis in young children. The loss of movement between the occiput and atlas increases the stresses at the AA joint, predisposing it to gradual degeneration or traumatic dislocation. Patients with occipitalization of the atlas may have associated anomalies such as the Klippel-Feil anomaly, basilar impression, or Chiari malformation ( ).
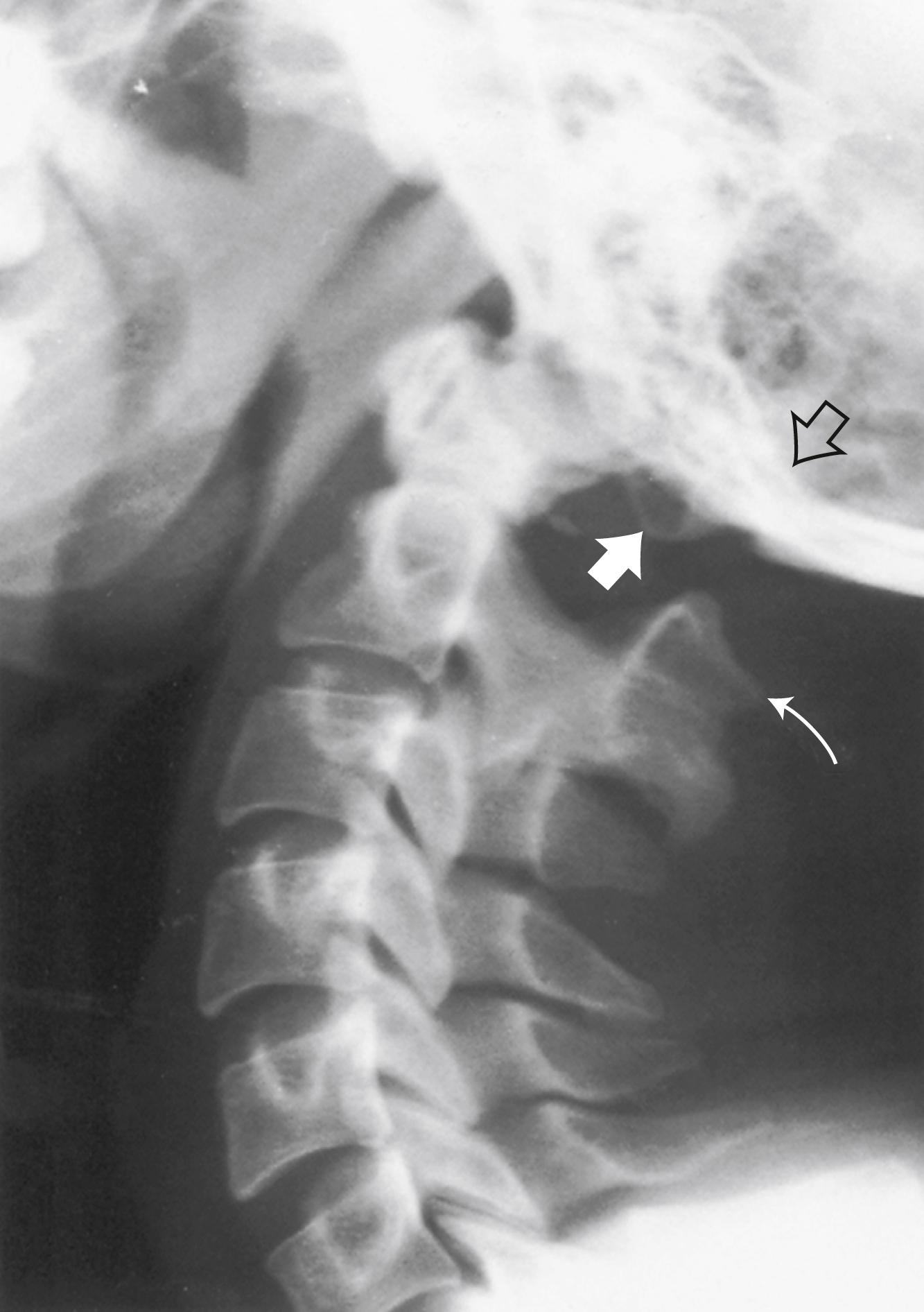
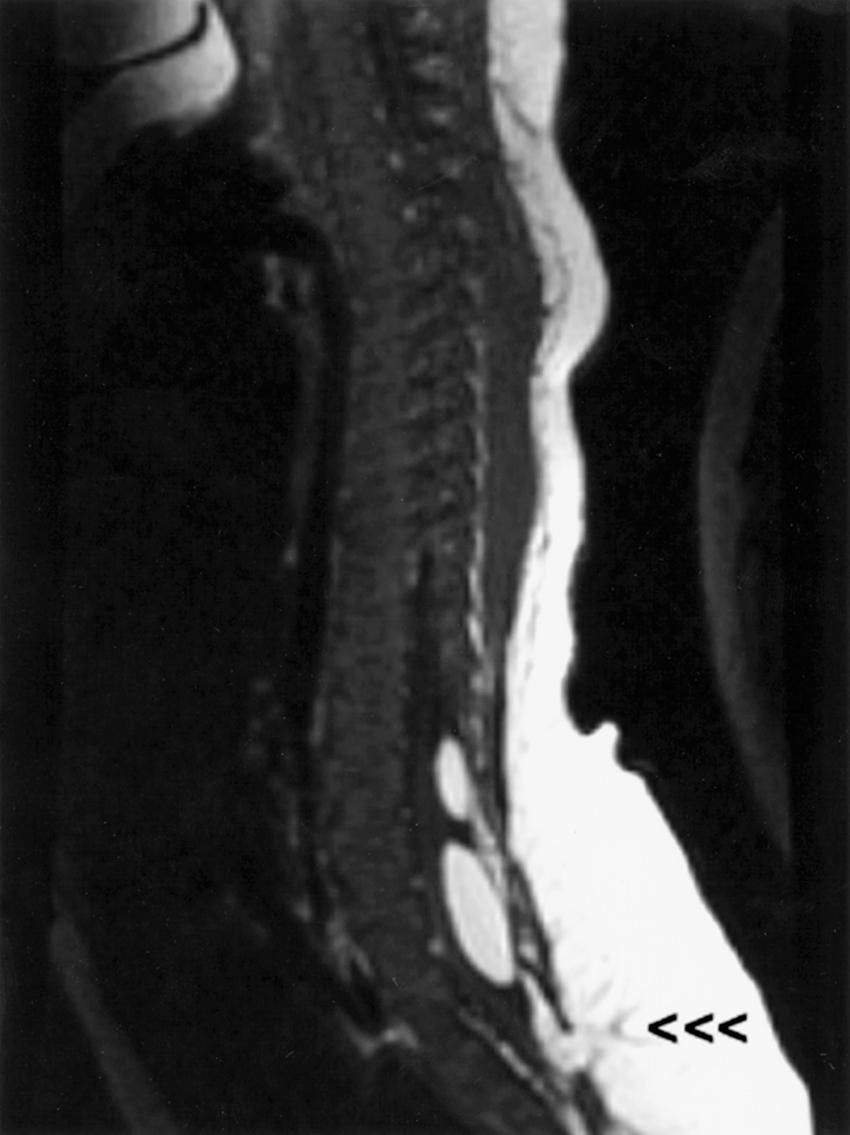
Basilar impression or invagination refers to caudal settling of the foramen magnum onto the cervical spine, generally with encroachment of the odontoid process into the foramen magnum, often resulting in brainstem and/or upper cervical spinal cord compression. Several radiological lines (Chamberlain, McGregor, McRae, digastric) ( eFig. 104.3 ) ![]() and measurements can be used to make the diagnosis. Congenital basilar impression may occur in isolation or may be associated with conditions such as achondroplasia, occipital dysplasia, Down syndrome, Hurler syndrome, Klippel-Feil anomaly, and cleidocranial dysplasia. Some instances of basilar impression are familial. The skeletal anomaly is often accompanied by anomalies of the neuraxis, including Chiari I or II malformation and syringomyelia ( ). Basilar impression can cause compression of the brainstem ( eFig. 104.4 )
and measurements can be used to make the diagnosis. Congenital basilar impression may occur in isolation or may be associated with conditions such as achondroplasia, occipital dysplasia, Down syndrome, Hurler syndrome, Klippel-Feil anomaly, and cleidocranial dysplasia. Some instances of basilar impression are familial. The skeletal anomaly is often accompanied by anomalies of the neuraxis, including Chiari I or II malformation and syringomyelia ( ). Basilar impression can cause compression of the brainstem ( eFig. 104.4 ) ![]() , the cerebellum, or (rarely) the vertebral artery, leading to vertebrobasilar ischemia. It is often asymptomatic, particularly when mild and unaccompanied by other anomalies.
, the cerebellum, or (rarely) the vertebral artery, leading to vertebrobasilar ischemia. It is often asymptomatic, particularly when mild and unaccompanied by other anomalies.
