Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A cognitive continuum exists from normal aging through mild cognitive impairment (MCI) to dementia. This continuum is better understood when realizing that it occurs on a background of some degree of cognitive decline with aging. While the theoretical ideal is to age without cognitive change, typically cognitive function declines over time. Research has provided normative data on cognitively normal individuals at each decade of life, but this approach has been criticized because these studies likely include individuals who subsequently develop cognitive impairment. Research on normal aging using biomarkers for both Alzheimer disease (AD) and non-AD related pathologies will hopefully improve these methodological issues. Despite the aforementioned limitations, a brief review of cognitive change with age is important.
Before age 60, a consistent pattern of cognitive change with age occurs. General knowledge and vocabulary are stable or improve while problem solving, speed of processing and reasoning decline ( ).
Age-related decline occurs primarily in cognitive speed, working memory, and encoding ( ). The pattern on neuropsychological testing associated with normal aging includes a decline in learning and acquisition performance with delayed recall relatively preserved ( ). Recognition performance also is preserved with age. Age-related cognitive decline is heterogeneous as a substantial minority may show minimal decline ( ). Cognitive reserve refers to different capacities for the brain to maintain cognitive functioning in setting of brain pathology or injury ( ).
Age-related cognitive decline is associated with different neuroanatomical changes compared to cognitive decline from AD. Loss of synaptic density occurs as a function of age independent of Alzheimer pathology ( ). While AD is characterized by early damage of the entorhinal cortex and relative preservation of the dentate, age-associated medial temporal lobe changes occur in the dentate with preservation of the entorhinal cortex ( ). Brain volume normally declines with age but at a significantly slower rate than AD patients. In addition to the hippocampus, which declines in volume by 1%–2% a year in normal aging ( ), the prefrontal cortex also undergoes an age-related decrease in volume ( ).
Many studies have shown the pathophysiological processes leading to dementia can begin decades prior to cognitive symptoms. An evolving understanding of the preclinical stages of dementia has resulted in significantly increased interest in targeting it as a possible therapeutic time window. In the preclinical phase of dominantly inherited AD, cerebrospinal fluid (CSF) amyloid beta 42 (Aβ42) decreases 25 years before expected symptom onset ( ). Recent studies have revealed that in the general population the sequence of biomarkers leading to dementia is more diverse than dominantly inherited AD. In addition to cognitively normal individuals with biomarkers compatible with preclinical AD, the Mayo Clinic Study of Aging identified a group of patients with evidence of neurodegeneration on fluorodeoxyglucose positron emission tomography (FDG-PET) or magnetic resonance imaging (MRI), but without cerebral amyloid deposition. This group, termed suspected non-Alzheimer pathophysiology (sNAP) did not have imaging evidence consistent with cerebrovascular disease or synucleinopathy as the cause of brain injury ( ; ). In general, these sNAP patients have a lower risk of becoming symptomatic after 5 years compared to patients with amyloid or amyloid plus neurodegeneration related biomarkers ( ). In synuclein-related neurodegenerative disorders, autonomic symptoms, rapid eye movement (REM) sleep behavior disorder, and anosmia can predate cognitive and motor symptoms by many years. Ioflupane dopamine transporter scanning appears to be a promising biomarker in these conditions ( ). Preclinical stages of frontotemporal dementia (FTD) have not been studied as much as AD. The available biomarker data will be reviewed subsequently in this chapter. The 2011 National Institute on Aging (NIA) ( ) preclinical AD criteria are summarized in Table 95.1 ( ; ).
| Diagnostic Category | Amyloid Beta (Positron Emission Tomography or Cerebrospinal Fluid) | Neuronal Injury ∗ | Cognitive Change | % of diagnostic category ( ) | 5-Year Risk of Dementia ( ) | |
|---|---|---|---|---|---|---|
| Normal AD biomarkers | Stage 0 (normal AD biomarkers) | − | − | − | 43% | 2% |
| Preclinical AD | Stage 1 (asymptomatic amyloidosis) | + | − | − | 16% | 11% |
| Stage 2 (amyloidosis plus evidence of neural degeneration) | + | + | − | 12% | 26% | |
| Stage 3 (amyloidosis, neurodegeneration, subtle cognitive change) | + | + | + | 3% | 56% | |
| sNAP † (neurodegeneration, no amyloidosis) | − | + | − | 23% | 5% | |
∗ Biomarkers of neuronal injury include increased cerebrospinal fluid (CSF) tau, hippocampal atrophy, and abnormal fluorodeoxyglucose positron emission tomography (FDG-PET) metabolism.
† Suspected non-Alzheimer pathway, not part of 2011 NIA criteria.
MCI refers to an in-between state of normal cognitive aging and dementia. In MCI, cognitive change is greater than expected for age but independence in the community and activities of daily living are preserved ( ; ). On average, MCI patients perform 1–1.5 standard deviations below matched normative data. In 2018, the American Academy of Neurology published evidence-based guidelines on the concept of MCI, documenting the prevalence of MCI to be 6.7% at 60–64 years, 8.4% between ages 65 and 69, 10.1% between ages 70 and 74, 14.8% between ages 75 and 79, and 25.8% for ages 80–84. The rate of progression from MCI to dementia was between 9% and 20% per year depending on the specific nature of the population ( ).
In 2004, Petersen published criteria for MCI ( Table 95.2 ; ). These criteria, proposed at the Key Symposium in Stockholm ( ), emphasized the concept as a syndrome between normal aging and dementia. Diagnosing MCI is the initial step, followed by a determination of etiology of the syndrome.
| aMCI | naMCI |
|---|---|
| Cognitive decline with intact ADLs, often corroborated by an informant | Cognitive decline with intact ADLs, often corroborated by an informant |
| Memory impairment | Nonmemory cognitive impairment (language, attention, executive function, visual-spatial) |
| Multidomain aMCI if other domains involved | Multidomain naMCI if more than one other nonmemory domain involved |
In 2011, the NIA and the Alzheimer Association published guidelines for the diagnosis of MCI due to AD ( ). More recently, the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5) described an analogous concept of “mild neurocognitive disorder.” A comparison of the recent MCI criteria is presented in Fig. 95.1 ( ; ). The American Academy of Neurology published guidelines recommending clinicians assess patients for MCI ( ).
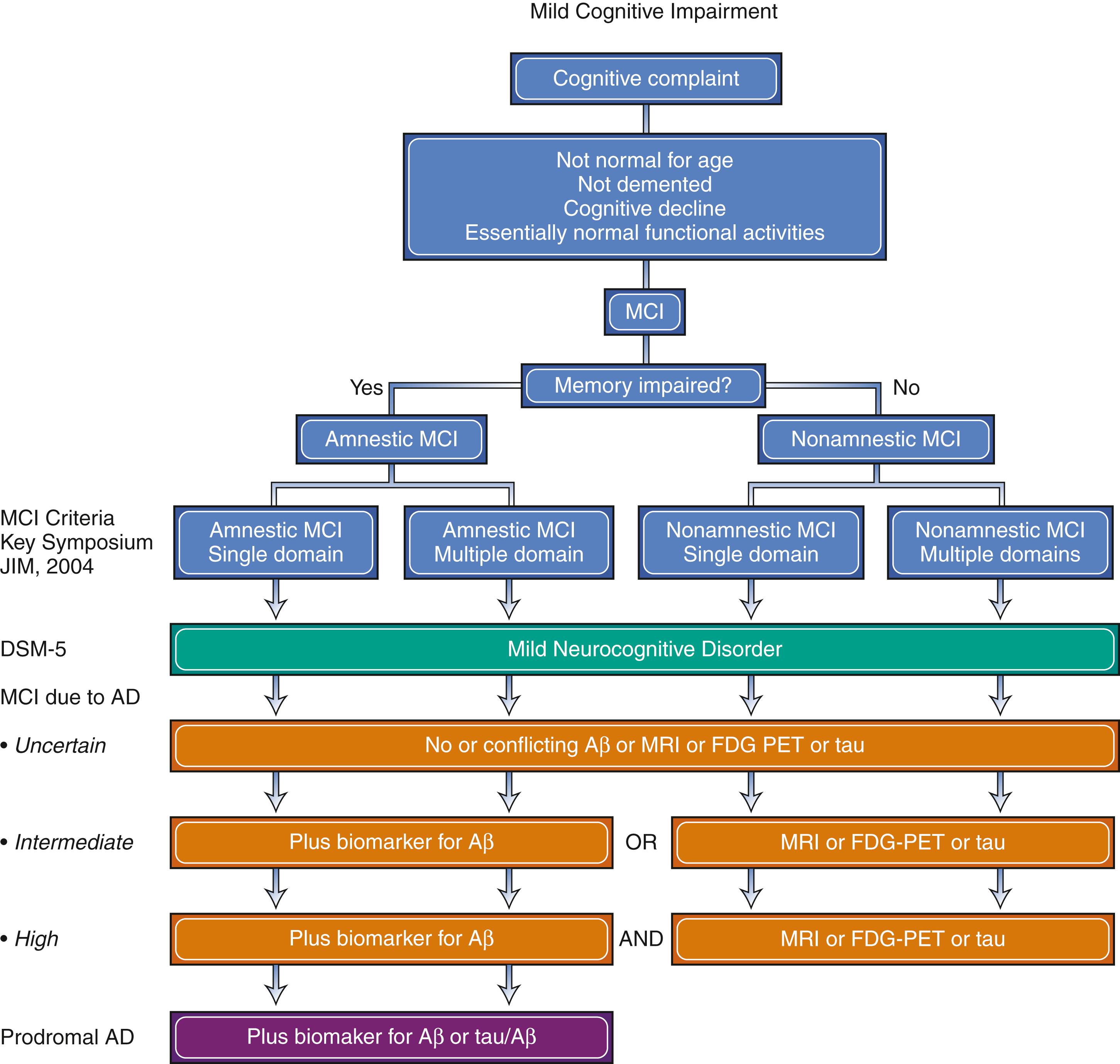
The concept of MCI is important because it identifies persons who are at great risk of developing dementia. While the annual risk of developing dementia in the elderly general population is approximately 1%–2%, MCI patients seen in the clinic setting have a 10%–15% annual risk. In population-based studies of MCI the annual risk of developing dementia is slightly lower at 5%–10% ( ; ; ). The prevalence of MCI in subjects age 70–89 is approximately 16% ( ). Identifying MCI patients allows for monitoring of progression, provides opportunity for appropriate counseling, and offers a possible therapeutic window for intervention in the future.
Several biomarkers predict the risk of converting from MCI to dementia. On structural MRI, MCI patients with hippocampal volumes on the 25th percentile are 2–3 times more likely to convert to dementia compared to MCI patients with hippocampal volumes on the 75th percentile ( ). In CSF, low Aβ 42 and high total tau (t-tau) and phospho tau (p-tau) levels are associated with progression in MCI patients ( ). Other risk factors for conversion include APOE ε4 allele ( ), temporal-parietal hypometabolism on FDG-PET ( ), and amyloid deposition on Aβ PET imaging ( ). One criticism of the MCI concept is that a proportion of patients diagnosed with MCI revert to normal. Interestingly, recent longitudinal studies demonstrated that those patients who fluctuate between normal cognition and mild cognitive impairment have a significantly higher risk of developing dementia over time. Therefore, a diagnosis of MCI even with reversion to normal has prognostic value ( ; ). This fluctuation is analogous to labile hypertension and glucose intolerance with respect to the ultimate development of hypertension or diabetes mellitus.
Subtyping MCI into amnestic and nonamnestic categories also has predictive value. Amnestic MCI (aMCI), which is more common, refers to memory impairment often noticed by family and even the patient but with intact cognitive skills in other domains (language, executive function, visual-spatial) and preservation of functional capacity. In contrast, nonamnestic MCI (naMCI) patients have declines in nonmemory cognitive domains such as language, executive function, and visual-spatial skills. The vast majority of aMCI patients progress to AD dementia ( ). Those with naMCI may progress to dementia with Lewy bodies (DLB) but can also progress to FTD, vascular dementia, and even AD dementia ( ; ).
While restricted insight into memory loss has been a distinguishing feature of individuals with cognitive impairment, recent studies have demonstrated that patients with cognitive complaints, good insight, and normal cognitive testing called subjective cognitive decline (SCD) are three times more likely than controls to develop MCI with AD-related biomarkers ( ; ). SCI has been associated with elevated levels of tau regionally in the entorhinal cortex and global elevation of Aβ ( ).
Dementia is an encompassing syndromic term for a decline in cognitive abilities of sufficient severity to interfere with function during daily activities (i.e., shopping, paying bills, cooking, driving, etc.). The term dementia does not imply an underlying etiology, although neurodegenerative diseases represent the most common causes. The decline from a prior higher level of functioning must be present in order to distinguish dementia from a developmental cognitive disorder. The cognitive deficit cannot be due to delirium or altered sensorium, which can be distinguished by the presence of marked fluctuations and acute-to-subacute temporal pattern, although dementia patients are more susceptible to delirium than the general population. The practice guideline on dementia from the American Academy of Neurology (AAN) recommended use of the DSM-IIIR dementia criteria (which were subsequently updated to the DSM-5 criteria). The DSM-5 criteria use the term major neurocognitive disorder to approximate dementia.
An estimated 35.6 million worldwide were living with dementia in 2010, with a prediction the number would double approximately every 20 years ( ). In the United States, the estimated prevalence of dementia among those 71 and older using an in-home visit is 13.9%. A sharp increase in dementia prevalence occurs with age ( ).
