Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
When the heart stops and cerebral blood flow is interrupted during a cardiac arrest, patients lose consciousness and may remain comatose after resumption of circulation. Such a global injury to the brain is understandably profound, and more than 70% of patients die or remain comatose 24 hours after cardiopulmonary resuscitation (CPR) ( ). Anoxia describes the complete lack of oxygen delivery (e.g., complete cessation of blood flow during cardiac arrest), whereas hypoxia describes what may occur during times of decreased oxygen delivery, but with some degree of continued blood flow. Hypoxic-ischemic brain injury—albeit less well defined and less clearly understood than anoxic-ischemic injury—can occur in patients with respiratory arrest or severe hypoxemia (e.g., asphyxia).
Approximately 100,000 patients a year in the United States are admitted to intensive care units with anoxic-ischemic brain injury after CPR ( ). Although the pathophysiology of brain injury caused by cardiac arrest is reasonably well understood, less is known about neuroprotection. For nearly two decades, there was enthusiasm that induced hypothermia could not only improve survival rates but also improve neurological outcomes ( ), but these beliefs have been challenged ( ). This chapter critically evaluates the current knowledge of anoxic-ischemic brain injury. Studies have reported tools for predicting outcomes, and guidelines for prediction of poor outcome have been developed by the American Academy of Neurology ( ). The accuracy of these predictors after the use of therapeutic hypothermia or targeted temperature management (TTM) is a subject of ongoing research.
One of the more vital questions for scientists and clinicians is whether there is a specific period during resuscitation in which interventions can modify the degree of anoxic-ischemic brain injury and improve clinical outcomes. Is the damage to the brain permanent and present at ictus, or are there processes at work that could potentially be influenced and modulated? Several clinical facts are important. First, with cardiac arrest, whether due to asystole or ventricular fibrillation, there is no measurable flow to the brain. Moreover, even with standard CPR techniques, only one-third of the pre-arrest cerebral blood flow can be attained ( ). In addition, the shockable rhythms (ventricular tachycardia and ventricular fibrillation) have a better outcome than nonshockable rhythms such as asystole, pulseless electrical activity, and bradyarrhythmias, reflected by restoration of adequate cerebral blood flow when ejection fraction of the ventricle improves ( ). Secondly, there might be a critical time period after which CPR may fail to restore neuronal function. This time interval is poorly defined, but we know that the neuronal oxygen stores are depleted within 20 seconds of cardiac arrest, and cerebral necrosis occurs as a result of ischemia. There is some uncertainty about whether hypoxemia alone could produce necrosis, and, although it can cause damage (preferentially in the striatum), necrosis is rarely seen even in patients with arterial Pa o 2 values less than 20 mm Hg.
After 2–4 minutes of anoxia, several biochemical mechanisms that result in irreversible neuronal damage may become operative ( Fig. 83.1 ). Selective neuronal vulnerability to this type of injury involves areas in the CA-1 sector of the hippocampus, the thalami, the neocortex, and the cerebellar Purkinje cells ( Fig. 83.2 ). Necrosis of the cortex involves layers three, four, and five and is pathologically known as laminar necrosis . The vulnerability of these areas may be explained by the presence of receptors for excitatory neurotransmitters or the high metabolic demands of these neurons. An important question is whether necrosis or apoptosis occurs. The cell death cascade that involves several modulatory and degradation signals has been documented in global cerebral ischemia, but whether these processes can be effectively manipulated remains unclear ( ). A caspase inhibitor did not affect neurological outcome after 6 minutes of cardiopulmonary arrest in rats ( ).