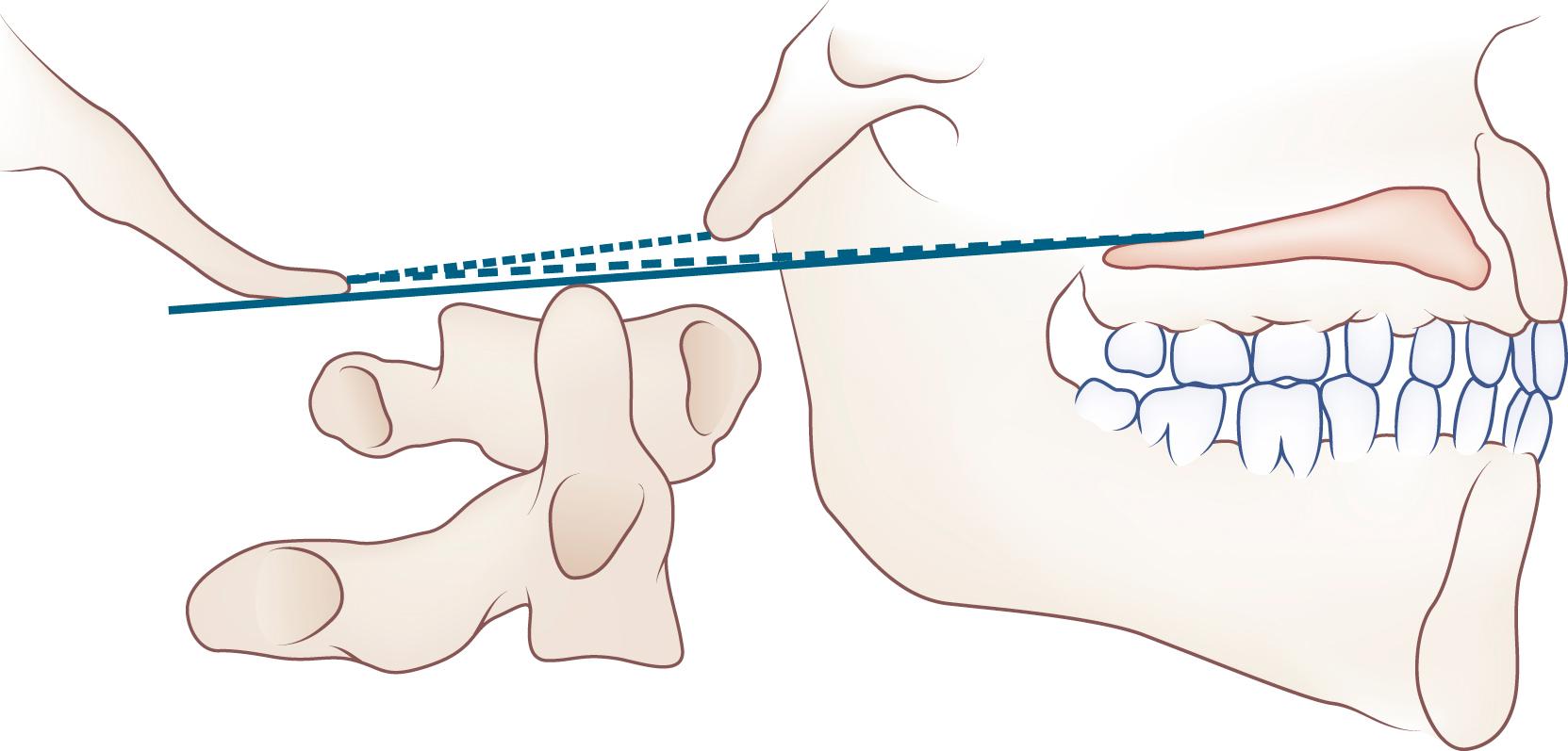
Platybasia , or flattening of the skull, refers to straightening of the angle between the clivus and the floor of the anterior fossa. It infrequently accompanies basilar impression and can also occur as an isolated radiographic finding without any adverse neurological consequences.
Patients with the Klippel-Feil anomaly (congenital synostosis of the cervical vertebrae) ( eFig. 104.5 ) ![]() typically have short necks, low hairlines, and limitation of cervical motion. The diagnosis is confirmed by radiographic demonstration of any two or more spontaneously fused cervical vertebrae. The condition is congenital, caused by failure of normal segmentation of the cervical vertebrae between the third and eighth weeks of fetal development. Although familial instances occur, most cases are isolated and idiopathic. The anomaly can cause direct nerve root, cervical spinal cord, and vertebral or spinal artery compression. Patients may have cervical ribs, predisposing them to thoracic outlet syndrome. Neck pain is common. Hearing loss is the most common cranial nerve symptom. Klippel-Feil syndrome is the anomaly most likely to cause mirror movements (synkinesia), particularly of the hands. Patients with mirror movements can have abnormal clefts or division of the spinal cord near the cervicomedullary junction, which can be detected by MRI ( ). Patients with Klippel-Feil anomaly can have a wide variety of associated abnormalities of brain, spinal cord, or skeletal development, especially congenital scoliosis or Sprengel deformity with unilateral scapular elevation. Patients may develop hydrocephalus, syringomyelia, or syringobulbia. However, many patients with Klippel-Feil anomaly have no neurological symptoms or signs.
typically have short necks, low hairlines, and limitation of cervical motion. The diagnosis is confirmed by radiographic demonstration of any two or more spontaneously fused cervical vertebrae. The condition is congenital, caused by failure of normal segmentation of the cervical vertebrae between the third and eighth weeks of fetal development. Although familial instances occur, most cases are isolated and idiopathic. The anomaly can cause direct nerve root, cervical spinal cord, and vertebral or spinal artery compression. Patients may have cervical ribs, predisposing them to thoracic outlet syndrome. Neck pain is common. Hearing loss is the most common cranial nerve symptom. Klippel-Feil syndrome is the anomaly most likely to cause mirror movements (synkinesia), particularly of the hands. Patients with mirror movements can have abnormal clefts or division of the spinal cord near the cervicomedullary junction, which can be detected by MRI ( ). Patients with Klippel-Feil anomaly can have a wide variety of associated abnormalities of brain, spinal cord, or skeletal development, especially congenital scoliosis or Sprengel deformity with unilateral scapular elevation. Patients may develop hydrocephalus, syringomyelia, or syringobulbia. However, many patients with Klippel-Feil anomaly have no neurological symptoms or signs.
Various congenital or acquired conditions can disrupt the integrity of the AA joint, leading to its dislocation ( Box 104.3 ). In horizontal subluxation, C1 usually moves anteriorly to C2. The movement can be assessed by measuring the separation between the dens and the anterior arch of C1 on flexion, extension, and neutral radiographs; in adults, the separation should not exceed 3.5 mm. This measurement is known as the atlantodental interval (ADI). An alternative measurement is the PADI, or posterior atlantodental interval, which is measured between the posterior border of the dens and the anterior surface of the posterior arch of C1. This measurement, therefore, represents the “space available for the cord” and more accurately reflects the risk of neurological compromise than the ADI. Patients with horizontal AA joint subluxation are likely to compress their spinal cords if the diameter of the spinal canal at the level of the dens is less than 14 mm. This is unlikely to occur if the diameter is more than 17 mm. The actual relationship between the cord and the subluxing bones is best imaged with MRI, which should include flexion and extension views if plain radiographs show significant subluxation on flexion/extension imaging. If MRI is contraindicated, then CT myelography is a consideration ( ). AA subluxation is very frequently encountered as part of the spectrum of cervical spine disease seen in rheumatoid arthritis (RA). The three cardinal cervical spine manifestations in RA are AA subluxation, subaxial spondylolisthesis (often multiple levels), and formation of retro-odontoid pannus. The pannus is an inflammatory mass of tissue that is often at least partially calcified, and can be very adherent to the dura, making its resection potentially very hazardous. The combination of AA subluxation and pannus formation can frequently result in significant myelopathy. Both conditions can be treated with surgical fusion of C1 and C2, which corrects the subluxation and also usually causes the pannus to resorb at least partially over several weeks to months. In patients with rapidly progressive myelopathy it might not be safe to wait for this process to alleviate the spinal cord compression, and direct resection of the pannus is required. This is a difficult operation, usually performed via transoral approach. (See also section below on RA.) Patients with congenital AA dislocation may have associated abnormalities such as Chiari I malformation or diastematomyelia. They can develop secondary syringomyelia. AA subluxation in patients with long-standing RA is a prime example of acquired abnormality of the AA joint and is discussed in more detail later in this chapter. Patients with AA subluxation may be asymptomatic, particularly if their spinal canal diameter is generous. However, they are vulnerable to spinal cord trauma during intubation or other neck motion under anesthesia, or as a result of a whiplash injury. Patients at risk for AA dislocation, such as those with Down syndrome or chronic RA, should have lateral flexion and extension cervical spine radiography performed before general anesthesia so the anesthesiologist can plan appropriate care during intubation.
Congenital
Os odontoideum (failure of odontoid to fuse with body of axis)
Isolated
With connective tissue dysplasias (e.g., Down syndrome, pseudoachondroplasia, multiple epiphyseal dysplasia, spondyloepiphyseal dysplasia, Morquio disease, Klippel-Feil anomaly, Conradi syndrome)
Hypoplastic dens
With connective tissue dysplasia
With incomplete segmentation (e.g., occipital assimilation of atlas, basilar invagination, incomplete segmentation of C2, C3, etc.)
Other anomalies of C2 (e.g., bifid dens, tripartite dens with os apicale, agenesis of all or part of dens)
Laxity of transverse atlantal ligament (e.g., Down syndrome)
Acquired
Traumatic, acute or chronic un-united dens fracture
Infectious
Neoplastic (e.g., neurofibroma)
Arthritic (e.g., rheumatoid arthritis, ankylosing spondylitis, renal amyloidosis)
Bone disease (e.g., vitamin D-resistant rickets and others associated with basilar invagination)
John Cleland first described a series of structural defects in the skull base and cerebellum in 1883. Hans Chiari classified the malformations into four types in 1891. Julius Arnold further expanded the definition of Chiari II malformations in 1894. In current usage, the terms Arnold-Chiari and Chiari malformations are often used interchangeably for all four types. Chiari malformations II, III, IV are discussed in the section on Spinal Dysraphism. In addition to the four classic types of Chiari malformations, several subtypes not in common use have been added: The Chiari 0 malformation and the Chiari 1.5 malformation (similar to CM-II, but without a cervical syrinx) ( ).