However, data from the Rotterdam study indicate the incidence of dementia may be declining ( ). This has been replicated in the UK ( ) and Rochester, MN ( ). One possible explanation is that this decrease is related to improved treatment of vascular risk factors.
Dementia can result from numerous causes, including brain injury (cerebrovascular disease or trauma) or infectious and metabolic diseases, but the most common causes are neurodegenerative diseases. Fig. 95.2 represents frequency of the predominant pathologies in a large dementia brain bank ( ). However, it is clear that while each patient has a predominant pathology, the majority of patients have multiple pathologies at autopsy. In fact, only 30% had AD pathology without other pathologies. Similarly, in the Rush Memory and Aging Project, over 50% of autopsied subjects with dementia had multiple pathological diagnoses ( ).
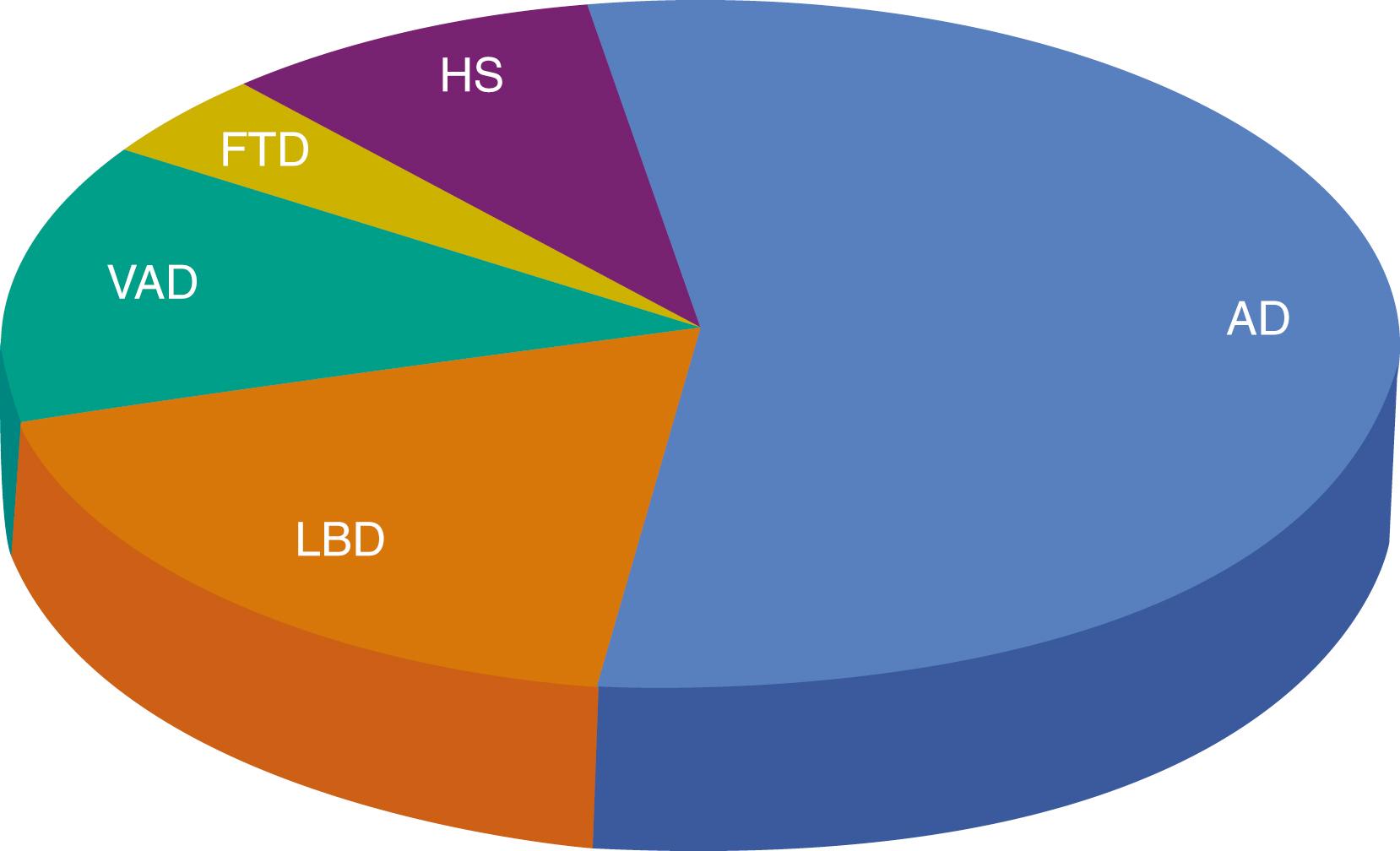
In a longitudinal clinicpathological study of aging, AD was the most common underlying pathology (65%) but occurred without other pathologies rarely (9%). On an individual level, AD pathology accounted for approximately 50% of cognitive decline on average, highlighting the importance of pathological heterogeneity in age-related cognitive decline ( ). In fact, even among patients diagnosed with AD-dementia, multiple pathologies are the rule rather than the exception ( , ). With age, amyloid accumulation plateaus ( ), tau, TAR DNA-binding protein 43 (TDP-43), and cerebrovascular pathologies continue to accumulate ( ; , ).
In 2001, the AAN published evidence-based guidelines for the diagnosis of dementia ( ).
The history is the most important component of the dementia diagnosis. Ideally, the history should be taken from not only the patient but also an individual who knows the patient well as lack of awareness of impairment commonly accompanies dementia. This individual can provide invaluable information regarding impairment in activities of daily living (ADLs). Other key elements of the history include identifying the presenting symptom, mode of onset, duration of symptoms, and rate of progression. Neurodegenerative causes of dementia typically present with an insidious onset and slow rate of progression, while the sudden onset of symptoms should raise suspicion for stroke, medication effect, infection, autoimmune process, or psychosocial stressors. Patients may have a subacute onset over weeks or months. The dementias presenting with this course will be discussed in Chapter 94 . Thorough evaluation of patients referred for cognitive symptoms to a memory clinic can identify a potentially reversible or partially reversible disorder in up to 9% of cases ( ) and a treatable coexisting disorder in up to 23% ( ). Several key components of the dementia history are reviewed in Table 95.3 . Background information such as age, level of education, occupation, social stressors, and cultural background can influence the presentation of dementia and should be taken into consideration. As a general framework, when an older patient presents with a progressive amnestic disorder with subsequent decline in other cognitive domains, AD dementia is the most common diagnosis. Alternatively, if the initial presentation is one of a change in language, personality, or behavior with relatively spared memory, FTD is the most likely consideration. The presence of parkinsonism, hallucinations, fluctuations, and REM sleep behavior disorder with dementia are most suggestive of DLB. Vascular cognitive impairment (VCI) ( ) can be temporally related to a stroke or develop gradually with prominent cognitive slowing and executive dysfunction with cerebral small-vessel white matter disease. Often vascular disease occurs together with other causes of dementia as a comorbid component of the clinical picture. In the setting of subacute dementia, consider Creutzfeldt-Jakob disease, autoimmune dementia, and their differential diagnosis.
| Cognitive Symptoms | Motor Symptoms | Autonomic Symptoms | Sleep | Behavioral |
|---|---|---|---|---|
| Impaired recent memory (repetitive questions/statements, forgets appointments, loses items easily) | Presence of tremor or myoclonus | Bowel/bladder symptoms | Presence of REM sleep behavior disorder | Change in personality, socially inappropriate behavior |
| Language difficulty (trouble understanding others, trouble getting words out, trouble finding words, uses incorrect words) | Trouble swallowing/slurred speech | Presence of orthostasis | Excessive daytime sleepiness | Loss of empathy/interest in hobbies, family |
| Visual spatial difficulty (getting lost more easily, trouble reading, difficulty recognizing familiar people) | Change in gait/falls | Sexual dysfunction (erectile dysfunction) | Evidence of sleep apnea (snoring, stopped breathing during sleep) | Compulsive behaviors, change in dietary preference |
| Executive/attentional dysfunction (poor judgment, difficulty problem solving, difficulty maintaining focus, trouble with calculations) | Muscle cramps atrophy, fasciculations | Change in sweating | Evidence of stridor | Disheveled, decreased interest in hygiene |
The past medical history can provide clues to the diagnosis or identify contributors to the cognitive decline. A careful head injury history is important because significant head trauma is a risk factor for dementia ( ). Repeated head injuries may suggest chronic traumatic encephalopathy (CTE) such as can occur in contact sports or the military ( ). Histories of stroke, hypertension, diabetes, high cholesterol, atrial fibrillation, smoking, or other vascular risk factors are important clues to vascular disease contributing to dementia. A history of cancer or autoimmune disease in the setting of a subacute cognitive decline may point to a paraneoplastic disorder or autoimmune dementia. A history of seizures, meningitis, or encephalitis, chemotherapy, brain radiation, sleep apnea, and other sleep disorders, depression and other psychiatric illness, and medication use may all be informative.
Knowledge about genetic causes and risk factors has rapidly increased in the last few decades. Thus a careful family history is essential. Identify not only first degree relatives but other relatives with dementia. It is also important to ask about Parkinson disease, amyotrophic lateral sclerosis (ALS), and psychiatric disease. Patients with one phenotype may have relatives with another. For example, one patient may have FTD and a relative may have ALS from the same gene mutation.
A thorough review of medications is essential as medication side effects exacerbate an underlying cognitive impairment or even mimic a dementia. A temporal association or worsening with starting a medication should be taken seriously and prompt consideration of a medication taper. While numerous medications are associated with cognitive side effects, the most common include anticholinergic agents (often present in medications for incontinence or antihistamines), benzodiazepines, zolpidem and other sedatives, opioids, and muscle relaxants. Table 95.4 is a partial list of medications that can be associated with cognitive symptoms.
| Sleep aids, cold medications, and antihistamines with an anticholinergic component: Diphenhydramine Acetaminophen/Dextromethorphan/Doxylamine succinate, Ibuprofen/Diphenhydramine |
Anticonvulsants Lamotrigine Phenobarbital Phenytoin Levetiracetam Topiramate Zonisamide |
Anticholinergics Benztropine Meclizine Scopolamine |
| Antihypertensives Beta-blockers |
Anticholinergics Tolterodine Oxybutynin |
Muscle relaxants Baclofen |
| Antidepressants Amitriptyline (anticholinergic property) Imipramine (anticholinergic property) |
Antipsychotics Quetiapine Olanzapine Risperidone Aripiprazole |
Cardiac Digoxin |
| Opiates Oxycodone Morphine Hydrocodone Fentanyl Propoxyphene Methadone |
Hypnotics Zolpidem Eszopiclone |
Immunosuppressants Tacrolimus Cyclosporine |
| Benzodiazepines Alprazolam Clonazepam Lorazepam Diazepam Temazepam |
Antibiotics Metronidazole Cefepime |
Mood stabilizer Lithium |
A thorough neuropsychiatric history provides important information by identifying potentially treatable symptoms and narrowing the differential diagnosis. According to the AAN guidelines, depression should be screened for in all dementia patients because it occurs frequently and is treatable ( ). While depression can mimic dementia, depression is also a risk factor for and occurs frequently with AD and DLB ( ; ). Traditional teaching suggested that patients with dementia have consistent memory deficits and executive dysfunction of which they are unaware or minimize, while depressed patients are more likely to complain about cognitive impairment and perform variably on cognitive testing due to attention deficits and poor effort on testing (although in practice, distinguishing the two is difficult due to the significant overlap). However, more recent work on the MCI stage of dementia due to AD has demonstrated that early neuropsychiatric features such as apathy, agitation, and dysphoria may be presenting features of a neurodegenerative process ( ). Important neuropsychiatric symptoms to review include change in mood (depression, mania), change in personality, and presence of delusions, obsessive behaviors, or hallucinations. The presence of certain neuropsychiatric features may narrow the differential diagnosis. For example, personality changes in behavioral variant FTD (bvFTD) or hallucinations or delusions in DLB. The Neuropsychiatric Inventory (NPI) can be used to screen for common neuropsychiatric manifestations of dementia ( ).
Many useful cognitive screening instruments have been developed. Common well validated instruments include the Mini-Mental State Exam (MMSE) ( ), the Blessed Orientation Memory Concentration Test ( ), the Kokmen Short Test of Mental Status (STMS) ( ), and the Montreal Cognitive Assessment (MOCA) ( ). Detailed cognitive testing by a neuropsychologist can be very helpful. The neuropsychologist provides an in-depth cognitive evaluation by administering a standardized battery of tests. These tests evaluate important cognitive domains such as attention and concentration, memory, language, visuospatial abilities, and executive function. They also gauge the psychiatric contributions to the clinical picture. Patients with different dementias have different strengths and weaknesses on these tests. The pattern of performance helps determine if the person is impaired, the severity of impairment, and the likely brain areas that are damaged. Neuropsychology can also be helpful in following a patient’s progression over time.
A general neurological exam is a key part of the evaluation of dementia. While the general neurological exam is typically normal in early AD, abnormalities on exam may indicate other neurodegenerative processes. The presence of parkinsonism may suggest DLB or another parkinsonian dementia. Focal findings on exam such as asymmetric reflexes or other lateralizing signs may suggest a vascular component to the dementia. A coexisting peripheral neuropathy may suggest a metabolic disturbance. Fasciculations can be seen in patients with suspected FTD to suggesting coexisting motor neuron disease. A language screening exam and testing for apraxia should also be performed. The presence of a gait abnormality may indicate normal pressure hydrocephalus (NPH), parkinsonism, or vascular disease.