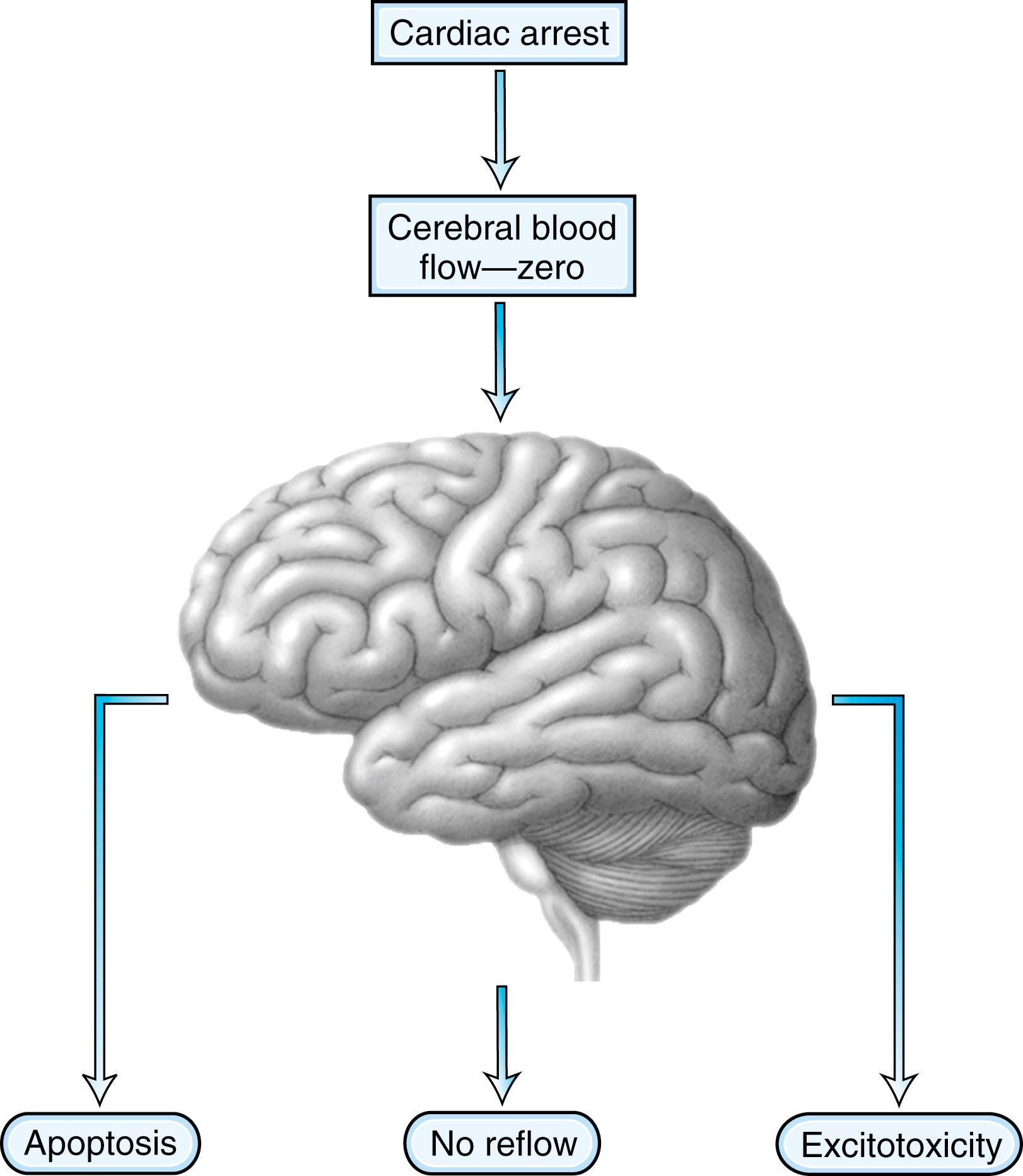
Another mechanism of neuronal and glial damage is excitatory brain injury. Glutamate efflux due to ischemic injury increases intracellular calcium concentration, which results in neuronal injury. The excess release of calcium leads to other processes that include activation of catabolic enzymes and endonucleases. Glutamate excitotoxicity has remained the major hypothesis to explain this type of neuronal injury and was made more probable after the documentation of neuroprotection with N -methyl- d -aspartate (NMDA) or α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonists.
In addition, research interest in anoxic brain injury has pointed toward a phenomenon called no reflow . This concept is based on the premise that after resumption of circulation, there are major microcirculatory reperfusion deficits. Coagulation may occur within these reperfusion zones, with intravascular fibrin formation and microthrombosis. This concept has served as a basis for experimental studies using recombinant tissue-type plasminogen activator (tPA) ( ). Despite our understanding of the pathophysiology of anoxic-ischemic injury based on careful animal experiments, the clinical reality of neuroprotection is discouraging. Clinical trials using barbiturates or calcium channel antagonists have been unsuccessful ( ). Induced hypothermia, which inhibits apoptosis and reduces free radical formation and excitatory neurotransmitters, was considered to be the only potentially beneficial intervention ( ), but even this has come into question and strict normothermia may be just as beneficial ( ). Patients who are comatose after CPR unfortunately often have a devastating outcome. Improvement of outcome might come from very early intervention and administration of neuroprotective agents at the onset of resuscitation, rather than when a patient enters the hospital.
Early awakening after CPR, clinical signs of localizing pain stimuli, and following commands are generally considered positive. However, the current literature provides no criteria on which a good long-term outcome can be reliably predicted. Most studies that have concentrated on the examination of the patient assume a poor outcome.