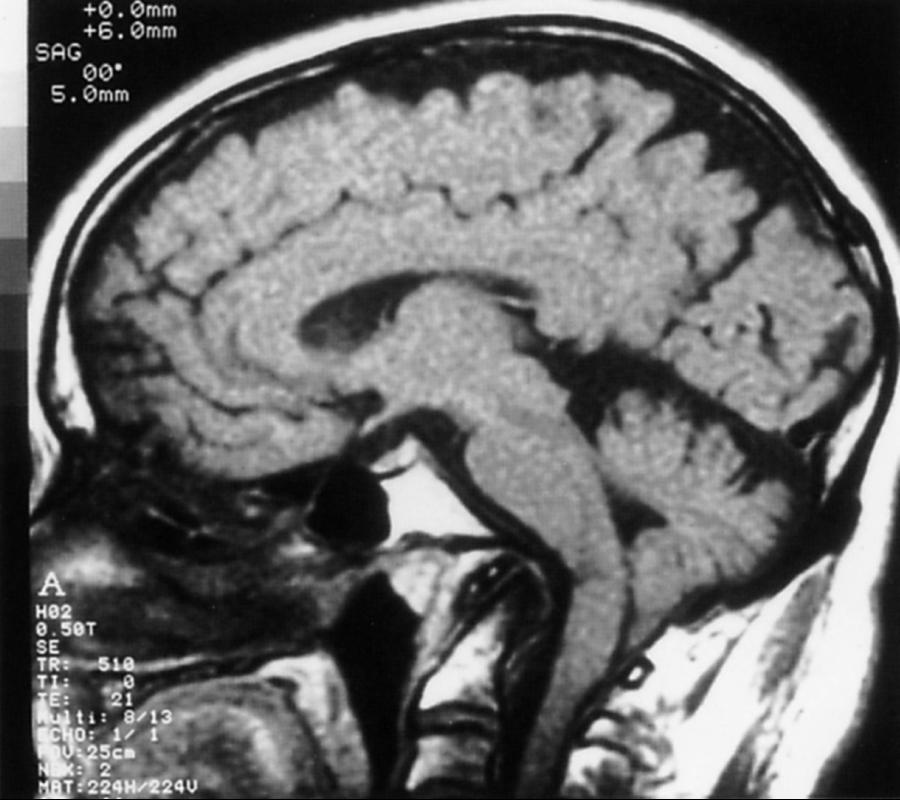
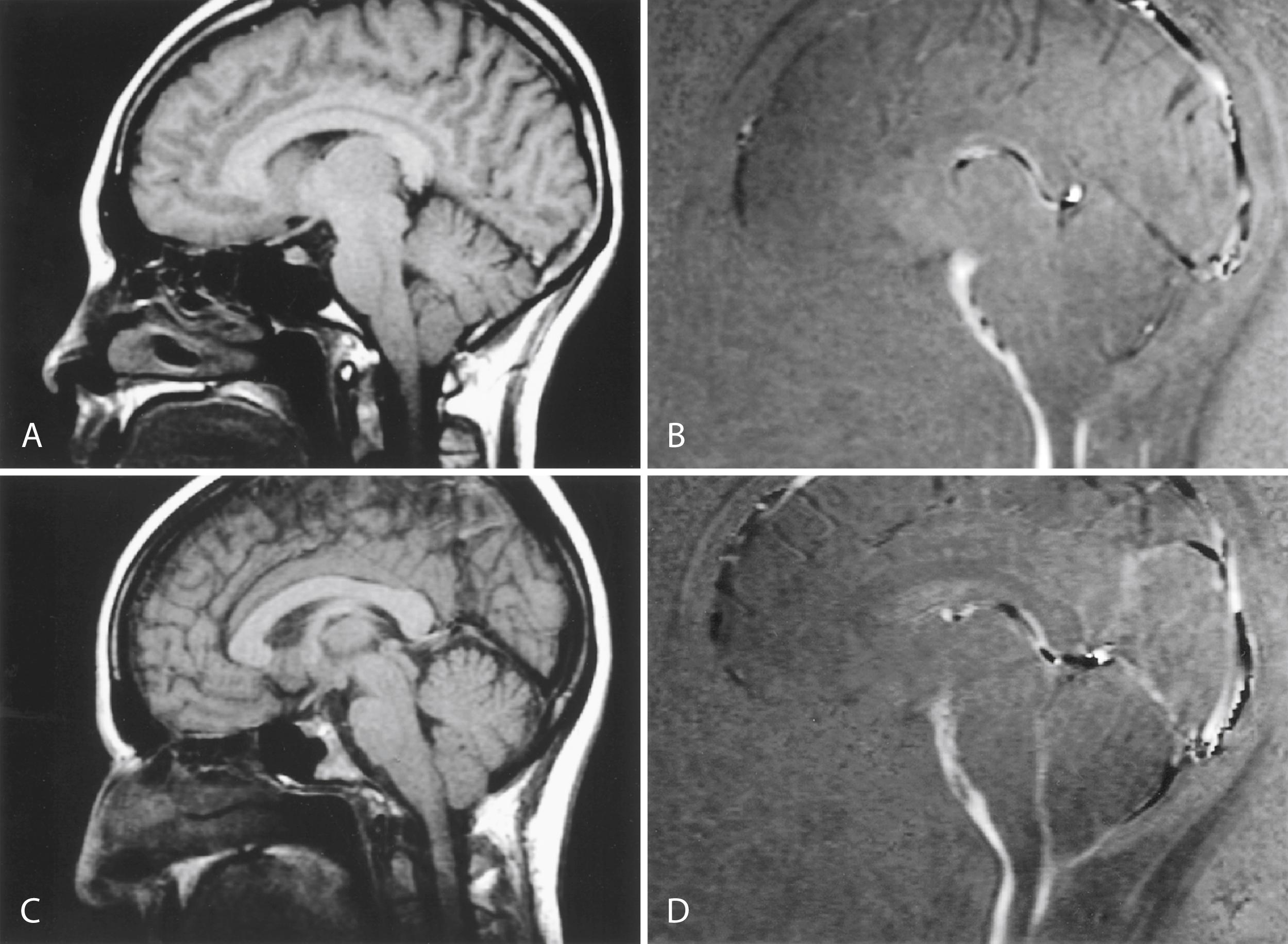
Chiari I malformation (CM-I) ( Fig. 104.6 ), the most common type, is characterized by abnormally shaped cerebellar tonsils with resultant tonsillar herniation through the foramen magnum (5 mm or more in young adults); this definition is anatomically precise, but can be confusing because there are many causes of abnormal tonsillar herniation that are not Chiari malformations. Classic CM-I is a congenital mesodermal malformation resulting in a hypoplastic posterior fossa, compressing neural tissue and forcing the cerebellar tonsils down through the foramen magnum. Distinguishing classic CM-I from other mechanisms of tonsillar herniation clarifies treatment despite overlapping clinical features ( ). Other potential disorders causing tonsillar herniation that are unrelated to skull-base hypoplasia include hydrocephalus, intracranial mass lesions, CSF leaks, prolonged lumboperitoneal shunting, hereditary disorders of connective tissue associated with occipitoatlantoaxial joint instability and cranial settling, tethered cord syndrome, and miscellaneous conditions such as craniosynostosis, acromegaly, and Paget disease. Milhorat and colleagues have correlated measurements of the posterior cranial fossa with clinical findings in 752 patients with Chiari malformations ( ; Table 104.2 ).
| Occipital Bone Size | PCFV | FM | |
|---|---|---|---|
| CM-I/classic type | Small | Small | Small |
| CM-I/craniosynostosis | Small | Small | Small |
| CM-II/with myelodysplasia | Small | Small | Large |
| CM-I/tethered cord syndrome | Normal | Normal | Large |
| CM-I/cranial settling | Normal | Normal | Normal |
| CM-I/intracranial space-occupying lesions | Normal | Normal | Normal |
| CM-I/lumboperitoneal shunt | Normal | Normal | Normal |
Patients with classic CM-I have a small posterior cranial fossa with constriction increasing below the Twining’s line , which extends from the anterior tuberculum sellae to the internal occipital protuberance. The foramen magnum is constricted transversely and has reduced outlet area ( Fig. 104.7 ). These findings suggest premature stenosis of the basi-exoccipital and exosupraoccipital synchondroses (see Fig. 104.7 , A ) which, if normal, would permit lateral expansion of the foramen magnum during somatic growth. Failure of the foramen magnum to expand normally during development may explain the conical shape of the posterior fossa in CM-I. Patients with achondroplasia can also have a stenosed foramen magnum and small posterior fossa, but the posterior fossa constriction is more generalized than in classic CM-I, suggesting an additional pathogenic mechanism in achondroplasia. Crouzon syndrome (see Fig. 104.7 , D ), Apert syndrome, nonsyndromic craniosynostosis, achondroplasia, acromegaly, and Paget disease are other causes of a small posterior fossa. In each of these conditions, the constricting small posterior fossa is the apparent cause of cerebellar tonsillar herniation.