The AAN practice parameter recommends routine assessment for vitamin B 12 deficiency and thyroid hormone abnormalities because these conditions are common, and can affect cognitive function. Treatment of vitamin B 12 deficiency and hypothyroidism may not completely reverse cognitive symptoms, but recognition of these conditions is important. Other routine lab tests include a complete blood cell count, electrolyte panel, glucose, liver function tests, and creatinine. Screening for syphilis should be done based on clinical suspicion ( ). Special circumstances, including age less than 65 years, seizures, rapidly progressive dementia, history of cancer or autoimmune disease, suspicion of central nervous system (CNS) infection, constitutional symptoms, history of drug abuse or immunosuppression, systemic infection, suspicion of vasculitis, or other atypical features, can guide further laboratory evaluation, including CSF examination. In neurodegenerative disease, the cell count, protein, and glucose concentrations in the CSF are within normal limits and specific markers of AD pathology; for example, Aβ42 total and phospho-tau, may be useful.
The AAN practice parameter recommends against electroencephalogram (EEG) in the standard evaluation of dementia ( ). However, EEG may be very useful in a patient with a history of seizures, assessment of rapidly progressive dementia, a history of spells, or suspicion of transient epileptic amnesia ( ).
Structural neuroimaging modalities including computed tomography (CT) and MRI can identify potentially treatable causes of dementia including subdural hematoma, hydrocephalus, and intracranial neoplasm. The AAN practice parameter paper recommends screening with either CT or MRI to identify these conditions ( ). In a study evaluating the usefulness of the AAN dementia guidelines, 3% of dementia patients had a surgically treatable finding on neuroimaging (NPH, subdural hematoma, neoplasm). Neuroimaging changed management in 15% of cases and clinical diagnoses in 19%–28% ( ). In the recent “Imaging Dementia-Evidence for Amyloid” Scanning (IDEAS) study, the use of amyloid PET among Medicare beneficiaries with cognitive impairment of unclear etiology led to a change in diagnosis and clinical management in a significant proportion of the participants ( ).
Recently, the DSM-5 was released, updating the prior criteria for dementia. DSM-5 introduces the terms “mild neurocognitive disorder,” which is similar to MCI, and “major neurocognitive disorder,” which is analogous to dementia. Major neurocognitive disorder represents a significant cognitive decline in at least one cognitive domain that interferes with daily function that is recognized by the individual, informant, or clinician and documented by neuropsychological testing. Mild neurocognitive disorder represents a cognitive decline which does not impair daily activities ( ). DSM-5 recommends a two-tiered approach: (1) syndrome characterization as outlined above and (2) an etiological determination.
In 1906 Alois Alzheimer, a German psychiatrist, reported the case of a woman in her 50s with paranoia and memory loss followed by aphasia whom he evaluated in a psychiatric unit. She eventually lost the ability to perform motor tasks. At autopsy, gross inspection revealed an atrophied brain with vascular changes. Microscopic sections prepared with Bielschowsky stain revealed the hallmark AD inclusions which would later be known as amyloid plaques and neurofibrillary tangles (NFTs). Although the patient had early onset of symptoms, Alzheimer’s case summarizes many of the key clinical features of AD dementia.
In 1984, the National Institute on Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association criteria were created conceptualizing Alzheimer disease as a clinicopathological entity for over 30 years ( ). With advancing technology allowing in vivo detection of amyloid with PET and CSF as well as measurement of neurodegeneration, in 2011 revised AD criteria were proposed by the National Institute on Aging-Alzheimer’s Association. Individuals were characterized by their clinical state (normal, MCI, and dementia) with biomarkers of amyloid and neurodegeneration providing the likelihood that their clinical state was due to Alzheimer disease. For example, if amyloid and neurodegeneration biomarkers were present in conjunction with the clinical syndrome of dementia, the diagnosis of dementia due to AD with high likelihood could be made ( ). But the diagnoses of MCI and dementia due to AD were still clinical-pathological conditions.
More recently, tau PET imaging and improvement in CSF assays has allowed detection of both hallmark AD proteins in vivo. This advance of being able to detect both amyloid and tau in conjunction with data from clinical trials, which suggested that almost a quarter of individuals enrolled in a trial targeting amyloid were considered amyloid negative ( ), led to a new research framework proposing that AD should be defined biologically by the presence of both amyloid and tau either with biomarkers during life or pathologically with a separation of the clinical syndrome from the disease definition. The advantages of this framework include the ability to classify individuals earlier in the disease process, prevention of individuals with an amnestic dementia being misclassified as AD dementia if they have an alternative pathology, and the ability for individuals with an atypical phenotype (nonamnestic) to be classified as AD. In this research framework, each individual would be characterized by 3 biomarker groupings: (1) β-amyloid deposition, (2) pathological tau, and (3) neurodegeneration [AT(N)]. In this research framework, neuritic plaques may be identified by decreased CSF Aβ42 or amyloid PET positivity and neurofibrillary tangles identified by a positive tau PET or elevated phospho-tau protein in the CSF ( ). The presence of an amyloid biomarker alone without tau would be referred to as Alzheimer pathological change and the absence of amyloid as non-Alzheimer pathological change. The authors cautioned that this framework is for research at this point in time and should not be used in routine clinical practice. This framework provides a universal terminology to allow comparison between research studies, avoiding misclassification and potential erroneous enrollment in randomized clinical trials of individuals with an amnestic dementia syndrome not due to Alzheimer pathology such as hippocampal sclerosis ( ). A cognitive staging scheme can be applied to an individual’s biomarker status ( ). This history of Alzheimer disease definition was reviewed in the 2018 Wartenberg lecture ( ). Table 95.5 provides proposed cognitive stages applied to the new AD biomarker framework.
| Cognitively Unimpaired | Mild Cognitive Impairment (MCI) | Dementia | |
|---|---|---|---|
| A – T – (N) – | Normal Alzheimer disease (AD) biomarkers, cognitively unimpaired | Normal AD biomarkers with MCI | Normal AD biomarkers with dementia |
| A + T – (N) | Preclinical Alzheimer pathological change | Alzheimer pathological change with MCI | Alzheimer pathological change with dementia |
| A + T + (N) – | Preclinical Alzheimer disease | Alzheimer disease with MCI (Prodromal AD) | Alzheimer disease with dementia |
| A + T + (N) + | |||
| A + T – (N) + | Alzheimer and concomitant suspected non-Alzheimer pathological change, cognitively unimpaired | Alzheimer and concomitant suspected non-Alzheimer pathological change with MCI | Alzheimer and concomitant suspected non-Alzheimer pathological change with dementia |
| A – T + (N) – | Non-Alzheimer pathological change, cognitively unimpaired | Non-Alzheimer pathological change with MCI | Non-Alzheimer pathological change with dementia |
| A – T – (N) + | |||
| A – T + (N) + |
According to the , 5.7 million Americans have AD dementia (2018) which represents 70% of dementia in the United States (not autopsy confirmed) ( ). The prevalence of Alzheimer dementia increases with age from 3% of people between the ages of 65 and 74 to 32% of people age 85 and older (Alzheimer’s disease facts and figures 2018). While MCI is more common in men, the prevalence of AD dementia is higher in women. This in part can be explained by a longer life span in women than men. The lifetime risk of developing Alzheimer dementia from the age of 45 is approximately 10% for men and 20% for women (Alzheimer’s disease facts and figures 2018). By 2060, the prevalence will increase to an estimated 15 million individuals in the United States ( ).
Age is the most important risk factor for AD dementia. The incidence of AD dementia increases with age: 2 new cases per 1000 for individuals age 65 to 74, 11 new cases per 1000 people age 75 to 84, and 37 new cases per 1000 people age 85 and older (Alzheimer’s disease facts and figures 2018).
Anticipated increases in cost to society for AD dementia are notsustainable. The total payments in 2018 for all individuals with dementia are estimated to be $277 billion (Alzheimer’s disease facts and figures 2018). The Alzheimer Association estimates that by 2050 annual costs for AD dementia will reach approximately $1.2 trillion ( ). Therefore, the burden on society will continue to be enormous unless a prevention or treatment is developed. The Health and Retirement Study has reported that AD is the costliest chronic disease in the United States exceeding those of cancer and heart disease ( ).
In addition to age and female gender, many other risk factors for AD dementia have been reported.
After controlling for confounders, systolic blood pressure (>160 mm Hg) and elevated cholesterol increase risk for AD dementia later in life ( ). In the Honolulu-Asia Aging Study, the use of beta-blockers for hypertension was associated with less cognitive decline, especially among diabetics ( ). In the Ginkgo Evaluation of Memory Study, the use of a diuretic blood pressure medication, angiotensin-1 receptor blocker, or angiotensin-converting enzyme inhibitor was associated with reduced risk of AD dementia in those with normal cognition at baseline ( ). The relationship between hypertension and dementia is complicated. Counterintuitively, some studies have shown that low blood pressure is more commonly seen in demented patients than high blood pressure ( ). A parsimonious explanation of this apparent discrepancy is that the association between hypertension and cognitive decline is age dependent. Mid-life hypertension and late-life hypotension are associated with AD dementia ( ). The relative risk of mid-life hypertension and dementia is 1.61 and it has been estimated that decreasing the prevalence of mid-life hypertension by 10% could result in a worldwide decrease of 160,000 AD dementia cases ( ). The recent SPRINT-MIND trial demonstrated that treating systolic blood pressure to a goal of less than 120 mm Hg compared to a goal of less than 140 mm Hg reduced the risk of mild cognitive impairment by approximately 19%, but the study did not meet its primary endpoint of showing a reduction in the risk of dementia ( ).
Type 2 diabetes is associated with hyperinsulinemia. Both insulin and Aβ are substrates for insulin-degrading enzyme. Therefore, hyperinsulinemia may result in accumulation of Aβ through competing with Aβ for insulin-degrading enzyme ( ). However, at autopsy, type 2 diabetes is associated with vascular brain disease not increased AD pathology ( ). Nonetheless, a meta-analysis estimated the relative risk of dementia related to diabetes was 1.39 ( ). It has been estimated that a 10% decrease in diabetes prevalence may decrease the number of worldwide dementia cases by 81,000. Recently, a study has demonstrated that elevated glucose in the absence of diabetes also increases the risk of dementia ( ).
A meta-analysis of 15 case-control studies demonstrated an increased risk of AD dementia with prior head injury in men ( ). The mechanism is unclear, but after severe head injury, levels of Aβ42 in the CSF decrease, which also occurs in preclinical AD ( ). The presence of the APOE4 allele may confer a higher risk of dementia after head injury ( ). In a population-based study, MCI subjects but not normal control subjects with history of head trauma had elevated brain Aβ deposition ( ).
The glymphatic system of the brain allows clearance of waste products. In mice, it has been shown that during sleep there is an increase in the interstitial space allowing for increased rate of clearance of β-amyloid ( ). Emerging evidence suggests that impaired sleep may alter β-amyloid dynamics in humans ( ; ). This is an active area of ongoing research.
Other risk factors associated with an increased risk of AD include smoking ( ), cerebrovascular disease ( ), anemia ( ), and obesity ( ).
In 1990, a study performed in Shanghai demonstrated an association between a lower educational attainment and dementia risk ( ). Subsequently, several other studies have demonstrated an association between low educational attainment and increased dementia risk ( ; ).
In addition to education, participation in certain leisure activities, including reading, dancing, playing board games, and playing musical instruments, is associated with a decreased dementia risk ( ).
These studies and others have led to the development of the cognitive reserve hypothesis. Which attempts to explain why those with certain life experiences, including higher educational attainment and increased leisure activity participation, are more resistant to neurodegenerative changes ( ).
Early-life cognitive abilities also may play an important role in dementia risk. In the nun study, autobiographical essays from nuns at a mean age of 22 were evaluated for idea density and grammatical complexity. Those with low idea density and grammatical complexity had lower cognitive scores later in life, and, in a small sample of nuns who came to autopsy, those with low early-life linguistic ability had AD pathology while those with linguistic talent did not have AD pathology ( ). Similarly in 1932, participants in the 1921 Scottish birth cohort took a test of intelligence at age 11. Lower mental ability at age 11 was associated with an increased risk of dementia ( ).
A Cochrane review of 11 studies of exercise in elderly non-demented participants concluded that exercise enhanced cognitive function ( ). In addition, Yaffe and colleagues ( ) reported that women with higher baseline levels of self-reported physical activity were less likely to decline cognitively. Similar findings were found in the nurses’ health study ( ) and a population-based study ( ). In addition to epidemiological studies, a single-blind prospective study of physical activity intervention in participants with subjective cognitive impairment but not dementia demonstrated modest but significant improvement in cognition at 18 months follow-up ( ). The mechanism of how exercise can improve cognition is unclear, but exercise and improvement in aerobic fitness correlate with increased hippocampal volumes ( ). Even in autosomal dominant AD, self-reported exercise of greater than 150 minutes per week was associated with lower amyloid load compared to those who exercised less than 150 minutes per week ( ).
In a nested case-control study from the Cardiovascular Health Study, consumption of 1–6 alcoholic beverages per week was associated with decreased odds of dementia relative to abstinence ( ). In contrast, heavy drinking (>3/day) was not associated with a lower AD dementia risk ( ). In fact, it is well described that alcoholic patients can develop dementia for multifactorial reasons. Korsakoff syndrome related to thiamine deficiency is primarily an amnestic disorder. Other neuropsychological features of alcoholics with dementia include impaired letter fluency, fine motor control, and delayed recall with relative preservation of recognition ( ).