Clinical neurological examination follows a standard procedure, with examination of brainstem reflexes, motor response to pain, specific attention to myoclonus, and spontaneous or elicited eye movements. Because the brainstem is far more resilient to anoxic-ischemic injury than the cortex, brainstem reflexes, including the pupillary reflex to light, are often normal. Absent pupil responses can be caused by a high dose of intravenous atropine used during resuscitation, although a pupil response might still be found when examined under the magnifying glass. Fixed, dilated pupils presenting 6 hours after resuscitation are a sign of poor prognosis, but this is rarely present in isolation and is usually an indication that the rest of the brainstem has also been involved in the anoxic-ischemic injury. The eye examination may provide useful supporting evidence of anoxic injury ( ). Sustained upward gaze is often indicative of a significant global bihemispheric injury that may include the thalamus. A proposed mechanism explaining this phenomenon is a complete disinhibition of the vestibulo-ocular reflexes from the cerebellar flocculus ( ). Although forced upgaze is usually associated with poor outcomes, it is still compatible with survival in approximately 12%–15% of cases ( ). In some patients, downward gaze can be elicited using rapid head shaking or attempting to elicit a vestibular ocular response ( ). Other eye abnormalities, including ping-pong gaze or periodic lateral gaze deviations, have not been specifically examined for their prognostic value ( ). Continuous blinking is often a common finding in comatose patients, although its anatomical substrate is unknown.
An important clinical sign is myoclonus status epilepticus , defined as continuous and vigorous jerking movements involving facial muscles, limbs, and abdominal muscles ( ). These jerks can often be elicited or aggravated by touch or hand clap and may also involve the diaphragm, which complicates ventilation. Myoclonus status epilepticus has classically been considered an agonal phenomenon indicating an almost invariably poor prognosis, although exceptional cases have been reported ( ). This sustained, diffuse, vigorous myoclonus should not be confused with occasional myoclonic jerks. The majority of these patients have a malignant burst-suppression pattern on electroencephalogram (EEG) and do not survive. In others, EEG may show a continuous background with polyspikes concordant with myoclonic jerks, and, in these cases, favorable outcome is possible ( ). Myoclonus status epilepticus must be distinguished from myoclonus due to intoxication or hepatic encephalopathy and from generalized tonic-clonic seizures. Convulsive status epilepticus is uncommon, as is nonconvulsive status epilepticus (NCSE).
The motor response to pain should be classified and described as absent to pain, extensor response, pathological flexion response, withdrawal to pain, or localization. Lack of motor response to nail-bed compression at the initial assessment does not necessarily predict poor outcome. It may represent the “man-in-the-barrel” syndrome that occurs after bilateral border-zone infarction in the anterior and middle cerebral watershed regions. Involvement in this territory will result in prolonged weakness of the arms, with normal findings in the lower limbs. The outcome in these patients is often better than that for other patients with ischemic-anoxic injury.
The outcomes for patients in coma range from death, including brain death, to persistent vegetative state (see Chapter 35, Chapter 5 ), to awakening with disabilities ranging from the minimally conscious state (see Chapter 6 ) to complete recovery (see Chapter 55 ).
Awakening from coma can be protracted and prolonged, although the vast majority of patients who will awaken will do so within the first few days, provided they are not kept sedated. In our series of patients, 94 of 101 patients with post–anoxic-ischemic injury awoke within 3 days after cardiac arrest and induced hypothermia did not seem to directly influence this ( ). However, awakening can occur even 3 months after onset, although rarely without a severe deficit such as an amnesic syndrome or other neurological findings ( Table 83.1 ).
| Clinical Syndrome | Mechanism | Outcome |
|---|---|---|
| “Man-in-the-barrel” syndrome | Bilateral watershed infarcts | Uncertain, may improve substantially |
| Parkinsonism | Infarcts in the striatum | Improvement possible |
| Action myoclonus | Cerebellar infarcts | In awake patients, could improve with medication |
The neurological examination can be confounded by an additional systemic injury associated with CPR. Several patients may have an associated acute renal failure or liver injury. In addition, medications may have been administered to counter pain or to facilitate mechanical ventilation. Often patients have been treated with fentanyl and lorazepam, both of which have long elimination half-lives ( Table 83.2 ). The use of therapeutic hypothermia (TH) or targeted temperature management (TTM) may further prolong medication effects, as hepatic metabolism and renal clearance are decreased, which may cause an enhanced and prolonged effect of medications ( ).
| Agent | Elimination Half-Life (h) |
|---|---|
| Morphine | 1.5–4 |
| Fentanyl | 2–5 |
| Alfentanil | 1.5–3.5 |
| Midazolam | 1–4 |
| Lorazepam | 10–20 |
| Propofol | 2 |
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here