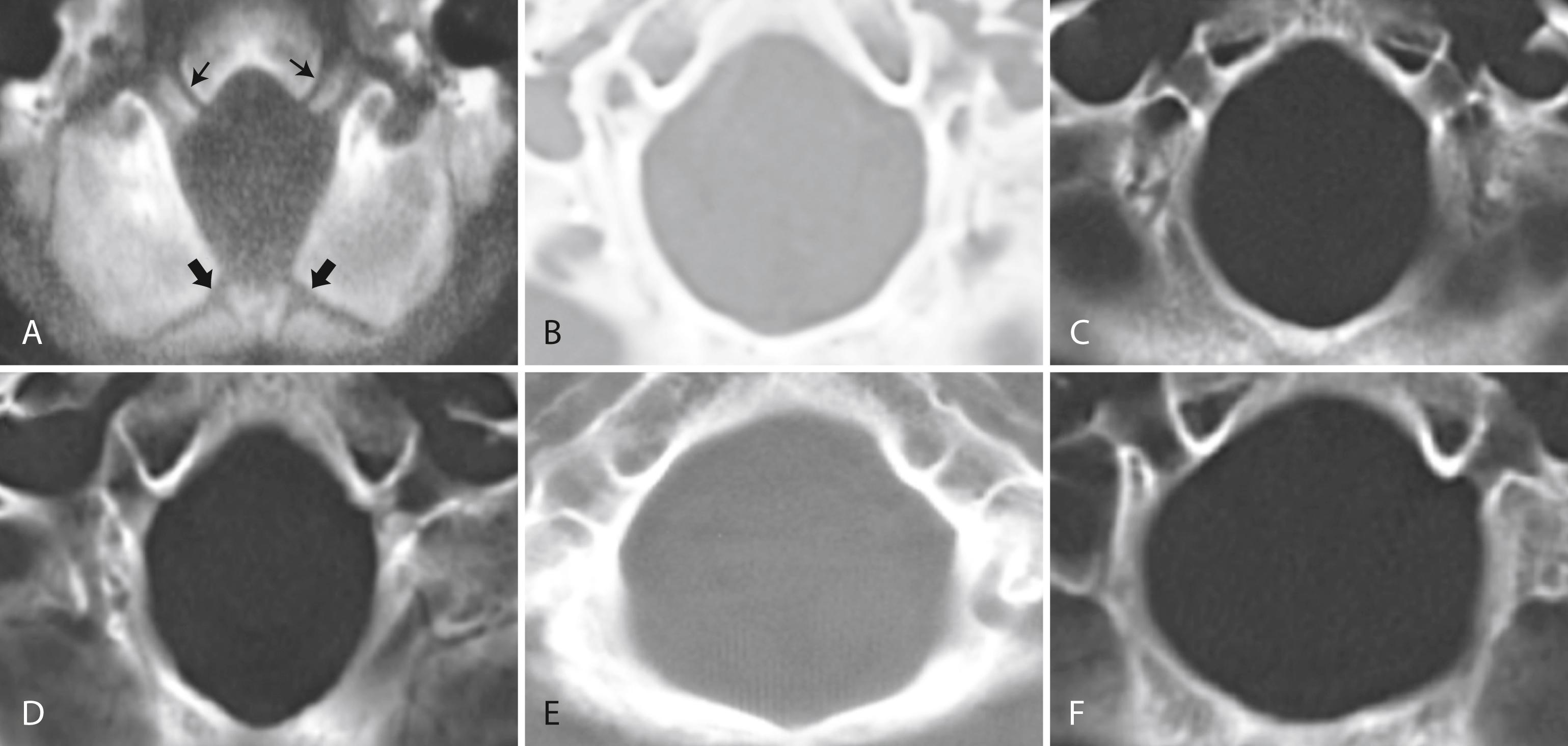
Some patients have a small posterior fossa with the conical configuration typical of classic CM-I, but do not have tonsillar herniation; this has been called Chiari 0 malformation . This designation remains controversial. It is thought that these patients can have symptoms similar to CM-I secondary to abnormalities in the flow of CSF within the skull and spinal canal ( ).
When patients with CM-I have other abnormalities such as hydrocephalus, basilar impression, occipitalization of the atlas, retroflexed odontoid processes, elongated styloid processes, or C1-level spina bifida occulta, the mechanism of the tonsillar herniation varies. In contrast to CM-I, patients with tonsillar herniation due to hydrocephalus, intracranial mass lesions, occipitoatlantoaxial joint instability, or prolonged lumboperitoneal shunting have normal occipital bone size, posterior cranial fossa volume, and foramen magnum size; in these conditions, mechanisms other than a small posterior fossa cause the tonsillar herniation. In patients with hydrocephalus or intracranial mass lesions, raised intracranial pressure causes compartmental shifts that push the brain caudally. In patients with occipitoatlantoaxial joint instability, cranial settling is the main cause of the herniation. In patients undergoing prolonged lumboperitoneal shunting, overdrainage of CSF apparently creates a pressure differential between the cranial and spinal compartments, drawing cerebellar tonsils downward. When the drainage is stopped, the pressure gradient can resolve, and the herniation often reverses. Low spinal fluid pressure can also cause tonsillar herniation in patients with spinal CSF leaks, dural ectasias, and myelodysplasia. Table 104.3 outlines five distinct mechanisms of cerebellar tonsil herniation; each mechanism has its own diagnostic and therapeutic implications.
| Cranial Constriction | Spinal Cord Tethering | Cranial Settling | Intracranial Hypertension | Intraspinal Hypotension |
|---|---|---|---|---|
| “Squeeze down” | “Pull down” | “Shake down” | “Push down” | “Suck down” |
| CM-I Craniosynostosis Achondroplasia Acromegaly Paget disease |
Tethered cord syndrome CM-II |
HDCT (e.g., EDS) Posttraumatic CCI Osteogenesis imperfecta |
Hydrocephalus Posterior fossa cysts, tumors Subdural hematoma Intracranial mass lesions |
Prolonged LPS CSF leaks Dural ectasia |
Measurements of the posterior cranial fossa enhance neuroradiological diagnosis of Chiari malformations. Computer analysis of standard MR and CT images is quick and easy. Diagnosis starts by assessing the size of the posterior fossa; an abnormally small posterior fossa shows that the CM-I is likely due to cranial constriction. A normal-sized posterior fossa necessitates a search for alternative mechanisms of tonsillar herniation such as cranial settling, spinal cord tethering, raised intracranial pressure, or intraspinal hypotension. Measurement of the foramen magnum helps in the differential diagnosis of tonsillar herniation because the foramen is enlarged in tethered cord syndrome and Chiari malformation II but stenosed in classic CM-I, craniosynostosis, and miscellaneous disorders such as achondroplasia.
Tonsillar herniation, once considered rare, is now easily recognized on sagittal brain MRI and has a prevalence of 0.1%–0.5% ( ). New genetic studies support a hereditary tendency, with a transmissibility rate approaching 12%. Women are affected three times more often than men.
Patients with CM-I may experience no symptoms or first have symptoms in adolescence or early adulthood ( ; Table 104.4 ). At least a quarter of patients first have symptoms following relatively minor head or neck injury. Most symptomatic patients have pressing occipital headache and neck pain. Other manifestations can include visual disturbances, neuro-otological complaints, cranial nerve dysfunction, cognitive difficulties, and sleep-related breathing disorders ( ). Patients with CM-I often have associated syringomyelia (discussed later), but motor, sensory, sphincter, and reflex disturbances suggestive of myelopathy can occur whether or not a syrinx is present. Clinical severity correlates generally but not perfectly with the extent of tonsillar herniation and the degree of obstruction to CSF flow. In more severe cases, the medulla descends below the foramen magnum; in which case brainstem dysfunction is more likely to be among the clinical findings ( ).
| Symptom | % of CM-I Patients (364) |
|---|---|
| Suboccipital headache (frequent retro-orbital component); exertional and postural accentuation | 81 |
| Ocular disturbances: floaters, blurring, photophobia, diplopia | 78 |
| Acoustic and vestibular complaints: dizziness/dysequilibrium, tinnitus | 74 |
| Dysesthesias: tenderness, numbness/tingling, burning | 59 |
| Chronic fatigue | 58 |
| Bulbar and coordinative problems: | 52 |
|
|
|
|
|
|
| Segmental pain | 44 |
| Impaired memory or concentration | 39 |
| Cervical pain | 34 |
| Low back pain | 24 |
| Urinary incontinence | 17 |
Deciding whether common symptoms such as memory concerns, back pain, or fatigue are caused by CM-I in an individual patient is a clinical challenge. Despite speculation and public interest, there is no scientific confirmation of an association between CM-I and fibromyalgia or chronic fatigue syndrome ( ).
Slight extension of the tonsils below the foramen is normal in childhood, and normal values decrease with increasing age ( Table 104.5 ). When young children have symptoms, they can have oropharyngeal dysfunction and scoliosis. Children younger than age 3 can have vomiting and gastric reflux as the sole symptoms of CM-I.
| Decade of Life | Distance Below Foramen Magnum (mm) |
|---|---|
| First | 6 |
| Second or third | 5 |
| Fourth to eighth | 4 |
| Ninth | 3 |
The posterior fossa or “Chiari” headache has diagnostically helpful characteristics: it is a suboccipital pressing head pain that is usually continuous, waxing and waning but not episodic, and radiating at times behind the eyes or to the vertex ( ). The headache is almost never hemicranial and is often exaggerated by physical activity and Valsalva maneuvers including bearing down with bowel movements, with laughter, crying, coughing, and sneezing. Exacerbations can be explosive rather than throbbing or pounding. There is no aura, but visual sparkles and scotomas can punctuate the peaks of headache. Neck pain without radicular features is common. Patients may have evanescent hand paresthesias and other generalized musculoskeletal complaints.