Dietary fat intake has been associated with AD dementia risk. While intake of saturated fats and trans-unsaturated fats is associated with a higher risk, intake of unsaturated, unhydrogenated fats may be protective against AD dementia ( , ). Weekly fish consumption and increased intake of omega-3 fatty acid may also be associated with decreased AD dementia risk. Similarly, adherence to the Mediterranean diet, which recommends fish intake, is associated with decreased risk of AD and decreased risk of converting from MCI to AD dementia ( , ). Cognitive outcome data from a clinical trial in which a Mediterranean diet was compared to a control diet demonstrated that a Mediterranean diet supplemented with olive oil or nuts was associated with better cognition than a control diet ( ).
AD dementia survival is shorter than predicted based on US population estimates, with a median survival from diagnosis of 4.2 years for men and 5.7 years for women ( ).
Typical AD dementia initially presents with an episodic memory impairment reflecting the selective vulnerability of the medial temporal lobe to AD pathology. Episodic memory relates to our ability to remember information specific to a time and place when that memory was formed (e.g., “What did you eat for dinner? What did you do on a trip?”). Recent episodic memory is particularly impaired in early AD.
While episodic memory is the hallmark of early AD, subsequent cognitive decline is heterogeneous as the pathology spreads to the association cortices. While individual presentations vary significantly, Feldman and Woodward have described the typical symptom progression in AD dementia: Mild AD (recent memory impairment, repetitive questions, loss of interest in hobbies, anomia, impaired instrumental ADLs), moderate AD (aphasia, executive dysfunction, impaired basic ADLs), severe AD (agitation, complete loss of independence, sleep disturbance) ( ).
Semantic memory dysfunction can be an early feature of AD but occurs after episodic memory involvement. Semantic memory is factual knowledge not linked to time or space context. Examples include naming or recalling animals, objects and tools, or landmarks. The preservation of semantic memory in a subset of early AD cases indicates that transentorhinal dysfunction is inadequate to disrupt semantic memory, which likely requires extension of pathology into the temporal neocortex ( ). A category fluency test (asking the subject to generate as many items as possible from a given category such as fruits, animals, or vegetables) is commonly used as a brief test of semantic knowledge. This not only tests the ability to remember the names of objects but the ability to search one’s mind for a category of objects. This test is often impaired early in the course of the disease and also tests executive function.
Executive function (planning, organization, problem solving, set switching) decline occurs in mild AD ( ). The executive dysfunction in AD dementia is mild until later in the disease course compared to bvFTD.
Language disturbance often occurs in the mild-moderate stage of AD dementia. Initial complaints often include word-finding difficulty. In time, other features of aphasia may develop, and in the late states, language output can be limited.
Decline in visuospatial skills is common, often manifesting early with the complaint of becoming lost or being disorientated in unfamiliar places.
Apraxia often occurs later in the course of typical AD, although it may be an early feature in atypical AD.
Strikingly, several abilities are preserved until very late in the development of AD dementia. For example, AD dementia patients have preserved motor learning (procedural) ( ), motor, and sensory skills.
A useful way to think of the clinical picture of AD dementia is to look at the pattern of both deficits and strengths the patient exhibits. Patients with AD dementia have episodic memory loss and develop anomia, executive dysfunction, and visuospatial difficulty in the setting of preserved ability to walk, see, hear, and feel. AD dementia is also characterized by the juxtaposition of “knowing how” (procedural memory) and “not knowing what” (declarative memory). The clinical picture of deficits and preserved abilities is explained by the anatomical distribution of the NFT pathology. demonstrated that tangle burden is greatest in the medial temporal lobe but spares the primary sensory and motor cortices until very late in the course, which is why AD dementia patients can see, hear, and move but not remember. Early AD is characterized by an isolated memory impairment resulting from significant pathology in the entorhinal cortex and hippocampus, which serves to disconnect the medial temporal lobe from other cortices. The spread of pathology to other association cortices results in accumulation of other symptoms: semantic memory involvement from spread to the anterior temporal lobes, executive dysfunction from spread to the frontal lobes, and visual-spatial dysfunction from spread to the occipitotemporal lobes. In contrast, the primary motor and sensory cortices are only affected late in the course. The preserved basal ganglia and cerebellum are involved in the procedural/motor learning (or knowing how).
Occasionally, AD can present with focal cortical syndromes without memory loss initially. The three most commonly described atypical presentations are posterior cortical atrophy (PCA), logopenic aphasia (LPA), and frontal variant of AD.
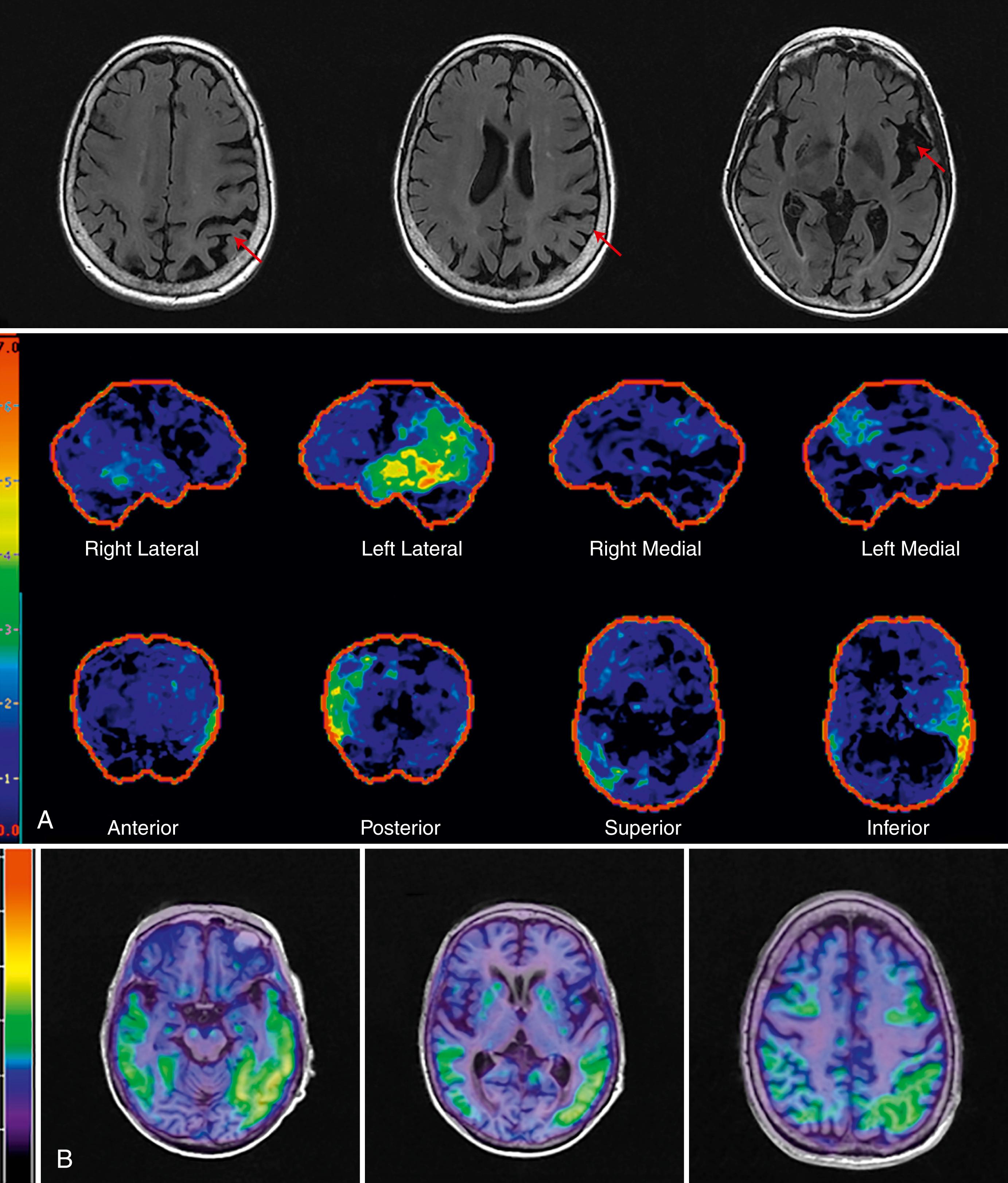
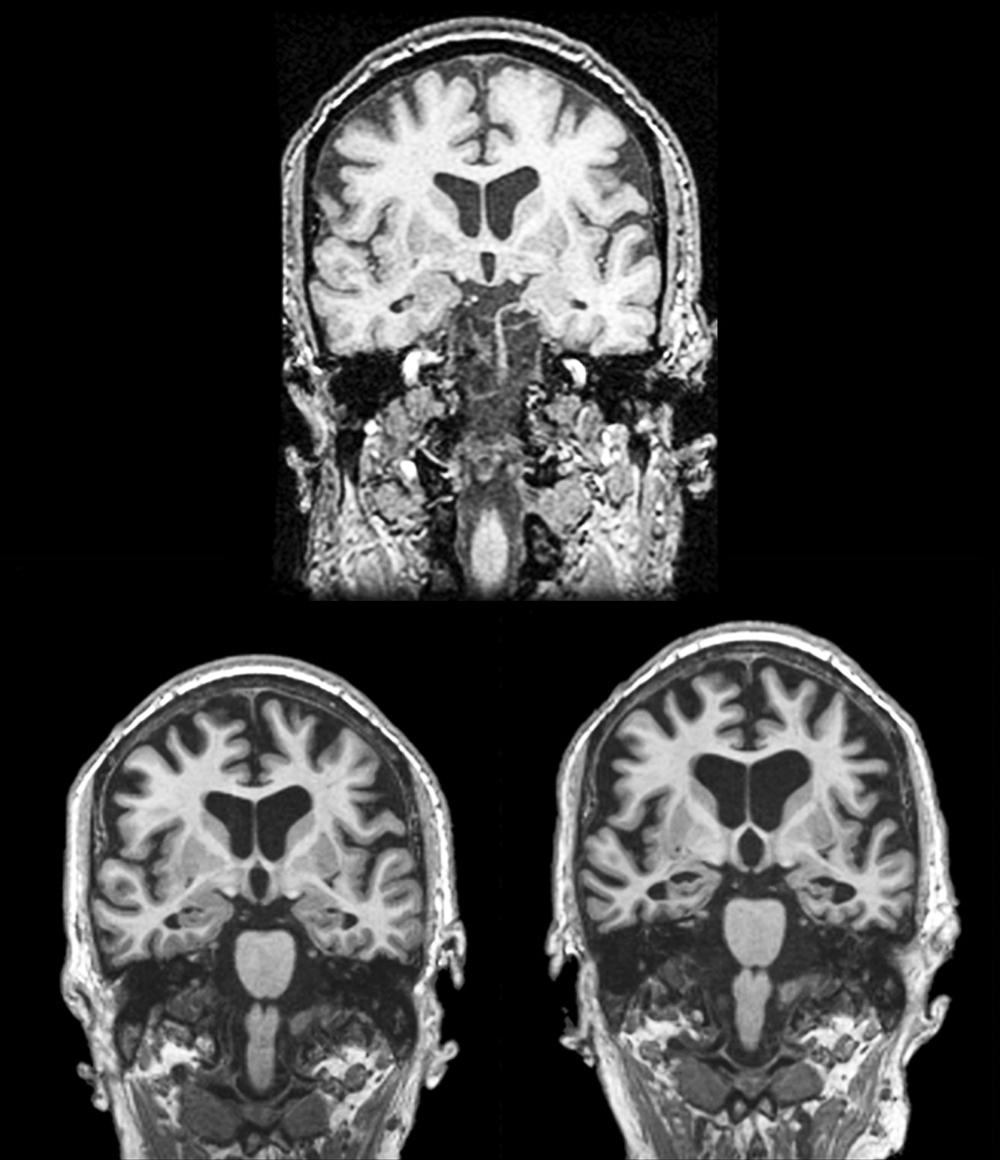
In 1988, Benson used the term posterior cortical atrophy to describe five cases with progressive dementia involving visual-spatial function, alexia, and partial Balint and Gerstmann syndromes with relatively preserved memory ( ). Later, autopsy series of PCA revealed Alzheimer pathology as the most common underlying etiology. While AD is the most common pathology, other pathologies in clinically diagnosed PCA cases include corticobasal degeneration and prion disease. PCA patients are often initially referred to optometry or ophthalmology for difficulty seeing, which may manifest as driving impairment or trouble reading. Not uncommonly, PCA patients undergo procedures such as cataract removal without significant benefit, or alternatively, they may be told there is nothing wrong with their eyes prior to definitive diagnosis. The mean age of onset of PCA is approximately 60 ( ). Compared to typical AD, insight is preserved ( ). In a large series of 40 PCA cases, complete or partial Balint syndrome (optic ataxia, oculomotor apraxia, simultanagnosia) was present at diagnosis in 88% of patients, complete or partial Gerstmann (left-right confusion, finger agnosia, agraphia, acalculia) was present in 62%, and visual field loss was present in 48%. Simultanagnosia is characterized by only being able to see one object at a time while viewing a scene. Ishihara plates used to assess color perception and complex scenes are good screening tools for the presence of simultanagnosia. Other important signs and symptoms include ideomotor apraxia, alexia, prosopagnosia, hemineglect (sensory or visual), achromatopsia, and dressing apraxia ( ). PCA is associated with the APOE ε4 allele ( ). Structural MRI reveals parieto-occipital atrophy and FDG-PET demonstrates associated parieto-occipital hypometabolism ( Figs. 95.3 and 95.4 , respectively). The distribution of amyloid is similar between PCA and typical AD ( ). In contrast, PCA patients have significantly more tau deposition in the occipital lobe compared to typical AD (see Fig. 95.4 ), corresponding to their clinical symptoms ( ). Adaptive equipment for the blind or those with low sight can be helpful. No randomized controlled trial supports the use of any drug therapies in PCA, although acetylcholinesterase inhibitors are commonly used since the most common underlying pathology is AD. Recent consensus criteria ( Table 95.6 ) for the diagnosis of PCA have been published emphasizing PCA as a clinicoradiological syndrome ( ). The new classification recognizes that PCA can present in a “pure” form or overlap with other degenerative diseases such as corticobasal syndrome.

Clinical Features
|
| Cognitive Features At least three of the following must be present as early or presenting features:
|
All of the following must be evident:
|
Neuroimaging:
|
LPA is one of the primary progressive aphasias. The distinguishing features of LPA include impaired naming, repetition, and word retrieval with phonological errors. Motor speech and grammar are spared. have suggested impairment in the phonological loop, which stores and rehearses verbal memory as the basis of the clinical presentation. In a study by , 64% of logopenic patients had AD pathology at autopsy, and these patients had greater tangle burden in the left hemisphere language areas and fewer tangles in the entorhinal cortex when compared to typical AD pathology. In a large multicenter study, amyloid pathology was present in 86% of those with LPA compared to 20% of those with nonfluent/agrammatic PPA and 16% of those with semantic variant PPA ( ). Phonological errors may be the strongest predictor of underlying AD pathology ( ). Structural MRI reveals left temporal-parietal atrophy. FDG-PET scans reveal temporal-parietal hypometabolism and tau PET demonstrates left greater than right temporal and parietal tau deposition ( Fig. 95.5, A and B ).
Rarely, patients with pathologically confirmed AD present with early impairment on tests of frontal lobe function including verbal fluency and Trails A. These patients may have behavioral and personality changes similar to patients with FTD. However, those with behavioral presentation of AD develop apathy as the most common behavioral feature, in contrast to hyperorality and perseverative/compulsive behaviors seen more commonly in bvFTD ( ). Interestingly, the NFT burden in these patients is increased in the frontal lobes ( ).
Other focal presentations with underlying AD pathology include corticobasal syndrome and, rarely, progressive agrammatic aphasia or semantic variant primary progressive aphasia, which are typically due to FTLD pathology ( ).
In a study using the Neuropsychiatric Inventory in AD, apathy (72%) was the most common neuropsychiatric symptom, followed by agitation (60%) and anxiety (48%) ( ). Identification of delusions is important because it often precedes physically aggressive behavior ( ). Common delusions in AD include paranoia and infidelity. Identification of neuropsychiatric features is important because these symptoms result in significant caregiver burden and can be targeted for treatment ( ).
For many years, the most commonly used criteria for the diagnosis of AD were the National Institute of Aging and Stroke–Alzheimer’s Disease and Related Disorders Association criteria ( ). However, over the ensuing years, it became apparent that the pathophysiological underpinning of AD began many years before the symptoms of dementia presented themselves. Therefore, in 2011, the National Institute on Aging–Alzheimer’s Association (NIA–AA) published revised criteria for the AD process. In this exercise, a distinction was drawn between the clinical symptoms of AD and the underlying pathophysiology. That is, prior to this point, AD was defined as a clinical–pathological entity, but that caused confusion in the field ( ). Therefore, the NIA–AA research criteria characterized the AD clinical spectrum in three phases: dementia, MCI, and preclinical. The dementia due to AD phase was quite similar to what had been defined in 1984 but was made more specific and suggested that biomarkers for AD may be useful in the future when they are validated. The new criteria, however, also recognized a milder symptomatic stage of the AD process which was termed MCI due to AD ( ). This stage recognized the growing literature on MCI that had been generated in the previous decade, documenting the existence of a clinical phase of the disease by which people may be mildly impaired from a cognitive perspective, usually with a memory disorder, but were otherwise intact in other cognitive domains and were functionally intact ( ). The most novel phase of the disease process was termed preclinical AD. In this phase, subjects were cognitively and functionally normal but harbored the underlying pathophysiological features of AD, such as amyloid deposition. This phase of the disease was meant to generate research on the preclinical aspects of the disease process to allow intervention when disease-modifying therapies become available. Subsequent research has demonstrated that these research criteria are reasonably accurate and data regarding the validation of the biomarkers are accumulating ( ; ). The role of biomarkers has been documented using both neuroimaging and cerebrospinal fluid measures ( ; ). Recent work has revealed a group of preclinical subjects without the typical AD biomarker profile (i.e., no evidence of Aβ deposition on PET or in the CSF) but evidence of neurodegeneration by FDG-PET or MRI, termed suspected non-Alzheimer disease pathophysiology or sNAP given the absence of Aβ deposition ( ). A similar condition, sNAP MCI has been described ( ). Fig. 95.6 compares the newest research diagnostic criteria for AD ( ; ) and reports the NIA–AA criteria, including the 2018 proposed research framework, which divorces the pathology from the clinical stage. This proposed framework is a significant departure from the 1984 and 2011 proposals since AD could only be called a disease if there was biomarker or autopsy evidence for the presence of amyloid (neuritic plaques) and tau (neurofibrillary tangles) independent of clinical state. One implication of this proposal is that a clinically unimpaired person could be labeled as having AD if there was biomarker evidence for amyloid and tau. Fig. 95.1 reports the NIA–AA criteria for MCI due to AD. The International Working Group has also incorporated biomarkers into updated criteria in 2007 ( ) and 2014 ( ).
Neuropsychology provides a more detailed understanding of cognitive constructs, thereby allowing identification of what cognitive functions are deficient or preserved. The pattern of neuropsychometric testing abnormalities can assist in predicting underlying anatomy and in the differential diagnosis of dementia. Testing is particularly helpful early in the course. Neuropsychology uses well-developed standards based on normative data that improve clinical utility and predictive value. The NIA workgroup consensus criteria of MCI due to AD ( ) recommend episodic memory evaluation to assist in predicting those with MCI at high risk of converting to AD dementia. Memory testing alone is insufficient, and simple bedside testing cognitive screens can be insensitive to early changes of neurodegeneration. The preservation versus impairment in memory subcomponent testing allows for differentiation among disorders. In brief, learning or encoding refers to the transfer of to-be-learned material from short-term sensory stores into consolidated traces in recent memory involving numerous integrated networks. Free recall pertains to the retrieval of that material without any cues or aids and recognition refers to the identification of the material from among several candidates. A classical memory test such as a verbal memory test consists of reading a list of words over multiple trials. An improvement in encoding over the learning trials (i.e., an increase in number of correct words per trial) is found in normal learning. An individual with an encoding problem may demonstrate a flat learning curve (i.e., the same number of words per trial). Despite the flat learning curve, an encoding problem might lead to preserved free recall and recognition of words with cues. A retrieval deficit is manifest when the person is unable to perform free recall of the material but is able to recall the items when retrieval cues are given. For example, if the person remembers the word “sweater” and is unable to free recall it but can recall it when cued, “It is an item of clothing,” then the person was demonstrating a retrieval failure since the word was encoded but not recalled without cues. With a retention or consolidation problem, the individual generally encodes normally with improvement in number of words learned over trials, but with delayed recall has significant difficulty recalling words and does not benefit from recognition cuing. Encoding problems may correspond to attentional deficits or a failure of medial temporal lobe structures such as the hippocampus to facilitate consolidation of the material. This pattern of poor learning and consolidation is commonly seen in AD. In contrast, a relatively pure retrieval problem would be more characteristic of parkinsonian disorders, vascular cognitive impairment, or other disorders not involving the medial temporal lobe. Other neuropsychometric tests can provide discriminating information. For example, category and letter fluency tests may also provide useful diagnostic pattern in AD. Typically, fluency for semantic categories (e.g., fruits, vegetables, and animals) is impaired relative to letter fluency performance (e.g., words beginning with a certain letter). This discrepancy in verbal fluency performance tends to reflect temporal lobe involvement in AD pathology and the relative preservation of subcortical circuitry. Confrontation naming of common objects is also impaired in early AD. Executive function tasks may also be impaired in early AD as evidenced by tasks requiring set-shifting and sequencing, including Trail Making Test Part B ( ). Tests of visual spatial function including figure copying can also be impaired early in AD spectrum disorders.
The combination of reduction in the CSF Aβ42 and elevation in the CSF tau protein has a sensitivity of 85% and specificity of 86% for the diagnosis of AD dementia ( ). More recent studies using Aβ42 and tau or phosphorylated tau suggest these biomarkers can improve diagnosis in difficult cases and predict conversion from MCI to AD dementia ( ; ; ). The current research framework includes elevated phosphorylated tau as evidence of pathological tau accumulation while the less specific total tau is used as a neurodegenerative biomarker ( ). While these biomarkers can provide important information, normal CSF Aβ42 and tau have been reported in autopsy-proven AD dementia patients ( ). Recent data suggest that novel CSF markers, for example, neurofilament light protein, may be good index of neurodegeneration ( ).
Various neuroimaging biomarkers are sensitive to different stages and markers of AD.
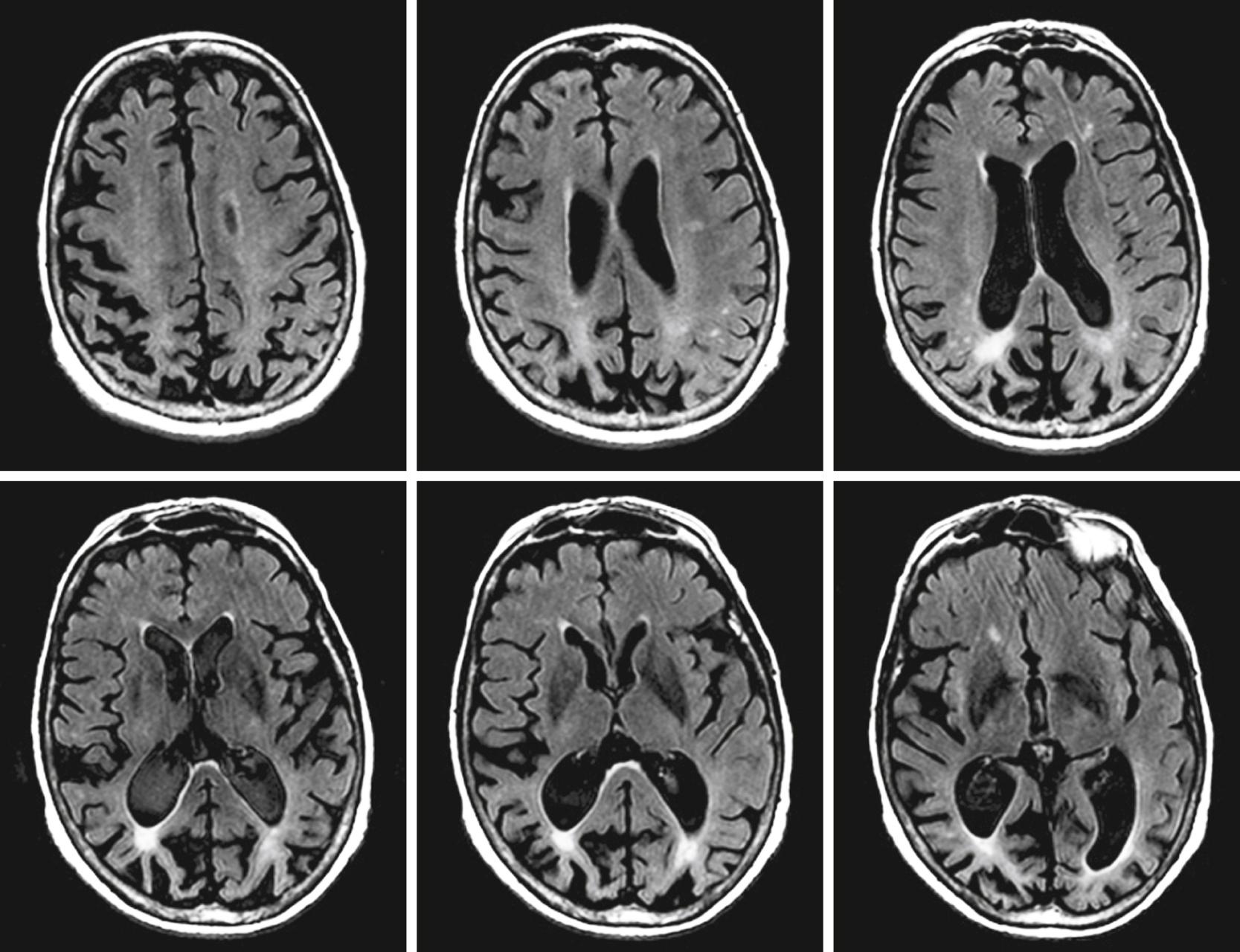
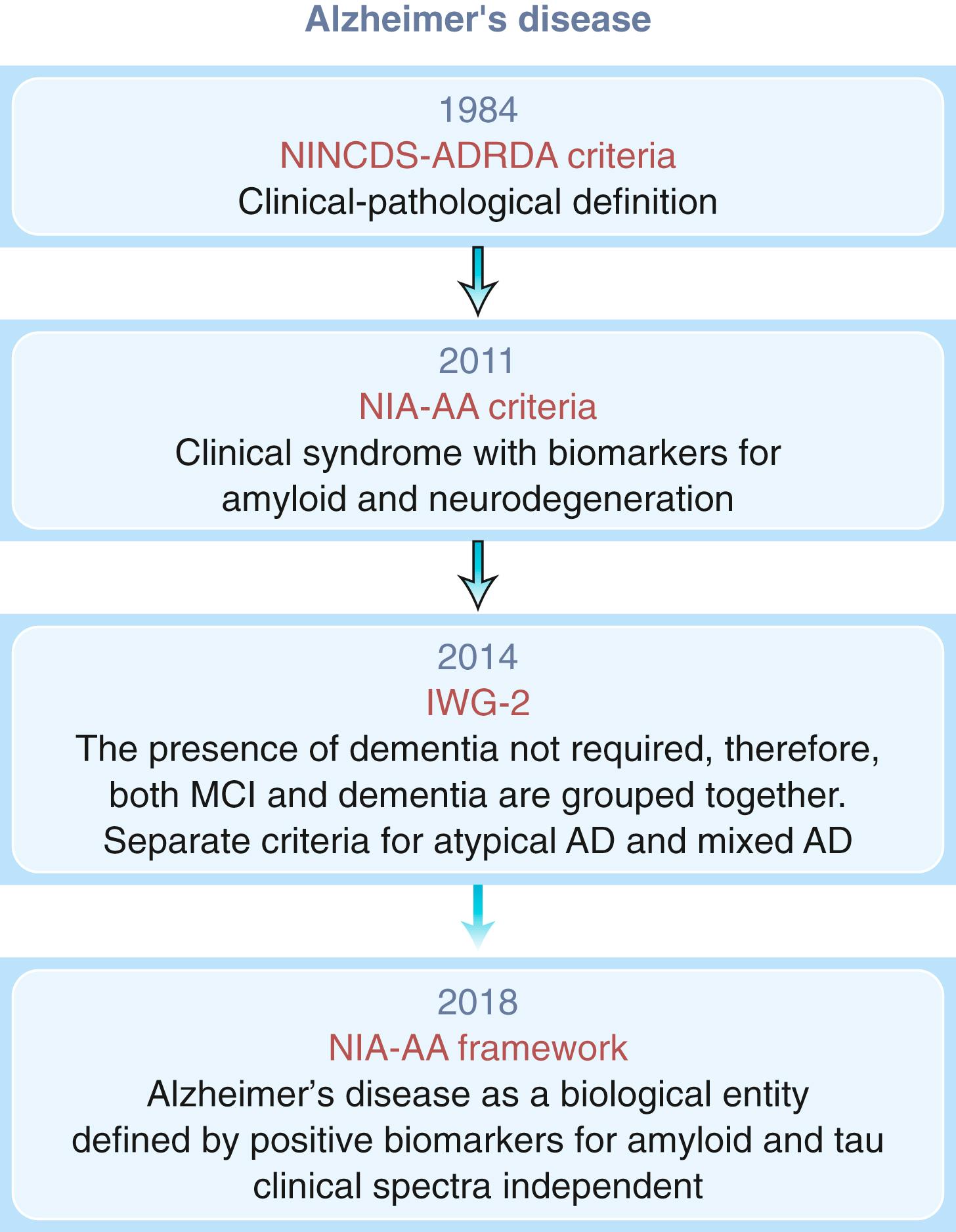
The AAN practice parameter recommends that a neuroimaging examination, either CT or MRI, be performed at the time of the initial dementia assessment ( ). While this recommendation was primarily suggested to exclude reversible and treatable causes of dementias, MRI provides much higher resolution than CT and has proven very useful in the differential diagnosis of dementia and as a biomarker of neurodegeneration in AD dementia. Medial temporal lobe atrophy of the hippocampus and entorhinal cortex with concomitant dilatation of the temporal horns is an early characteristic of AD dementia ( Fig. 95.7 ) and can predict conversion from normal cognition to MCI and MCI to AD dementia ( ). Reduction in hippocampal volumes correlates with NFT pathology at autopsy and cognitive decline ( ). In aMCI, atrophy is limited to the medial temporal lobe structures while with AD onset, atrophy spreads to the lateral temporal and parietal cortices. This change corresponds with Braak staging, which is why structural MRI serves as a biomarker of neurodegeneration ( ).