CM-I patients with “Chiari” headaches and little else may coincidently have migraine without aura, which can result in a diagnostic dilemma. Both headaches occasionally can share some similar features such as occipital location and perimenstrual accentuation. “Chiari” headaches are not likely to respond to migraine abortive agents such as the triptans or prophylactic agents including beta-blockers and serotonin reuptake inhibitors (SRIs). However, “Chiari” headaches like migraine may respond to topiramate, presumably because it is a carbonic anhydrase inhibitor and lowers CSF pressure. Patients may have tonsillar herniation on MRI and not yet have any CM-I–related symptoms. However, these patients can have migraine. It is therefore important to first rule out migraine as a cause of headache before aggressively treating a CM-I malformation. This is even more important in those patients diagnosed with Chiari 0. Neurological examination of patients with CM-I may show subtle abnormalities but is often completely normal. The most typical finding is a vestibular-generated dysequilibrium and difficulty in tandem standing and walking. Nystagmus is difficult to appreciate even with Fresnel lens examination. Objective findings frequently elude sophisticated vestibular testing, which only occasionally reveals nystagmus of possible central or peripheral etiology. Classic downbeat nystagmus is rarely seen even with tonsillar herniation as striking as 20 mm.
The malformation is best seen on a T2-weighted sagittal MRI scan of the brain and cervical spine, which allows for assessment of the shape of the posterior fossa and may detect an accompanying syrinx as well as the extent (if any) of brainstem compression. Flow of CSF at the foramen magnum can be evaluated using phase-contrast MRI and cardiac gating to acquire images throughout the cardiac cycle ( ). Symptomatic Chiari I malformations can cause an increase in peak systolic CSF flow velocity and decreased uniformity of flow.
The severity and nature of symptoms of CM-I vary greatly, so naturally therapy should also vary from patient to patient. The minority of patients require surgery. Headache symptoms sometimes respond to carbonic anhydrase inhibitors, simple approaches to associated paracervical pain, or head elevation during sleep. Minor traumatic events can aggravate symptoms, and patients should avoid activities that risk head and neck injury.
Management of labor and delivery in patients with CM-I is somewhat controversial. Although avoiding epidural or spinal anesthesia has been recommended by some, there is a literature suggesting these techniques can be employed safely in CM-I parturients for vaginal or cesarean deliveries ( ). For women with syringomyelia, a cesarean section is advisable to avoid intrathoracic and abdominal pressure alterations associated with vaginal deliveries ( ). Surgical decompression of the posterior fossa is appropriate treatment for the minority of patients who have significant functional impairment due to persistent headache despite medical therapy or have progressive syringomyelia. It is not indicated for symptoms limited to chronic fatigue, musculoskeletal pain, or vertigo, or for prophylaxis against worsening of headache or of syringomyelia. discusses treatment in detail and provides a comprehensive management algorithm.
Spinal dysraphism is congenital failure of the primitive neural tube to close during fetal development and includes a number of disorders of fusion of dorsal midline structures of the spinal canal or skull ( ). Neural tube defects are one of the most common congenital malformations, with 70,000–100,000 disabled individuals in the United States alone ( ). The neural tube normally closes during the first 3 weeks following conception. There are genetic causes, but environmental causes are important in most cases. The most extreme form is anencephaly, characterized by absence of the entire cranium at birth; the undeveloped brain lies at the base of the skull as a small vascular mass without recognizable nervous structures.
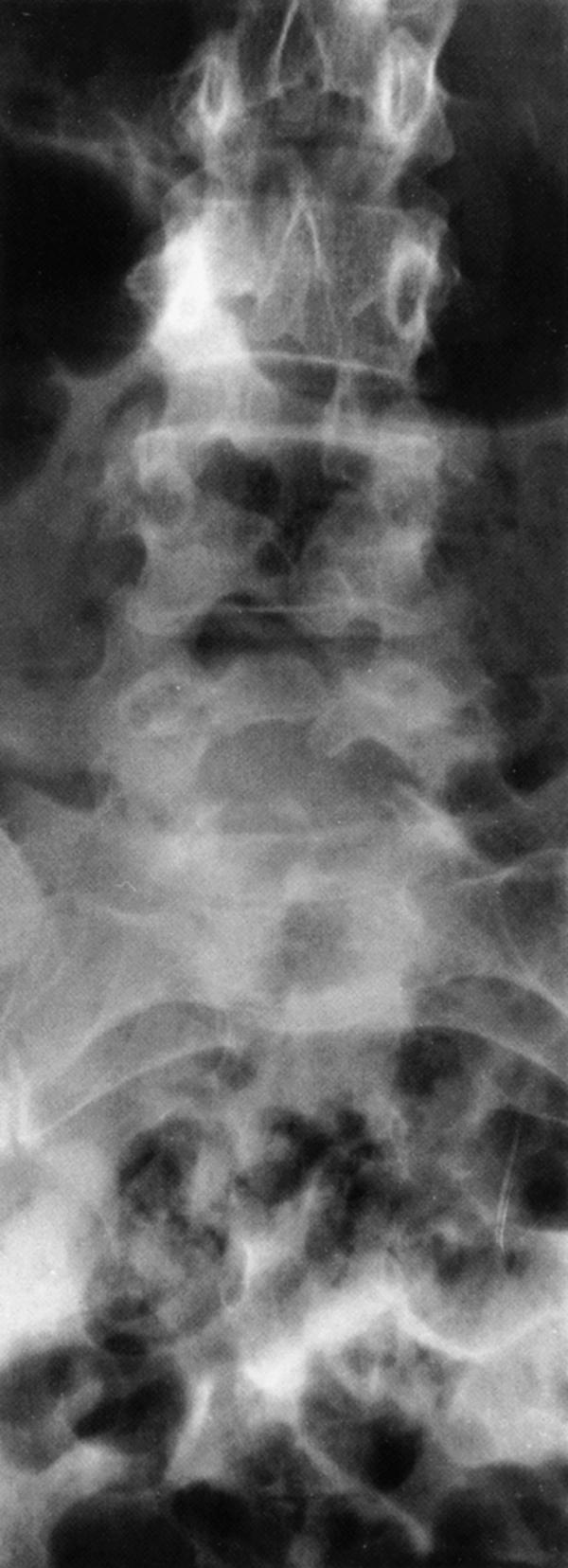
Spina bifida occulta is the most common and least symptomatic (usually asymptomatic) form of dysraphism. In this anomaly, the vertebral elements fail to fuse posteriorly, but the thecal and neural elements remain within the spinal canal. It is most common at posterior elements of L5–S1 and is usually noted as an incidental finding on spinal plain radiography ( eFig. 104.8 ) ![]() . Cutaneous abnormalities may be associated ( Box 104.4 ). Orthopedic foot deformities, urinary or rectal sphincter dysfunction, or focal neurological abnormalities can indicate that the spina bifida occulta is associated with compression or malformation of neural tissues or with spinal cord tethering.
. Cutaneous abnormalities may be associated ( Box 104.4 ). Orthopedic foot deformities, urinary or rectal sphincter dysfunction, or focal neurological abnormalities can indicate that the spina bifida occulta is associated with compression or malformation of neural tissues or with spinal cord tethering.
Asymmetrical gluteal fold
Dermal sinus or dimple
Hairy tuft
Hemangioma
Lipoma
Nevus
Pilonidal sinus
Rudimentary tail
Spinal aplasia cutis
In myelomeningocele (spina bifida) and meningocele (spina bifida cystica) , congenital defects of midline closure are accompanied by eventration of meninges and even of neural tissue. Spinal defects are often visible on examination of the back of the newborn ( eFig. 104.9 ![]() , Figs. 104.10 and 104.11 ). At times the skin and vertebral canal are open, and a sac of meninges is directly visible. The defect is most common in the lumbar region. If the sac contains nerve roots or spinal cord, it is a myelomeningocele ; if neural elements are absent from the sac, it is a meningocele . These brain and spinal cord malformations may be associated with CSF leakage into adjacent structures, posing a risk of meningitis. Neurological deficits are directly related to the anatomical extent of the malformation and vary from insignificant to grave.
, Figs. 104.10 and 104.11 ). At times the skin and vertebral canal are open, and a sac of meninges is directly visible. The defect is most common in the lumbar region. If the sac contains nerve roots or spinal cord, it is a myelomeningocele ; if neural elements are absent from the sac, it is a meningocele . These brain and spinal cord malformations may be associated with CSF leakage into adjacent structures, posing a risk of meningitis. Neurological deficits are directly related to the anatomical extent of the malformation and vary from insignificant to grave.
Either defect is often accompanied by hydrocephalus or by Chiari II malformation, in which the cerebellar vermis and caudal brainstem descend through an enlarged foramen magnum ( ). The extent of brainstem herniation is variable, including portions of the medulla or even of the pons. Hydrocephalus and syringomyelia are common accompanying features, and patients often have various associated anomalies such as a small posterior fossa, kink in the medulla, and polymicrogyria. These infants are at risk for later development of tethered cord syndrome, spinal dermoid, and epidermoid inclusion cysts. In Chiari III malformation, the displaced cerebellar and brainstem tissue extends into an infratentorial meningoencephalocele. An important cause is maternal folate deficiency, and most cases could be prevented if women with childbearing potential routinely took folic acid daily. Other risk factors include family history of neural closure defects and maternal treatment with some antiepileptic drugs such as valproic acid and carbamazepine. Pregnant women can be screened at 14–16 weeks for serum α-fetoprotein (AFP) levels, which are elevated about 85% of the time when the fetus has neural closure defects. The defects also can be detected by fetal ultrasonography. Ultrasound detects greater than 90% of neural tube defects at 18–20 weeks (coupled with AFP >95%).
Planning treatment for affected infants, potentially including surgery, is difficult. Initial surgical treatment in utero or in the neonatal period can provide cosmetic repair and decrease the risk for meningitis ( ). Also hydrocephalus can be shunted. Any existing myelopathic or radiculopathic neurological deficit is likely to persist after surgery. Some patients, especially infants with progressive brainstem dysfunction, are treated with decompression of the rostral spinal canal. Less than 30% of such patients survive beyond the first year, and long-term problems including mental retardation and paraplegia are often severe. Few patients with myelomeningocele are mentally normal, but most of those with lumbar meningocele are.