The utility of volumetric measurements of the entorhinal cortex, while promising, is controversial. Although medial temporal lobe structures become atrophic in early AD and correlate with episodic memory performance, later in the disease atrophy rates are greater in the temporal, parietal, and frontal cortices and are associated with deterioration in other cognitive domains including language, praxis, and visuospatial ( ). Medial temporal lobe atrophy is not specific for AD and can be seen in other degenerative and vascular processes. In particular neurodegenerative hippocampal sclerosis of aging can have a similar imaging appearance to Alzheimer dementia ( ). During the dementia stage, significant global atrophy occurs typically most significantly in a temporal-parietal distribution with coinciding ventriculomegaly.
The presence of white matter hyperintensities observed by FLAIR or T2 MRI also appears to contribute to cognitive impairment in AD ( ).
Hypointense signal on MRI gradient-echo sequences represents hemosiderin deposition reflective of cerebral microbleeds. Newer techniques, such as susceptibility-weighted imaging, are more sensitive for these findings. In the Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort, cerebral microbleeds were present in approximately 33% of cases and increased with Aβ load as measured by amyloid PET ( ). When cerebral microbleeds occur in a lobar distribution in elderly patients, they often represent cerebral amyloid angiopathy (CAA). In a population-based study, β-amyloid load on PET was associated with lobar but not with deep cerebral microbleeds ( ). Identification of coexisting CAA and AD has clinical importance because patients with AD and CAA have worse cognition and higher mean tangle and plaque rates than patients with just AD changes alone ( ; ). Specific cognitive correlates of CAA include decreased perceptual speed and episodic memory ( ). CAA preferentially involves the occipital lobe.
Functional brain imaging using single-photon emission computed tomography (SPECT) and FDG-PET can suggest disease specific patterns. While functional imaging studies have not been endorsed by criteria for MCI or AD due to dementia ( ; ), their value is recognized in selected cases. Especially when the structural imaging scan is not informative, functional imaging modalities may provide additional useful information ( ; ).
Decreased blood flow in a temporal-parietal distribution seen on SPECT correlates with hypometabolism seen on FDG-PET and is suggestive of AD.
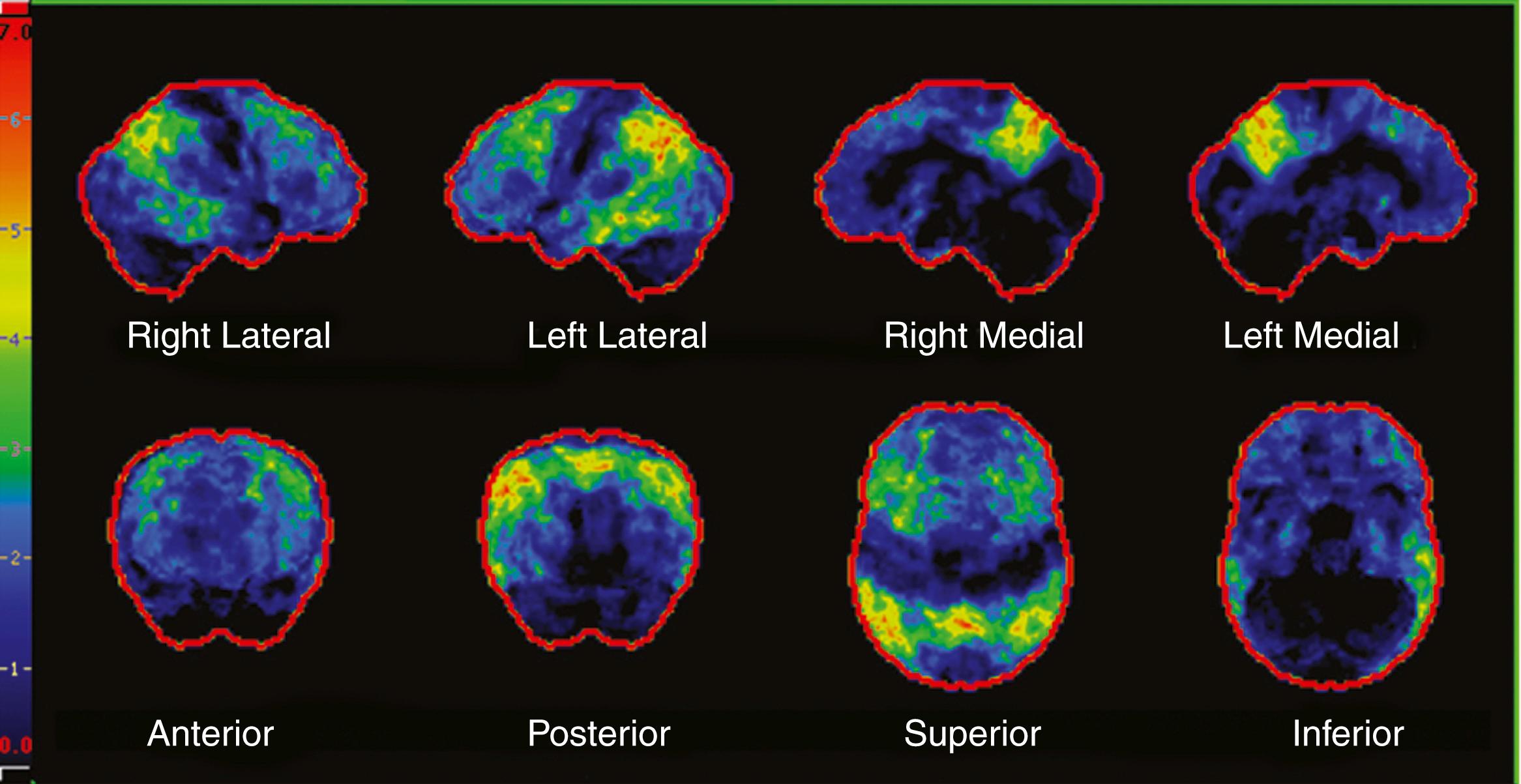
In aMCI, hypometabolism is primarily in the hippocampus and posterior cingulate. In AD dementia, the hypometabolism includes these regions as well as the temporal-parietal regions ( ). Recent studies have indicated that FDG-PET can be used as an aid in the diagnosis of AD dementia, in particular in differentiating AD from FTD ( ; ), which has led to the Centers for Medicare and Medicaid Services approving reimbursement for evaluating this differential diagnosis. Several studies have validated the use of FDG-PET as a biomarker in AD, resulting in its inclusion as a biomarker in the most recent AD criteria ( ). In MCI ADNI participants, FDG-PET predicted conversion to AD dementia ( ). Of particular interest, FDG-PET in cognitive normal homozygous carriers for APOE ε4 demonstrates hypometabolism in the posterior in the cingulate, temporal, and parietal cortices ( ). Fig. 95.8 demonstrates an FDG-PET in a patient with typical AD dementia.
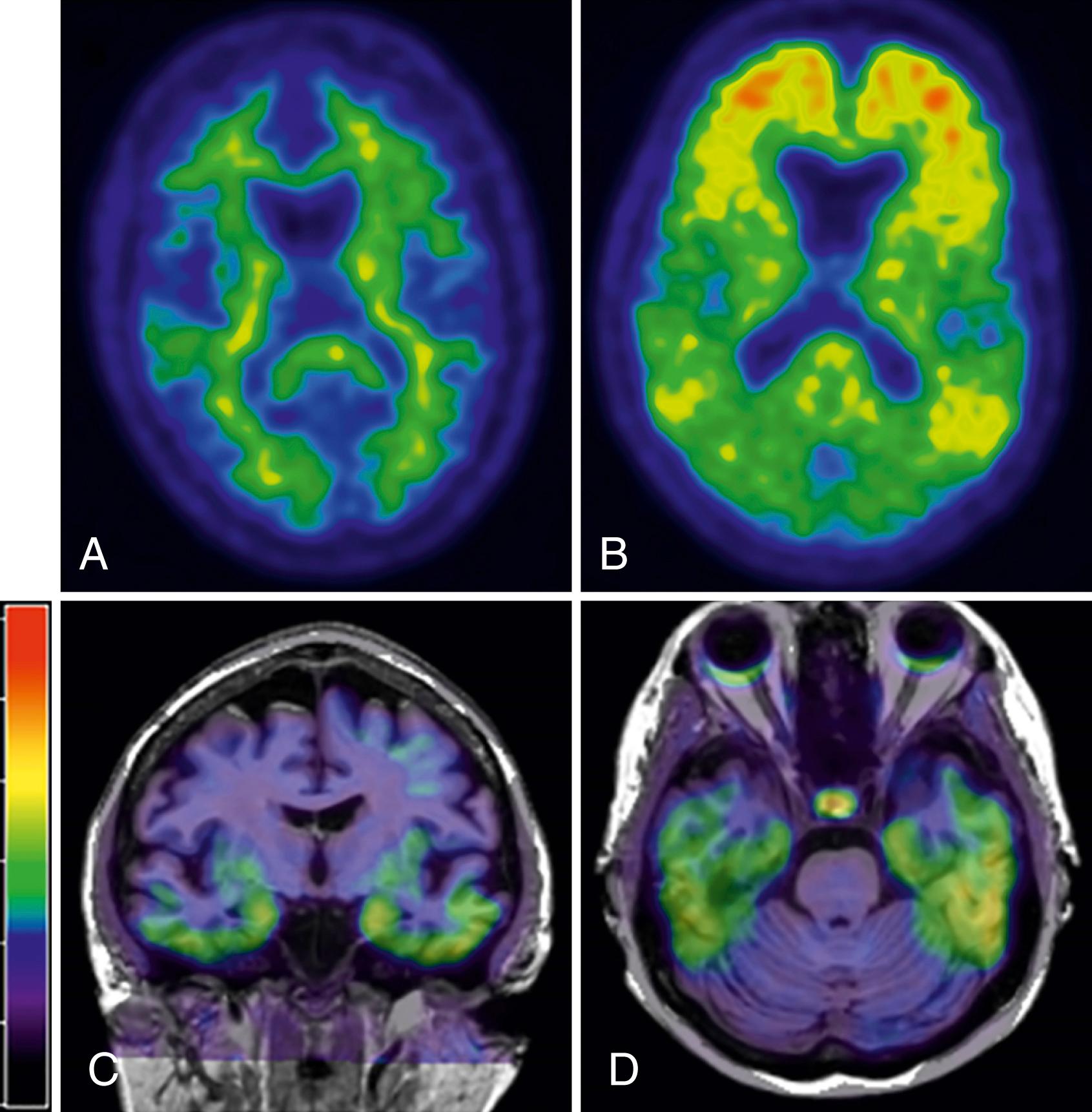
The development of Pittsburgh Compound B (PiB) has allowed measurement of amyloid burden in living subjects ( ). While not currently recommended for routine clinical use, this imaging modality is already being used in clinical trials for identifying preclinical AD, MCI due to AD, and monitoring effectiveness of amyloid-targeted therapies. Appropriate-use criteria have been published which make recommendations regarding the clinical setting in which amyloid PET could be considered ( ). The deposition of amyloid as measured by PiB-PET occurs primarily in the frontal and temporal-parietal regions (see Fig. 95.9 for examples of PiB-PET imaging ) . Since its discovery, numerous studies have demonstrated possible clinical utilities. PiB-PET outperformed FDG-PET in discriminating FTD from AD ( ). PiB binding cross-sectionally correlates with cognitive function ( ). Longitudinally, however, once, symptomatic, the deposition of β-amyloid plateaus demonstrating that PiB utility may be greatest in the preclinical stages ( ).