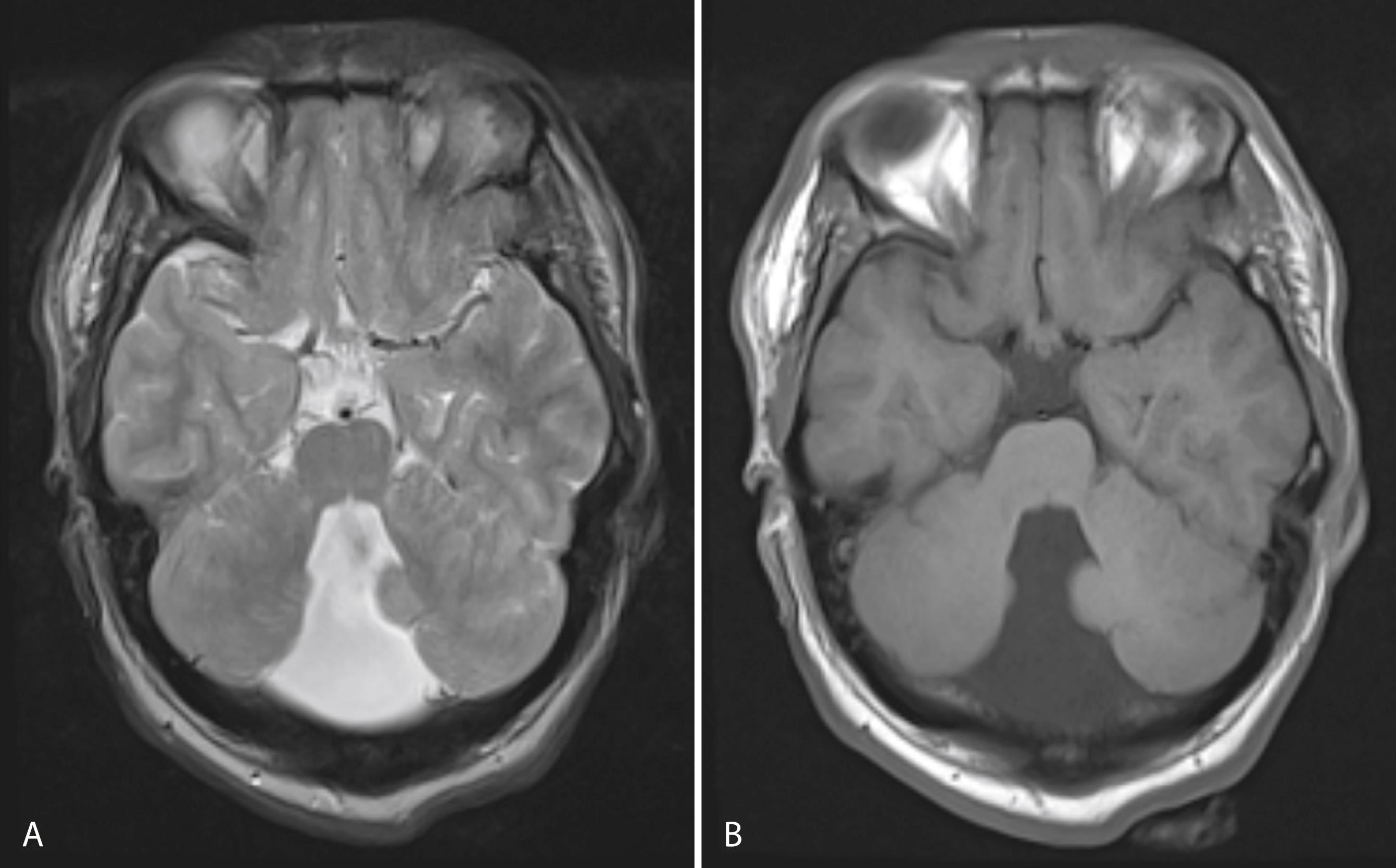
Dandy-Walker syndrome results from the failure of development of the midline portion of the cerebellum. A cyst-like structure associated with a greatly dilated fourth ventricle, expanding the midline, is often seen ( Fig. 104.12 ). The malformation typically causes the occipital bone to bulge posteriorly and displaces the tentorium and torcula upward. The cerebellar vermis is aplastic, and the corpus callosum may be deficient or absent. There is usually dilation of the aqueduct as well as the third and lateral ventricles. Chiari IV malformation is characterized by cerebellar and brainstem hypoplasia rather than displacement and is probably a variant of the Dandy-Walker malformation.
Congenital abnormalities of the spinal cord or cauda equina can result in spinal cord tethering, in which stretching and tension develops within the cord tissue as the spinal column elongates during early life, resulting in the conus medullaris being found at an abnormally low vertebral level ( ; Box 104.5 ). Imaging studies, such as spinal MRI, showing the conus medullaris caudad to the lower endplate of L2, can be evidence of tethering. A child or even an adult with these abnormalities can develop progressive neurological dysfunction due to traction on the cord or nerve roots. One presentation is lower motor neuron dysfunction in one or both lower extremities, but patients can also have sensory loss, upper motor neuron signs, orthopedic foot deformities, and scoliosis. A tethered spinal cord can, in addition, cause isolated sphincter dysfunction as subtle as intermittent urinary incontinence.
The so-called occult tethered cord syndrome is an area of controversy ( ). Uncontrolled surgical series suggest that children with neurogenic voiding dysfunction and normal spinal MRI might also have cord tethering. For some children, voiding dysfunction reportedly improved after lysis of the filum terminale, which microscopically can be abnormally thickened, fatty, and fibrous, even though the filum and conus medullaris appear normal on MRI. A few cases of cerebellar tonsillar herniation seem to be due to occult cord tethering; other features are syrinx development below the T5 level and scoliosis ( ). In contrast to patients with classic CM-I, patients with this variation of CM-I with cord tethering have normal posterior fossa volume and an enlarged foramen magnum. Supporting the role of cord tethering as a cause of the tonsillar descent are reports of increasing herniation of the cerebellar tonsils with somatic growth, cerebellar prolapse following Chiari decompression surgery, and anatomical improvements including ascent of the conus medullaris, ascent of the cerebellar tonsils, and resolution of brainstem elongation following section of the filum terminale.
Split spinal cord malformation (SSCM), formally termed diastematomyelia, is a congenital malformation of the spinal cord characterized by sagittal division of a portion of the cord into two hemicords. In most instances, the division is located in the lower thoracic or lumbar regions. SSCM is often accompanied by skin abnormalities such as a tuft of hair at the level of the lesion. Two types of SSCM are described: type I, in which each hemicord has its own dural sheath, and type II in which both hemicords are enclosed in a single dural sheath. Neurological deficits, scoliosis, and congenital foot deformities are more common in type I. Bony and cartilaginous spurs between the hemicords are also more common with type I. Finally, surgical repair is more effective in type I and can be combined with distal untethering if a tethered cord is present as well. The spur tethers the spinal cord, leading to progressive neurological dysfunction when the vertebral column lengthens during growth. The diagnosis can often be suspected on plain radiography, which shows widening of the interpedicular distance and a posterior bony bridge at the level of the lesion. MRI or CT myelography can confirm the diagnosis ( eFig. 104.13 ) ![]() . Surgical therapy consists of attempts to free all structures tethering the cord by removing the spurs and dura in the cleft and cutting the filum terminale if abnormally tethered.
. Surgical therapy consists of attempts to free all structures tethering the cord by removing the spurs and dura in the cleft and cutting the filum terminale if abnormally tethered.
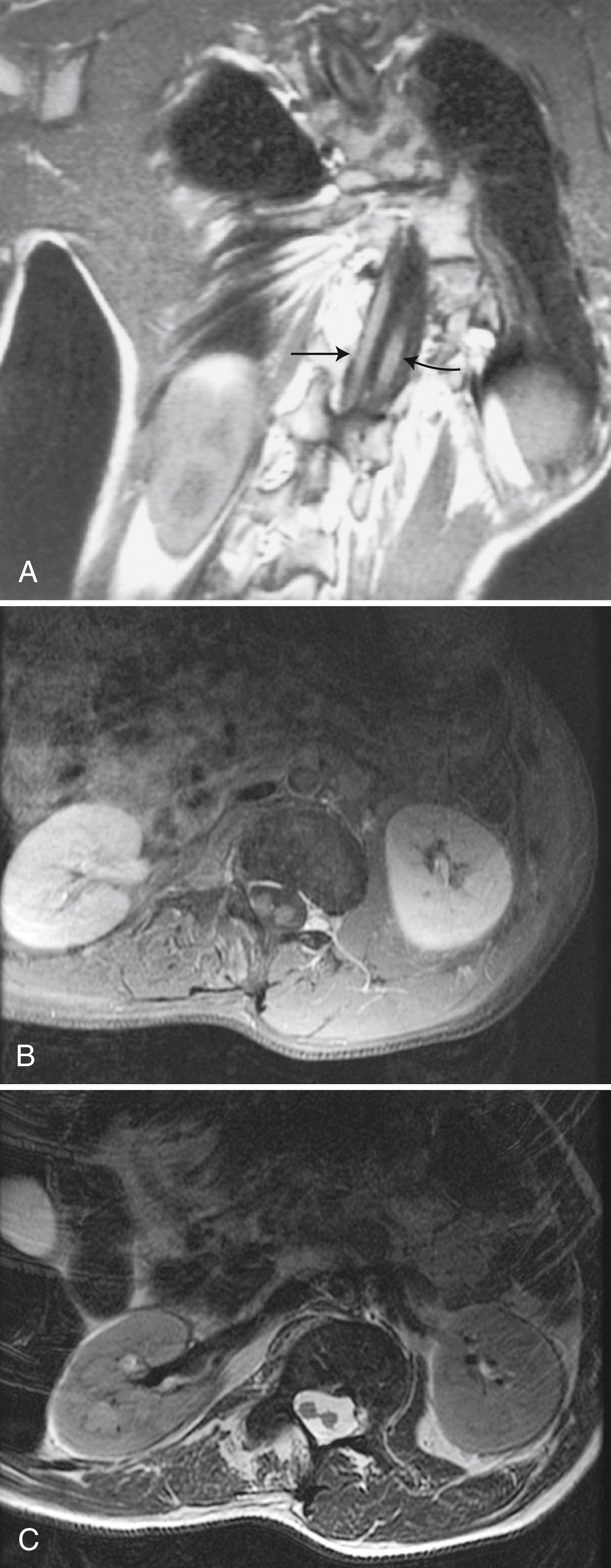
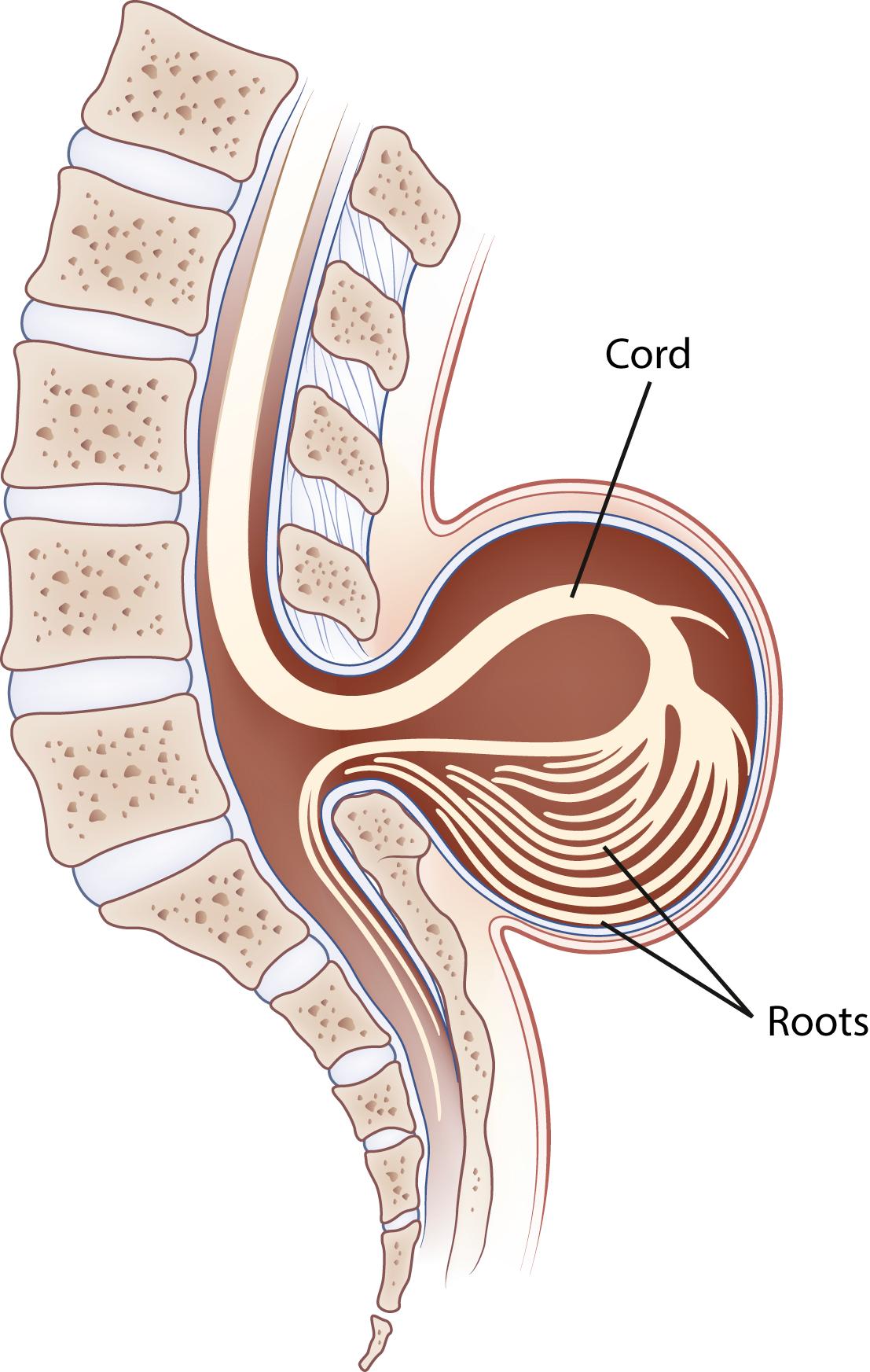
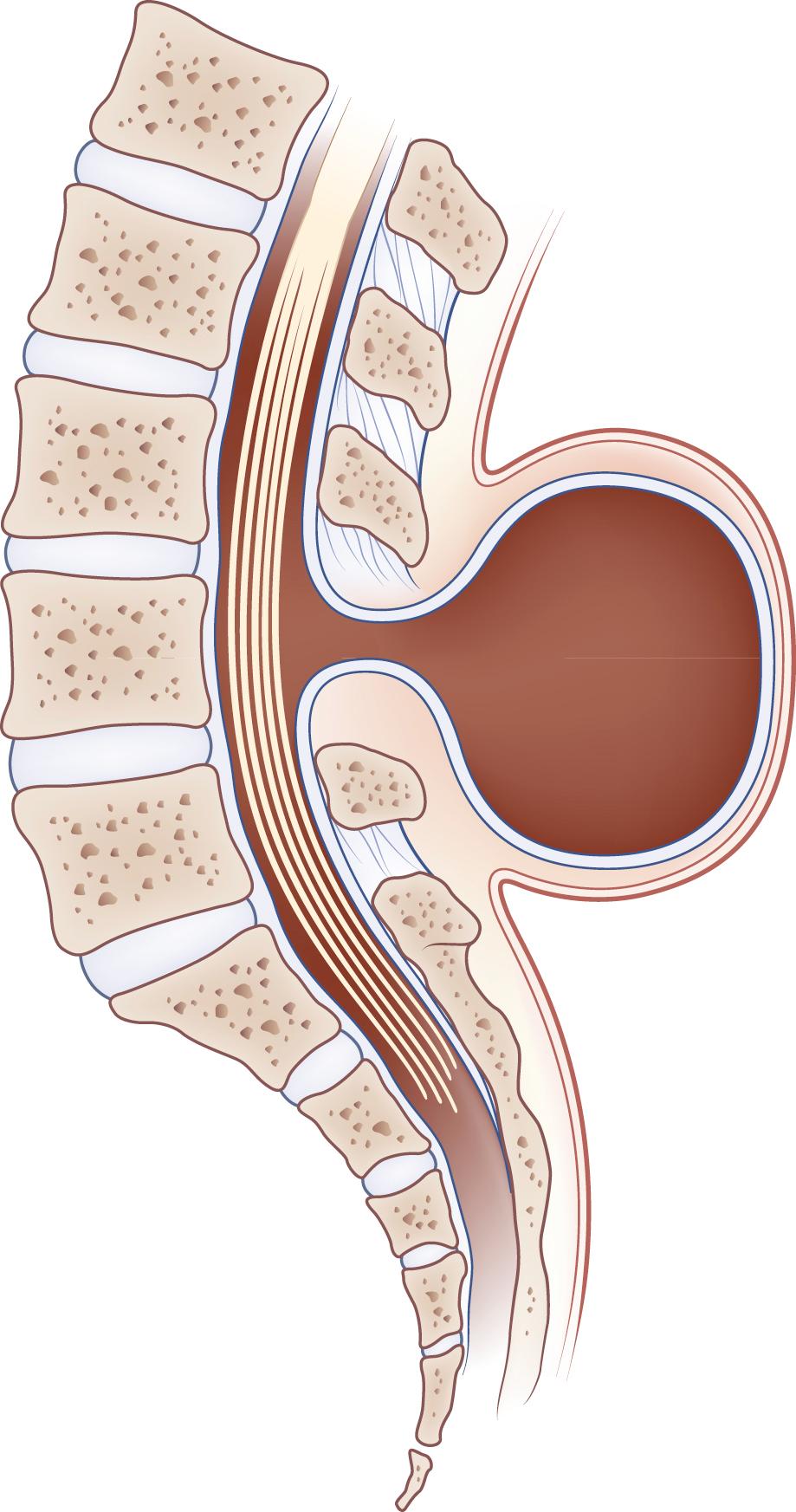
Hydromyelia is an abnormal dilation of the central spinal canal with “excess” CSF contained within the ependymal lining. When fluid dissects into the surrounding white matter, forming a cystic cavity or syrinx, the term syringomyelia is applied. A syrinx , then, is a cavity in the spinal cord (syringomyelia) or brainstem (syringobulbia) ( Figs. 104.14, 104.15, and 104.16 ). Hydromyelia and syringomyelia often coexist, and many physicians use the terms interchangeably.