In a population-based study of cognitively normal individuals over age 70, approximately one-third have significant β-amyloid load ( ). The prevalence of a positive amyloid PET scan among cognitively unimpaired individuals in the general population increased from 2.7% in individuals between ages 50 and 59 years to 41% in those individuals between ages 80 and 89 years ( ). The high rate of amyloid-positive scans in the cognitively normal population along with the absence of any disease-modifying therapy would suggest amyloid imaging in cognitively normal elderly individuals should be reserved for research purposes until disease-modifying therapy is available.
Several F-18 analog amyloid tracers have been developed and approved for clinical use by the Food and Drug Administration (FDA) florbetapir, flutemetamol, and florbetaben. The F-18 agents provide comparable results to PiB imaging, but a longer half-life allows for transportation to clinical centers. Florbetapir performs well compared to autopsy confirmation ( ). In the ADNI study, Aβ deposition, as measured with florbetapir, correlated with cognitive decline in cognitive normal and MCI participants. In the cognitive normal group this decline sometimes occurred in the absence of FDG abnormality ( ). Multiple studies have demonstrated the prognostic importance of amyloid PET. The incident risk of developing mild cognitive impairment increased greater than twofold among cognitively unimpaired individuals who were amyloid PET positive compared to those who were amyloid PET negative ( ).
Functional MRI (fMRI) measures the blood-oxygen-level dependent (BOLD) signal, essentially relying on the fact that neuronal activation in a region produces associated increases in blood flow to that same region. Dementia caused by neurodegeneration is caused by the disruption of specific, large-scale neural networks ( ). fMRI is one technique to study these neural networks. The default mode network (DMN) refers to connected regions of brain that are active when an individual is at rest or not focused on the external environment ( ). In AD, the DMN is selectively targeted. The core regions of the brain activated in this default state include the medial prefrontal cortex, inferior parietal lobule, posterior cingulate gyrus, hippocampal formation, and lateral temporal cortex. These changes on fMRI occur early in the disease process. In cognitively normal APOE ε4 subjects, there is a decrease in connectivity relative to controls ( ). These changes occur in the absence of brain amyloidosis, as measured by amyloid PET and Aβ42 levels in the CSF ( ). Therefore, fMRI changes may be a very early marker of the pathophysiology of AD. While current use of fMRI in dementia is limited to research, it has provided substantial knowledge about degenerative disease and is actively being investigated as a biomarker.
Recently, several tau imaging tracers have been developed The FDA approved Tauvid (flortaucipir F18) for patients being evaluated for AD. Since tau comprises the other hallmark of the AD pathological process, neurofibrillary tangles, the ability to image it in vivo would be extremely useful. Tau is also implicated in a variety of other disorders and the ability to characterize this protein would be advantageous in diagnosis and following putative treatments.
The presence of tau PET signal in the inferior temporal cortex is closely linked to clinical symptoms ( ). Tau PET reliably distinguishes AD dementia from non-AD dementias ( ). Since flortaucipir binds to AD-type tau (3R,4R), it is not surprising that tau-PET levels in the temporal pole of tau mutation (microtubule-associated protein tau [MAPT]) cases were lower than in AD dementia cases, with the exception of tau mutation cases, whose MAPT mutation occurs outside of exon 10 (V337M and R406W) and, therefore, develop AD-like tau and have tau-PET signal close to the AD dementia levels ( ). Many issues exist regarding these tracers, such as specificity for tau and various tau isoforms, but it is a promising technique. For example, in semantic variant primary progressive aphasia, which is most often associated with TAR DNA-binding protein (TDP)-43-positive inclusions, tau-PET signal has been seen in areas of atrophy, possibly reflecting off-target binding ( ; ). Tau PET has allowed investigation into how patterns develop with age. In cognitively unimpaired individuals in the preclinical stage of AD, in addition to the expected medial temporal tau involvement, tau is present in extra-medial temporal regions and extra-temporal regions arguing against a region-region spread of tau pathology that has been previously proposed ( ) (see Fig. 95.9 bottom row for examples of tau-PET imaging).
The advent of biomarkers for tracking the progression of AD has vastly increased our knowledge about its temporal progression and will play a key role in clinical trial design and execution. Several key biomarker studies in conjunction with data of unselected autopsies published over a short period of time have provided evidence that the pathophysiological processes underlying AD start decades before cognitive decline. This knowledge has shifted the focus of disease therapies to the presymptomatic or early symptomatic phases of the disease. One influential hypothetical model for the sequence of these biomarkers was first proposed by Cliff Jack in 2010 ( ) and revised in 2013 ( ). This model represents the pathophysiological processes underlying AD, and is summarized in Fig. 95.10 . The Dominantly Inherited Alzheimer Network (DIAN) study has provided important information regarding the timing of biomarkers in autosomal dominant AD largely consistent with the model described above ( ). This sequence can be summarized by the following: CSF Aβ42 declines over two decades before clinical symptoms; Aβ-PET abnormalities begin about 15 years before symptoms; brain volume loss and increased CSF tau also occur 15 years before symptoms; FDG-PET abnormalities occur 10 years before symptoms ( ).
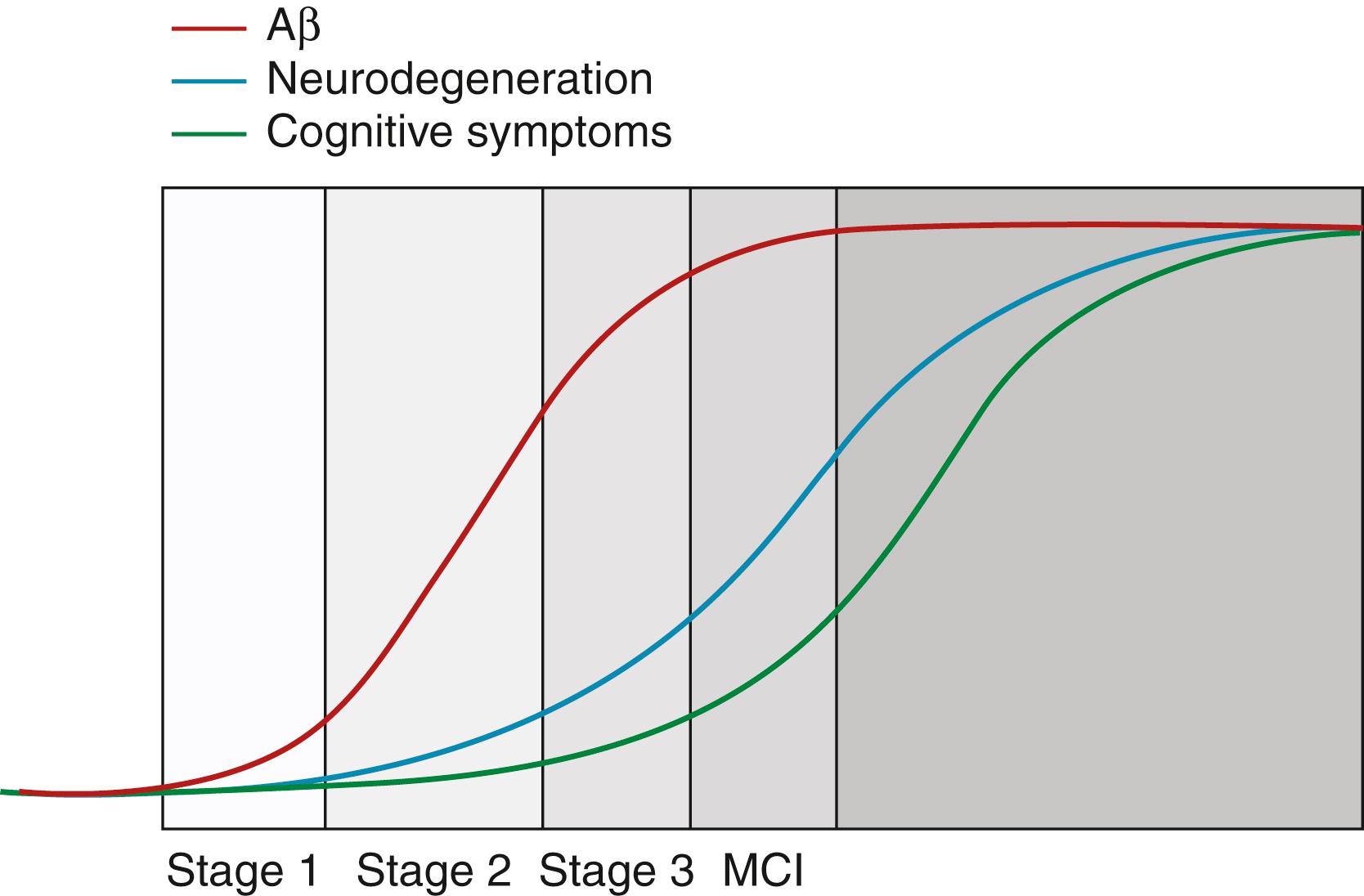
While this sequence is predictable for dominantly inherited AD, the Mayo Clinic Study of Aging has demonstrated that a substantial proportion of cognitively normal subjects have neurodegeneration biomarkers but not amyloid biomarkers, as was noted earlier in this chapter ( ; ). In preclinical AD, 42% of incident Aβ-PET positive cases also have positive neurodegeneration biomarkers first. This suggests that at least two biomarker profile pathways to preclinical AD exist, and some of the cases labeled sNAP are on the AD pathway but with a different biomarker progression ( ). In the same framework, progression in MCI to dementia has also been characterized and a similar group of MCI sNAP subjects have been identified, implying that neurodegenerative pathologies other than AD may be operating in some MCI subjects, and while most progress to AD dementia, some do not ( ).
Recently, longitudinal tau-PET studies have demonstrated the rate of accumulation of tau over time. Cognitively unimpaired individuals accumulate tau at a rate of 0.5% per year compared to cognitive impaired individuals who accumulate at a rate of 3% per year ( ).
In a large twin study from Sweden based on 392 pairs of twins, the genetic component of AD was estimated to be 58%–79% ( ). Family history of AD can provide important risk information. The lifetime risk of AD dementia in first-degree relatives is approximately 39% and this risk increases to 54% by age 80 if both parents have AD dementia ( ).
Three rare, early-onset, fully penetrant gene mutations have been described to cause Alzheimer dementia. While mutations in these genes are rare, studying them has been instrumental in our understanding of AD. All three increase brain Aβ levels and form an important part of the amyloid hypothesis for AD (discussed later). This provided the basis for several animal models and biomarker development including development of CSF and PET Aβ.
Mutations in amyloid precursor protein (APP) were the first mutation to be described to cause AD. Chromosome 21 became a chromosome of interest for AD, since patients with trisomy 21 (Down syndrome) develop AD pathology after age 40. Investigators looked at the brains of patients with AD dementia and Down syndrome and found Aβ in both. Since Aβ is the product of APP (located on chromosome 21), APP became a candidate gene ( ; ; ; ). All APP mutations that cause AD change the Aβ42 to Aβ40 ratio. Recently, certain mutations in APP have been described to be protective against AD ( ) by decreasing the production of Aβ. The mean age of onset for APP mutation families is approximately 50. In addition to early age of onset, the clinical presentation of APP mutations carriers may be distinguished from sporadic AD by the presence of myoclonus, seizures, early dyscalculia, cerebral white matter changes, and even corticospinal tract signs ( ).
The majority of early-onset familial AD cases are mapped to chromosome 14 ( ). In 1995, mutations on chromosome 14 were found in PSEN1 in autosomal dominant AD families ( ). Interestingly, presenilin is part of the γ secretase complex that cleaves APP ( ). Clinical features of PSEN1 families can include significant aphasia in addition to myoclonus and seizures ( ).