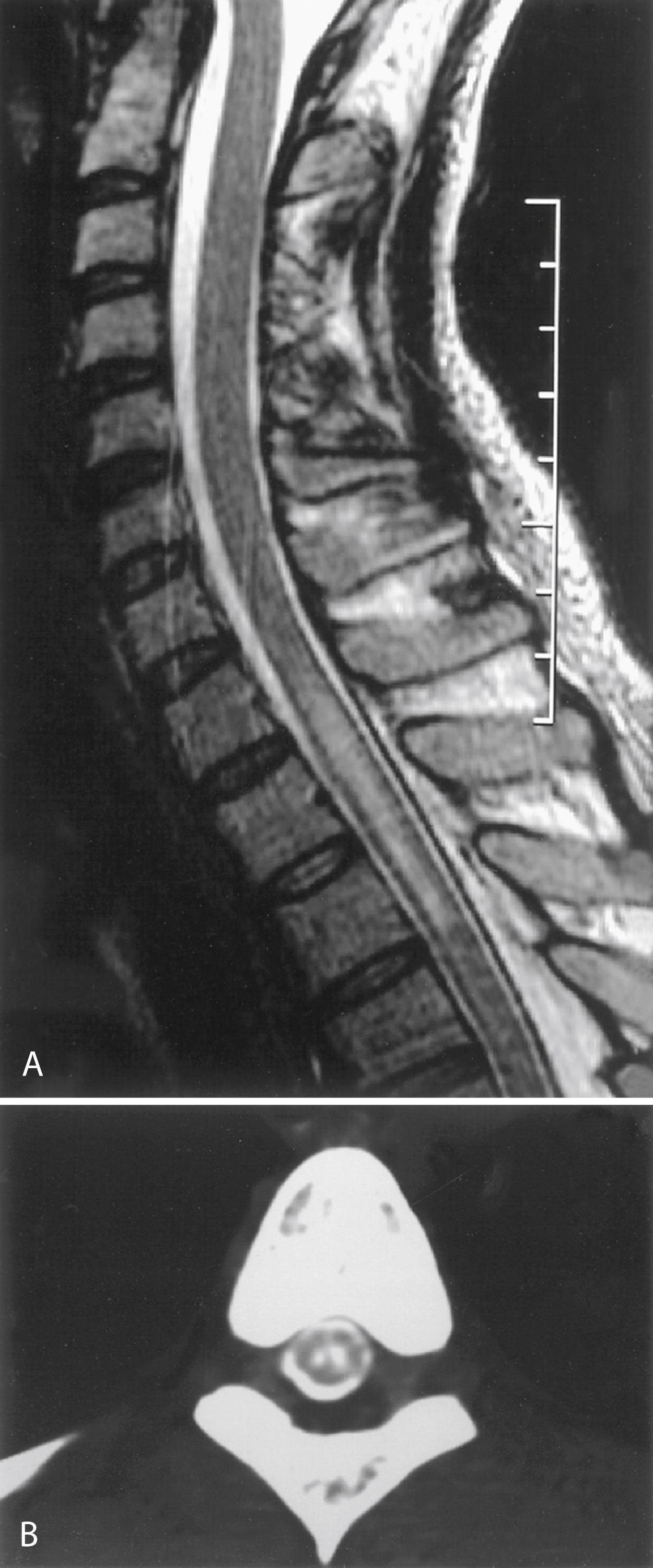
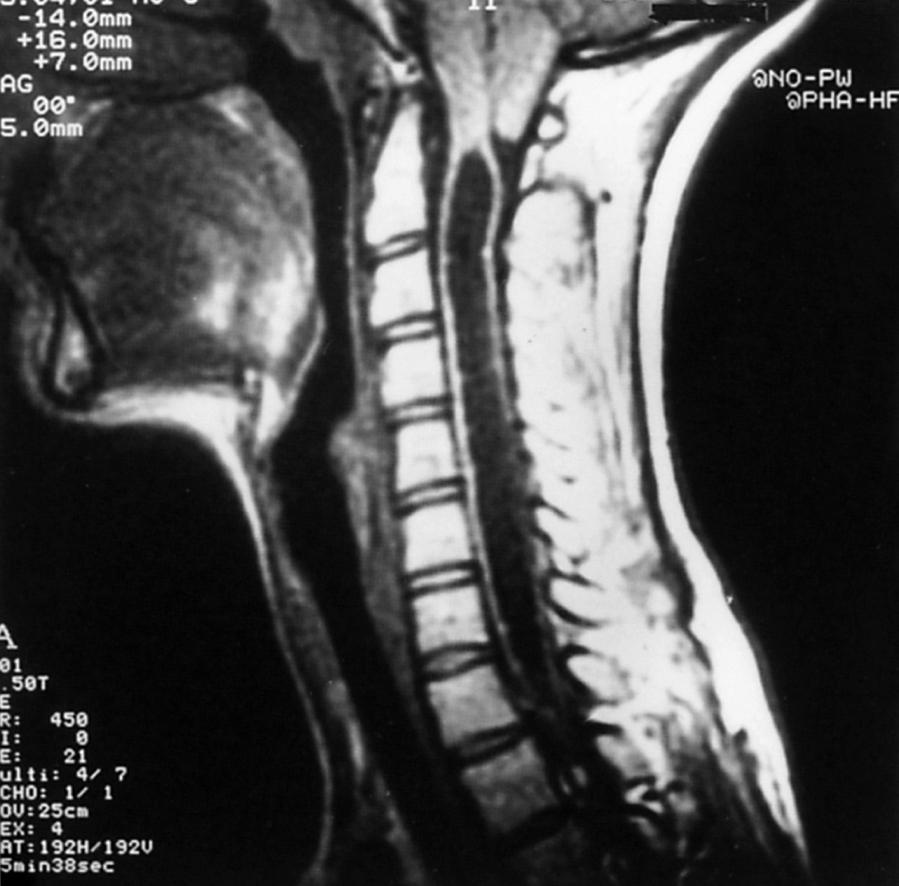
The widespread use of spinal MRI has greatly increased the detection of cervical syringomyelia, which often produces nonspecific symptoms such as neck pain and at times no symptoms at all. Estimated prevalence in the United States is between 1:1300 and 1:1900.
The central canal of the spinal cord is normally wide open during embryonic life and becomes atretic after birth. It is occasionally patent in adults (see Fig. 104.14 ). Cervical or thoracic MRI in an adult will occasionally reveal an incidental asymptomatic hydromyelia, which is typically linear or fusiform on sagittal images; extends over several levels, sometimes discontinuously; and is round, central, and up to 4 mm in diameter on axial images ( ).
The prototypical presentation of a symptomatic syrinx is the presence of lower motor neuron signs at the level of the lesion, usually in the upper extremities, or in the case of syringobulbia, the lower cranial nerves. Furthermore, it can present as a myeloradiculopathy with lower motor neuron findings in the upper extremities and upper motor neuron findings in the lower extremities. In addition, there is often a dissociated, suspended sensory loss with impaired pain and temperature sensation, but preserved dorsal column sensation (i.e., light touch). However, few patients show this total picture. The clinical features vary with the size, location, and shape of the cavity; the rapidity of its evolution; and any associated neurological conditions such as a Chiari I malformation. Symptoms are more related to the pace of evolution of the syrinx than to its absolute size. Otherwise healthy patients with slit-like syrinx cavities may present with severe localized spinal and radicular pain. Other patients with syrinx cavities displacing as much as 90% of the spinal cord mass may be virtually asymptomatic.
Pain is a prominent symptom in most patients with syringomyelia. Common complaints include neck ache, headache, back pain, radicular pain, and areas of segmental dysesthesia. Painful dysesthesias are most likely to occur at or adjacent to the caudal extent of the syrinx cavity. Some patients have trophic changes corresponding to segmental loss of pain sensation. Syringomyelia can cause neuropathic monoarthritis (Charcot joint), most commonly in a shoulder or elbow.
Most syringes are in the cervical spinal cord. Those developing from hydromyelia are usually associated with Chiari I or II malformations, communicating hydrocephalus, or abnormalities at the craniovertebral junction. Asymptomatic hydromyelia and syringomyelia may be incidentally discovered by MRI while investigating unrelated cranial symptomatology, such as a Chiari malformation. A syrinx associated with a spinal cord tumor or trauma can occur at any level of the spinal cord. Although either CT or MRI can demonstrate a syrinx, MRI is more sensitive for complete evaluation of the cord and surrounding soft tissues. In patients in whom MRI is contraindicated, CT myelography may be useful in discerning syringomyelia, which will commonly fill with contrast on delayed images due to communication with the CSF through the central canal of the spinal cord (see Fig. 104.15 ).
The terms communicating and noncommunicating syringes indicate whether the syrinx is in communication with the CSF pathways. However, it is often difficult to determine this, even at autopsy, so these terms are mainly of use in discussions of etiology. It is better to classify syringomyelia according to its associations.
Abnormalities of the cervicomedullary junction and posterior fossa, such as Chiari anomalies types I and II and the Dandy-Walker malformation, are the most common cause of nonidiopathic syringes. Up to 70% of nonidiopathic syringes are associated with CM-I. The mechanism of formation of these syringes is controversial ( ). Patients with cervical syringomyelia and no Chiari malformation often have a small posterior fossa or disturbed flow of CSF near the foramen magnum ( ). Syringes can extend beyond hydromyelia as an outpouching of the dilated central canal (see Figs. 104.15 and 104.16 ). One hypothesis is that the posterior fossa abnormalities interfere with the passage of CSF from the fourth ventricle through the foramina of Luschka and Magendie into the subarachnoid space. The consequence is transmission of bulk flow and the various pressure waves of the CSF (arterial, venous, respiratory) down the central canal of the spinal cord, leading to dissection of a syrinx into the substance of the spinal cord. Noncongenital abnormalities at the cervicomedullary junction that sometimes cause syringomyelia include arachnoiditis and meningiomas.
In CM-I, the large majority (80%) occur within the cervical cord. The thoracic cord can, however, be affected in isolation, and, more rarely, a lumbosacral syrinx may appear associated with spinal cord tethering secondary to a thickened and restrictive filum terminale.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here