PSEN2 was discovered shortly thereafter ( ). PSEN 2 mutations are the rarest of the autosomal dominant mutations. PSEN2 is part of the γ secretase complex that cleaves APP. Most of these individuals are descendants of families from the Volga River region of Russia. Similar to the other familial early-onset mutations, PSEN2 families have a higher rate of seizures than in sporadic AD ( ).
Apolipoprotein E (APOE) is the most important genetic risk factor for late-onset AD ( ). APOE has three isoforms (E4 associated with high risk, E3 associated with neutral risk, and E2 which is protective). About 20% of all late-onset AD is thought to be related to APOE ε4 ( ). APOE ε4 affects AD risk and age of onset in a dose-dependent way; for example, E4 homozygotes have a mean age of onset of 68 with a lifetime AD risk of 91%; in E4 heterozygotes, the mean age of onset is 76 with a 47% lifetime risk ( ). In contrast, E4 noncarriers have a mean age of onset of 84 with an approximately 20% lifetime frequency ( ; ).
Trem2 variants have recently been identified as rare risk variants for AD dementia ( ). The odds ratio is similar to APOE ε4 but it is rare, which is why it was not seen in previous genome-wide association studies. Its population-attributable risk is lower than APOE ε4 due to its lower frequency.
Many other risk loci have been associated with AD risk, but their overall impact is thought to be small. These include CD33 molecule, ATP-binding cassette, subfamily A, member 7 (ABCA7), sortilin-related receptor L (SORL1), clusterin (CLU), phosphatidylinositol binding clathrin assembly protein (PICALM), as well as many others ( ).
Genetic testing for APP, PSEN1, PSEN2, and APOE is commercially available in Clinical Laboratory Improvement Amendments (CLIA) laboratories. Routine genetic testing is not recommended by the practice parameter of the AAN, but if patients have testing, genetic counseling prior to testing is essential. Testing may have significant implications for patients and families in terms of family planning, financial costs, and risk of depression. Genetic testing currently does not change treatment of the patient but may provide opportunities to participate in research studies such as DIAN.
All three early-onset AD genes (APP, PSEN1, PSEN2) play a role in Aβ metabolism which has provided the foundation for the amyloid hypothesis of AD. The basic hypothesis is that abnormal Aβ metabolism altering the Aβ42/Aβ40 ratio in the brain causes Aβ oligomer formation and aggregation to form fibrils, which form the amyloid plaque. This oligomer formation and aggregation results in a cascade of events, including tau protein tangle formation, increased inflammatory response, and oxidative injury, to cause neurotoxicity and neurodegeneration. More recent work has focused on the pathogenicity of soluble Aβ oligomers with converging basic scientific evidence that they play a major role in neurotoxicity. For example, human Aβ oligomers injected into the hippocampus of rats inhibit long-term potentiation ( ) and result in synaptic dysfunction which impairs memory formation ( ).
Aβ is derived from APP through proteolytic processing. Aβ is produced normally in the body but under normal circumstances it is removed efficiently by a number of mechanisms. These include breakdown by extracellular proteases, such as insulin-degrading enzyme, and receptor-mediated endocytosis followed by lysosomal degradation and drainage through the cerebral vasculature and into the CSF via the glymphatic system ( ).
The amyloid hypothesis proposes that AD can result from too much Aβ production or a change in ratio with more Aβ42 than Aβ40 produced, or impaired clearance of Aβ. Understanding APP processing is central to the amyloid hypothesis ( ).
APP is a type 1 protein with the amino terminal in the extracellular space. APP is cleaved by three enzymes (α, β, and γ secretase) ( ) and can undergo processing either through the amyloidogenic pathway (γ and β secretases) which produces Aβ, or the nonamyloidogenic pathway (α secretase) ( Fig. 95.11 ).
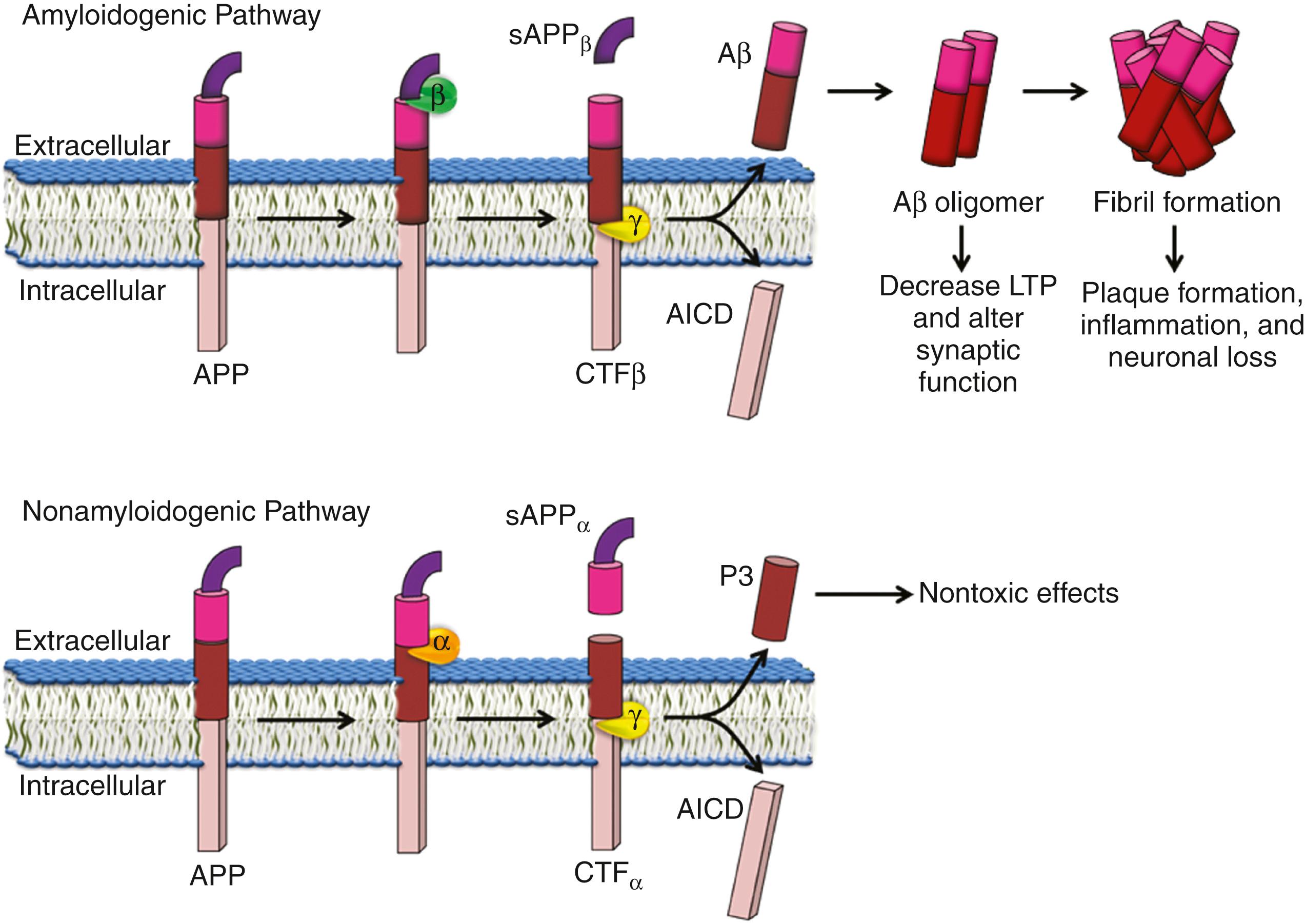
In the amyloidogenic pathway, APP is first cleaved by β secretase. This cleavage produces a β C-terminal fragment, which stays on the membrane and soluble APP Aβ. This β C-terminal fragment is then cleaved by γ secretase in the transmembrane region producing APP intracellular domain (AICD) and Aβ peptide, which is then released into extracellular space. PSEN 1 and 2 are part of the γ secretase complex ( ). Other components of the γ secretase complex include nicastrin, APH-1. This aforementioned cleavage occurs by sequential events eventually producing β-amyloid fragments, either Aβ1-42 (about 10%) or Aβ1-40 (about 90%). Aβ can aggregate and these aggregates may form oligomers which may be toxic. Aβ1-42 has a greater tendency to aggregate and is found in greater concentration in plaques, while Aβ1-40 is the predominant form in vascular amyloid deposits.
In the nonamyloidogenic pathway, APP cleavage is mediated by α secretase. This cleavage is in the middle of the β-amyloid peptide above the surface of the membrane, thereby preventing the formation of Aβ. This cleavage generates soluble APP α (sAPPα) and α C-terminal fragments, which can also undergo cleavage by γ secretase, producing a nontoxic peptide called P3.
APOE functions primarily in the transport of lipids/cholesterol from astrocytes to neurons. The presence of the APOE ε4 allele is associated with decreased CSF Aβ42 and increased brain Aβ burden seen on Aβ ( ). Despite these biomarker changes, the mechanisms of E4 leading to AD are numerous and include both Aβ- related and Aβ-unrelated mechanisms. Aβ-dependent mechanisms include interfering with cerebral Aβ clearance and promoting Aβ aggregation. Aβ-independent mechanisms include promoting abnormal lipid transport, thereby altering synaptic plasticity and the inflammatory response ( ).
Accumulating evidence suggests that a common mechanism across neurodegenerative diseases may include the trans-synaptic spread of tau and other misfolded proteins to anatomically connected regions in a prion-like manner where the protein is released and taken up by the anatomically related neuron ( ).
Recent efforts have been made to investigate how large-scale neural networks may be involved in the pathophysiology of AD and other neurodegenerative disorders. It has long been known that systems of connected neurons are selectively vulnerable to neurodegenerative disease, but the pathophysiology behind this association is debated. In contrast to disease models that emphasize the molecular misfolding of proteins spreading within connected systems, complex systems models emphasize the causal role of dynamic functional activity within brain systems interacting with molecular physiology ( ).
Alzheimer disease is defined by two pathological findings: extracellular plaques composed primarily of amyloid and intraneuronal neurofibrillary tangles composed primarily of hyperphosphorylated tau. Both involve abnormal conformational changes in proteins. Proteolytic events are critical in APP processing that leads to Aβ. In neurofibrillary pathology, phosphorylation of tau is a critical event.
Macroscopically, AD is characterized by diffuse brain atrophy including significantly decreased weight at autopsy. Some areas are preferentially affected, including multimodal association areas, while others, like primary motor, somatosensory, auditory, and visual cortices, are relatively spared. The limbic areas including hippocampi and cingulate gyrus are severely affected. Substantia nigra (unlike in parkinsonian disorders) is relatively spared while locus coeruleus is severely affected. Work from Braak looking at autopsies of young adults suggests the earliest place neurofibrillary tangle pathology occurs is in the locus ceruleus, with tangles occurring in the third or fourth decades without involvement of tangles in the limbic areas or the presence of amyloid plaques ( ). Fig. 95.12 summarizes typical examples of Alzheimer pathology.
Aβ deposition in the brain occurs in a sequential manner starting in the cortex, followed sequentially by the hippocampus, basal ganglia, thalamus, and basal forebrain before in the final stages reaching the brainstem and cerebellum ( ). Plaques are complicated heterogeneous lesions composed of extracellular protein deposits and certain cellular components. Aβ derived from APP is the sine qua non of plaques, which are in turn the defining pathological finding in AD. Despite the strong association of Aβ with plaques, in reality plaques contain many other components. Other associated components of the plaque include APOE, alpha-1 antitrypsin, complement factors, and immunoglobulins. Plaques can be divided into diffuse (Aβ mainly) and neuritic plaques (includes damaged tau containing axons and dendrites, i.e., neurites). Cell components seen in plaques include neurites, microglia, and astrocytes. The typical neuritic plaque has a dense core of Aβ and less compact rim consisting of neurites, microglia, and astrogliosis at the periphery. Diffuse plaques have less diagnostic specificity and can be seen in a variety of different disorders. They contain Aβ but do not stain with tau-containing neurites and are not associated with synaptic loss, reactive astrocytes, or microglia.
The major component of the NFT is tau within neurons and their cell processes. First, pretangles form in the neuron cytoplasm, often concentrated around the membrane. Then, tau is organized into fibrils as NFTs. When the cell dies, the tangle may be situated extracellularly. Normal tau is a microtubule-associated protein and is not visible within the brain. In NFTs, tau has become hyperphosphorylated and abnormally conformed. This tau can be detected with immunostains that bind abnormally conformed tau. have described a fairly predictable spread of NFT pathology through the brain occurring in six stages which can be reduced to three, consisting of transentorhinal (stages I, II), limbic (III, IV), and isocortical (V, VI) stages. Braak staging has recently been modified to include brainstem regions preceding medial temporal involvement ( ).
Several other associated pathological findings may be seen with AD. CAA, often present in leptomeningeal vessels, capillaries, small arterioles, and middle-sized arteries, is seen in over 80% of AD cases. In contrast to cerebral plaques consisting primarily of Aβ42, CAA’s major component is Aβ40. Granulovacuolar bodies, typically found in the hippocampus, are small dense granules within the vacuole and can be labeled with acid phosphatase, tubulin, ubiquitin, and neurofilament. Hirano bodies (eosinophilic rod bodies), also typically found in the hippocampus, are often in neuronal processes and contain actin and actin-binding proteins. The significance of granulovacuolar bodies and Hirano bodies is incompletely understood.
Numerous studies have shown that dementia duration and severity correlate better with NFTs compared to amyloid plaques ( ; ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here